Abstract
Factor VIII (FVIII) administration elicits specific inhibitory antibodies (Abs) in about 25% of patients with hemophilia A. The majority of such Abs reacts with FVIII C2 domain. mAbBO2C11 is a high-affinity human monoclonal antibody (mAb) directed toward the C2 domain, which is representative of a major class of human FVIII inhibitors. Anti-idiotypic Abs were raised to mAbBO2C11 to establish their neutralizing potential toward inhibitors. One mouse anti-idiotypic mAb, mAb14C12, specifically prevented mAbBO2C11 binding to FVIII C2 domain and fully neutralized mAbBO2C11 functional inhibitory properties. Modeling of the 3-D conformation of mAb14C12 VH and alignment with the 3-D structure of the C2 domain showed putative 31 surface-exposed amino acid residues either identical or homologous to the C2 domain. These included one C2 phospholipid-binding site, Leu2251-Leu2252, but not Met2199-Phe2200. Forty putative contact residues with mAbBO2C11 were identified. mAb14C12 dose-dependently neutralized mAbBO2C11 inhibitory activity in mice with hemophilia A reconstituted with human recombinant FVIII (rFVIII), allowing full expression of FVIII activity. It also neutralized in an immunoprecipitation assay approximately 50% of polyclonal anti-C2 Abs obtained from 3 of 6 unrelated patients. mAb14C12 is the first example of an anti-idiotypic Ab that fully restores FVIII activity in vivo in the presence of an anti-C2 inhibitor. The present results establish the in vitro and in vivo proof of concept for idiotype-mediated neutralization of a major class of FVIII inhibitors.
Introduction
Hemophilia A is characterized by a functional deficiency of coagulation Factor VIII (FVIII), a cofactor for the formation of the intrinsic tenase complex, which is required for thrombin generation. Patients with hemophilia A suffer from spontaneous or posttraumatic bleeding into joints, muscles, or soft tissues. Replacement therapy using either plasma-derived or recombinant FVIII is complicated in about 25% of the patients by the development of specific antibodies (Abs) that neutralize FVIII function, also called inhibitors.1,2 The presence of an FVIII inhibitor precludes further specific replacement, thereby threatening the patient's life. To date, patients with FVIII inhibitor are treated by administration of bypassing agents, including recombinant FVIIa.3 Administration of high doses of FVIII over extended periods of time is considered in some cases, but such a therapy is extremely costly and limited to patients with recent and low inhibitor titers.4 Neutralizing anti-FVIII inhibitors nevertheless remains a major challenge and alternative; more specific strategies are warranted.
One of such strategies would be to directly neutralize the function of inhibitor Abs. Methods to inhibit the binding of inhibitory Abs to FVIII have been proposed, in which FVIII-derived peptides compete for the binding to inhibitor,5 but they have been limited practically by the absence of precise molecular information on such Abs and the risk of interfering with normal FVIII activity.
Despite its high molecular weight, FVIII presents only a limited number of B-cell epitopes, which are directly or indirectly involved in the function of the molecule.6 The majority of Abs react toward the C2 domain of FVIII, which mediates interactions with von Willebrand factor (VWF) and phospholipids (PLs). We recently derived a human monoclonal antibody (mAb), mAbBO2C11, from the peripheral memory B-cell repertoire of a patient with severe hemophilia and a FVIII inhibitor,7 and we demonstrated that mAbBO2C11 reacts with high affinity to the C2 domain of FVIII. Furthermore, the crystal structure of a complex of C2 and mAbBO2C11 Fab fragment demonstrated that the antibody recognizes a large conformational C2 epitope.8 Thus, mAbBO2C11 belongs to a prominent class of FVIII-specific Abs that inhibit the binding of FVIII to VWF and PL. Neutralizing anti-FVIII inhibitors nevertheless remains a major challenge. Alternative, more specific strategies are warranted.
Such neutralization could be achieved by using specific antiidiotypic Abs. An Ab idiotype refers to the ensemble of determinants that are located within its variable part. Anti-idiotypic Abs are second-generation Abs directed toward the variable part of pathogenic Abs and, as such, are highly specific. On the basis of previous findings that anti-idiotypic Abs may neutralize FVIII inhibitor Abs in the plasma of healthy individuals9,10 and of patients with hemophiliaAunder tolerance induction by administration of high doses of FVIII,11 it was predicted that idiotypic regulation could be used therapeutically to interfere with the function of FVIII inhibitors.12
Recent evidence has indicated that only a proportion of anti-FVIII Abs exert an inhibitory activity13 and that FVIII inhibitors could emerge from only a limited number of B-cell precursors, at least with regard to light chain-specific Abs.14 Taken together, this information suggests that a limited number of anti-idiotypic Abs could be all that is required to control FVIII inhibitors.
Starting from mAbBO2C11, we have generated mouse antiidiotypic Abs, and we provide in the present paper the in vitro and in vivo proof of concept, establishing the basis for idiotype-mediated neutralization of a major class of FVIII inhibitors.
Materials and methods
Reagents
Full-length human recombinant FVIII (rFVIII) was obtained as material for laboratory use only (rFVIII; Bayer, Berkeley, CA). Plasma-derived VWF was obtained from the Département Central de Fractionnement (DCF; Brussels, Belgium) and further purified as described.15 mAbBO2C11 IgG4 specific for the FVIII C2 domain has been fully described.7,8 Human mAbLE2E9 specific for the C1 domain15 and mouse mAbs directed toward different human FVIII domains or VWF were produced in our center (Center for Molecular and Vascular Biology [CMVB]).
Animals
C57BL/6 FVIII-/- with a target deletion in exon 16, initially obtained from Dr S. Antonarakis (Geneva, Switzerland), and BALB/c mice were bred in our animal facilities. All animal experiments were conducted according to the Ethical Committee Rules for Animal Experiments of the University.
Anti-idiotypic mAb production and characterization
mAbBO2C11 (10 μg) emulsified in complete Freund adjuvant (CFA) were injected subcutaneously in BALB/c mice, followed by 2 boost injections in incomplete Freund adjuvant (IFA) made at 2-week intervals. Antibody titers were measured by direct binding to mAbBO2C11-coated microtitration plates. Clones were obtained after fusing splenocytes with the SP2 myeloma cells and expanded under limiting dilution. mAb14C12 Fab fragments were prepared by digestion with papain agarose beads (Pierce, Rockford, IL) and purified by passage over a protein-A Sepharose column. Real-time kinetic interaction between mAb14C12 and mAbBO2C11 was evaluated by surface plasmon resonance by using a Pharmacia Biosensor BIAcore instrument (Pharmacia Biosensor AB, Uppsala, Sweden). Purified mAbBO2C11 (5 μg/mL in 10 mM sodium acetate buffer, pH 5.0) was immobilized on the activated surface of a CM5 sensor chip. mAb14C12 was infused at various concentrations over the mAbB02C11-immobilized sensor chip surface. Association and dissociation rate constants were determined as reported.7 For sequencing, mRNA from the mAb14C12 hybridoma was isolated by using the Quick Prep Micro mRNA Purification Kit (Amersham Pharmacia Biotech, Uppsala, Sweden). cDNA was synthesized with First-strand cDNA Synthesis Kit (Amersham Pharmacia Biotech). The cDNA encoding the heavy (VH) and light chain (VL) variable regions were amplified by polymerase chain reaction (PCR) using specific primers. PCR products were isolated from 1.5% agarose gel using the QIA quick Gel Extraction Kit (Qiagen, Hilden, Germany) and cloned using the pGEM-T Easy Vector system (Promega, Madison, WI). Plasmid DNA from positive colonies was isolated by using the High Pure Plasmid Isolation Kit (Roche Diagnostics, Mannheim, Germany) and sequenced in both directions with Sequenase (US Biochemical, Cleveland, OH).
Binding of mAb14C12 to mAbBO2C11 and its neutralization
Binding of mAb14C12 to mAbBO2C11. Microtitration plates were coated with 2 μg/mL mAbBO2C11. Samples containing mAb14C12 were added to the plate, and bound mAb14C12 was detected by addition of a horseradish peroxidase (HRP)–labeled goat antimouse IgG (Bio-Rad, Hercules, CA). Optical density (OD) was evaluated at 490 nm in a microtiter plate reader Emax (Molecular Devices, Sunnyvale, CA).
Inhibition of mAbBO2C11 binding to FVIII by mAb14C12. The concentration of mAbBO2C11 required to reach 80% of maximal binding to FVIII-coated plates was determined. This determination was carried out by using plates coated with 2 μg/mL rFVIII overnight at 4°C. All Ab solutions were diluted in casein buffer (citrate buffer containing 0.5% casein, 9 g/L NaCl, pH 7.2). Bound mAbBO2C11 was detected by using an HRP-conjugated mAb specific for human Fc (Southern Biotechnology, Birmingham, AL). For inhibition assays, 60 μL of 2 μg/mL mAbBO2C11 was mixed with an equal volume containing various concentrations of mAb14C12. The mixture was preincubated for 1 hour at 21°C. Aliquots of 50 μL were applied in duplicates to FVIII-coated plates for a further incubation of 2 hours at 21°C. mAbBO2C11 binding was detected as earlier, and inhibition was calculated from the ratio of OD values.
Neutralization of mAbBO2C11 inhibitory activity in a functional FVIII assay. To evaluate the capacity of mAb14C12 or of its Fab fragment to restore FVIII function in the presence of the inhibitor mAbBO2C11, we first determined the concentration of mAbBO2C11 required to inhibit 80% of FVIII activity in a functional chromogenic assay (Dade Behring, Marburg, Germany) by using 1 IU/mL rFVIII. The same determination was made with a total immunoglobulin G (IgG) fraction and with affinity-purified polyclonal anti-FVIII Abs obtained from the mAbBO2C11 donor. The amount of mAbBO2C11 (or of polyclonal Abs) required to inhibit 80% of FVIII activity was mixed with an equal volume of various concentrations of mAb14C12 or of its Fab fragment. The mixtures were incubated for 1 hour at 37°C before addition of rFVIII. An aliquot of the mixture was retrieved after a further 1-hour incubation at 37°C and added to the chromogenic assay reagents. Control experiments included rFVIII incubated alone or with an Ab of unrelated specificity.
3-D modeling and alignment of mAb14C12 with the C2 domain
The 3-D model of the variable parts of mAb14C12 was established by submitting the corresponding amino acid sequences to the Web Antibody Modelling (WAM; University of Bath, Swindon, United Kingdom; http://antibody.bath.ac.uk/). This algorithm takes into account both conserved structures of framework regions corrected for variations in particular beta strands and the large sequence and structure variability of complementarity determining regions (CDRs), which fall into one of the canonical classes,16 as well as the particular sequence of the CDR3. The method combines database and conformational searches, energy screening, and structural-determining residue filtering. Alignment of C2 domain and variable parts of mAb14C12 was carried out by combining manual alignment of secondary structures with the use of the Kabsch-Sander algorithm,17 and global sequence alignment was calculated with the Myers-Miller algorithm.18 The result was then refined by juxtaposing the 3-D structure of C2 domain19 and the WAM model of mAb14C12 Fv.
Inhibition of FVIII binding to VWF and PL
The capacity of mAb14C12 to interfere with the binding of FVIII to either VWF or PL was investigated as follows. Microtitration plates were coated with an anti-VWF Ab followed by purified VWF as described.15 mAb14C12 (1.25 μg/mL in phosphate-buffered saline [PBS] containing 0.5% casein) was mixed with an equal volume of FVIII at a final concentration of 1 IU/mL. Fifty microliters of the mixture was then added to the VWF- or PL-coated plates, and the plates further were incubated for 2 hours at 21°C. The binding of the FVIII to VWF was detected by the addition of 2 μg/mL biotin-labeled mAb15 (an anti–heavy chain–specific antibody), followed by avidin-peroxidase and a specific substrate. To assess FVIII binding on PL, plates were coated with phosphatidyl serine diluted at 10 μg/mL in methanol, and the capacity of mAb14C12 to inhibit the binding of FVIII to PL was investigated by following the same procedure as for the VWF assay. Control experiments included the substitution of mAb14C12 by mAbBo2C11, known to inhibit the binding of FVIII to both VWF and PL.7
Binding of mAb14C12 to anti-FVIII antibodies of unrelated donors and mouse
Cloning of the FVIII C2 domain cDNA fragment and expression in a reticulocyte transcription/translation system. The DNA fragment encoding for the C2 domain was generated by PCR using primers bound by the restriction sites HindIII and Not1. Sense primer, named according to the first FVIII amino acid residue encoded, was as follows: 2124, 5′-GATGCGAAGCTTGTCTTCTTTGGCAATGTGGATTCA-3′. The antisense primer, named according to the last encoded amino acid residue, was as follows: 2332, 5′-TTCTCGACTTGCGGCCGCGTAGAGGTCCTGTGCCTCGCAGCC-3′.
The PCR product was subcloned in frame into Signal pIgplus (R&D Systems Europe Abingdon, United Kingdom) and controlled by sequencing in both directions with the use of an ABI genetic Analyser 3.10 (Applied Biosystems, Foster City, CA).
A mixture containing all amino acids except methionine was added, together with 35S-methionine (Amersham, Bucks, United Kingdom). The presence of C2 in the supernatant was checked by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by autoradiography, showing a single band with the expected molecular mass of 23 kD.
Immunoprecipitation assay. The capacity of polyclonal anti-FVIII human Abs to recognize C2 was evaluated as described.7 The assay was adapted to determine whether mAb14C12 could prevent the binding to the C2 domain. Assays were carried out for plasma samples from 7 unrelated patients with hemophilia A inhibitor, including the patient from whom mAbBO2C11 was derived. Thus, mAb14C12 was diluted to final concentrations of 50, 150, or 450 μg/mL in 500 μL 50 mM Tris (tris(hydroxymethyl) aminomethane)–HCl buffer, pH 7.5, containing 150 mM NaCl, 0.1% Nonided NP-40, 1 mM EDTA (ethylenediaminetetraacetic acid), 0.25% gelatin, and 5% bovine serum albumin (BSA; NET-gel buffer). Undiluted plasma (20 μL) containing polyclonal anti-FVIII antibodies was added to each vial, and the mixtures were incubated for 1 hour at 4°C. From 1 to 3 μL L-(35S)methionine-labeled FVIII C2 domain was then added to the mixture for a further incubation of 1 hour at 4°C. Protein-A Sepharose (20 μL; Amersham Pharmacia Biotech) was then added to each vial, and the suspensions were again incubated for 1 hour at 4°C. Sepharose beads were centrifuged and washed twice with NET-gel buffer. Bound antigen/antibody complexes were eluted from beads by boiling for 2 minutes in 30 μL SDS gel-loading buffer. Supernatant (2 μL) was taken and diluted in 20 mL Lumasafe (Lumac LSC, Groningen, The Netherlands) for radioactivity counting.
As positive control, an aliquot of 2 μL mAbBO2C11 (1 mg/mL in PBS) was added to each of the mAb14C12 solutions, and the experiment was carried out as described earlier for polyclonal antibodies.
Binding of mAb14C12 to anti-FVIII mouse mAbs. Microtitration plates were coated with PBS containing 2 μg/mL mouse mAb to various FVIII domains, including the C2 domain. Plates were washed and further incubated for 2 hours at room temperature with biotin-labeled mAb14C12, followed by washing and sequential addition of streptavidin peroxidase and its substrate. mAb14C12 binding was evaluated by OD reading described earlier.
In vivo assays
Effect of mAb14C12 on FVIII clearance. To ensure that mAb14C12 did not modify FVIII clearance, C57BL/6 FVIII-/- mice were injected with 10 μg/mL mAb14C12 or an IgG2a mAb of unrelated specificity, followed 15 minutes later by an intravenous injection of 1 IU rFVIII. Blood samples were collected by venipuncture after 180 and 360 minutes. Residual FVIII activity was evaluated by using a functional chromogenic assay (Dade Behring).
Neutralization of mAbBO2C11-mediated FVIII inhibition by mAb14C12. The assay was run essentially as described,20 except for the use of mAb14C12 instead of peptides. Briefly, C57BL/6 FVIII-/- mice were reconstituted by intravenous injection of 1 IU rFVIII, which gives a concentration of 0.5 IU/mL and a reaction half time (t1/2) of 180 minutes.21 Residual FVIII activity was measured ex vivo by using a functional chromogenic assay on blood obtained by cardiac puncture. Three mice were used under each experimental condition. Mice were injected intravenously with 100 μL mAbBO2C11 at concentrations that ranged from 10 to 2.5 μg/mL in PBS followed 30 minutes later by 1 IU rFVIII, and the concentration resulting in 90% inhibition of FVIII activity (ie, 0.5 μg) was selected for neutralization assays. Controls included injection of a human IgG4 antibody of unrelated specificity. In a first series of experiments, complexes of mAbBO2C11 and mAb14C12 were formed prior to injection. Thus, 10, 1, or 0.1 μg mAb14C12 was mixed with 0.5 μg mAbBO2C11 in 100 μL PBS for 30 minutes at 37°C before the injection, followed by 1 IU rFVIII. Control experiments included mice injected with saline, mAbBO2C11 alone, mAb14C12 alone, and mAbBO2C11 mixed with an IgG2a mAb of unrelated specificity. In a second series of experiments, the capacity of mAb14C12 to combine with, and neutralize, mAbBO2C11 in vivo was evaluated. Thus, groups of 3 FVIII-/- mice were injected intravenously with 0.5 μg mAbBO2C11 or saline. After 30 minutes, each mouse received a second 100 μL intravenous injection of a mixture containing 1 IU rFVIII plus various amounts of mAb14C12, ranging from 0.1 to 10 μg. Blood samples were collected as described earlier for assessing residual FVIII activity.
Results
Anti-idiotypic Ab, mAb14C12, neutralizes in vitro the FVIII inhibitory activity of human mAbBO2C11
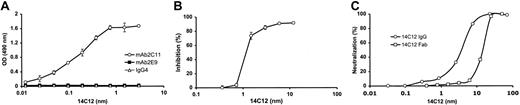
BALB/c mice were immunized with mAbBO2C11, a human anti-FVIII inhibitor Ab that recognizes a major determinant of the C2 domain.7 The supernatants of 8 clones reacted with mAbBO2C11 but not with an unrelated IgG4κ or with another human mAb specific for FVIII C1 domain. Two of these clones produced Abs that inhibited the binding of mAbBO2C11 to FVIII in a dose-dependent manner and neutralized the FVIII inhibitory activity of mAbBO2C11 in a functional assay. Figure 1 shows the results for one of the 2 mAbs, mAb14C12 (IgG2a). It can be seen that mAb14C12 specifically binds to mAbBO2C11, whereas it does not bind to a human anti-C1 mAb (Figure 1A). mAbBO2C11 binding to FVIII can be inhibited in a dose-dependent manner (Figure 1B). Fifty percent of the neutralization of the inhibitory activity was obtained in a chromogenic FVIII activity assay at a 1:2 mAbBO2C11/mAb14C12 molar ratio (Figure 1C). Fab fragments were prepared from mAb14C12 and shown to neutralize 50% of mAbBO2C11 inhibitory activity at a 1:8 mAbBO2C11/mAb14C12Fab molar ratio (Figure 1C), thereby confirming that the neutralization properties of mAb14C12 were brought about by residues located in the Ab variable parts.
Specificity and dose-dependent neutralization properties of mAb14C12. (A) mAb14C12 binds to microtitration plates coated with mAbBO2C11 but not to a human IgG4κ Ab of unrelated specificity or to an anti-FVIII C1 domain mAb (mAbLE2E9). (B) mAb14C12 inhibits the binding of mAbBO2C11 to plates coated with rFVIII. In panels A and B each point represents the mean of 2 determinations ± SD. (C) mAb14C12 or its Fab fragment neutralizes the FVIII inhibitory activity of mAbBO2C11 in a functional chromogenic assay as measured by single determination.
Specificity and dose-dependent neutralization properties of mAb14C12. (A) mAb14C12 binds to microtitration plates coated with mAbBO2C11 but not to a human IgG4κ Ab of unrelated specificity or to an anti-FVIII C1 domain mAb (mAbLE2E9). (B) mAb14C12 inhibits the binding of mAbBO2C11 to plates coated with rFVIII. In panels A and B each point represents the mean of 2 determinations ± SD. (C) mAb14C12 or its Fab fragment neutralizes the FVIII inhibitory activity of mAbBO2C11 in a functional chromogenic assay as measured by single determination.
The efficient neutralization of mAbBO2C11-mediated inhibitory activity prompted us to measure the binding affinity of mAb14C12 for mAbBO2C11. This measure was carried out by surface plasmon resonance, which showed association and dissociation rate constants of 5 × 105 M-1s-1 and 5 × 10-5s-1, respectively. Interestingly, these values are comparable to those calculated for the binding of mAbBO2C11 to FVIII,7 in keeping with the molar ratio of mAbBO2C11/mAb14C12 required to neutralize the inhibitory activity of mAbBO2C11 at equilibrium.
As mAbBO2C11 represents a significant part (± 70%) of the total inhibitory activity present in the plasma of the patient from whom it had been derived,7 we checked whether the capacity of mAb14C12 to neutralize mAbBO2C11 inhibitory activity could also be observed when using the plasma, a total IgG fraction, or a polyclonal preparation of affinity-purified anti-FVIII Abs, all obtained from the patient from whom mAbBO2C11 was derived. Thus, the volume of plasma or amount of polyclonal IgG antibodies was determined, which inhibited by 80% the activity of 1 IU/mL FVIII, as described in “Neutralization of mAbBO2C11 inhibitory activity in a functional FVIII assay.” To this plasma volume or amount of polyclonal IgG an aliquot of mAb14C12 was added containing different concentrations of this antibody. Incubation of the mixtures and assay of FVIII residual activity were carried out as described in “Materials and methods.” A maximum of 60% neutralization of FVIII inhibition was obtained (data not shown), whereas full neutralization was observed with the positive control containing mAbBO2C11. This finding confirmed that mAbBO2C11 represented a major part of the repertoire of inhibitor antibodies in this patient and that efficient neutralization could be achieved directly in plasma.
mAb14C12 shows extended homology with the C2 domain
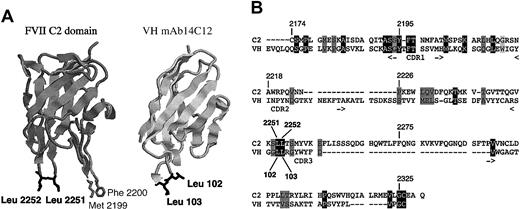
The sequence of mAb14C12 was aligned with that of the FVIII C2 domain. A significant homology was found between the C2 domain and the VH region. Figure 2 shows that 31 amino acid residues were found either identical or similar. This included 13 residues associated with CDR regions, but clustering to the CDR1 (6 residues) and CDR3 (6 residues). Interestingly, a significant contribution is observed from residues located in framework regions (18 amino acids). Moreover, only 2 residues (marked with an asterisk) of the VH region are mutated from the germ line sequence of mAb14C12. All 31 residues are surface exposed on C2, according to the crystal structure of the C2 domain19 (Figure 2), as well as on mAb14C12 VH region. A salient feature of this alignment is that it involves 1 of the 2 C2 PL binding sites made of Leu2251 and Leu2252 (total surface of 180 A), which correspond to Leu102 and Leu103 in mAb14C12 VH CDR3, whereas the second site (Met2199 and Phe2200) is not represented. Taken together these observations indicate that the VH region of mAb14C12 in its germ line configuration carries an extended homology of the C2 domain.
Homology between the C2 domain of FVIII and VH region of mAb14C12. (A) The sequence homology between the variable parts of mAb14C12 and the C2 domain of FVIII, including one PL binding site (Leu2251-Leu2252), is primarily carried by the VH region. (A) Views of a model structure of the FVIII C2 domain on which amino acids Leu2251 and Leu2252, highlighted in black, can be aligned with identical residues Leu102 and Leu103 of the VH mAb14C12. (B) Sequence alignment resulting from combination of data obtained by secondary structure alignment, global sequences alignment, and 3-D structural comparison are shown. Homologue residues between C2 domain and mAb14C12 are highlighted in black; similar residues are highlighted in gray. For mAb14C12, CDR position is indicated above the sequence.
Homology between the C2 domain of FVIII and VH region of mAb14C12. (A) The sequence homology between the variable parts of mAb14C12 and the C2 domain of FVIII, including one PL binding site (Leu2251-Leu2252), is primarily carried by the VH region. (A) Views of a model structure of the FVIII C2 domain on which amino acids Leu2251 and Leu2252, highlighted in black, can be aligned with identical residues Leu102 and Leu103 of the VH mAb14C12. (B) Sequence alignment resulting from combination of data obtained by secondary structure alignment, global sequences alignment, and 3-D structural comparison are shown. Homologue residues between C2 domain and mAb14C12 are highlighted in black; similar residues are highlighted in gray. For mAb14C12, CDR position is indicated above the sequence.
mAb14C12 does not interfere with FVIII binding to either VWF or PL and does not reduce FVIII clearance rate from the circulation
The extended homology between the C2 domain and mAb14C12 VH, which involved FVIII VWF and PL binding sites, prompted us to verify whether mAb14C12 was able to inhibit the binding of FVIII C2 domain to VWF and/or to PL. No such inhibition was observed for either VWF or PL in the presence of mAb14C12, whereas control experiments carried out in the presence of mAbBo2C11 showed complete inhibition in both cases (Table 1). As mAb14C12 carries only one of the PL binding sites of C2 (made of Leu2251 and Leu2252), we also checked whether the antibody could interfere with the binding of a mutant of FVIII with a deletion of Ala2201, known to alter the PL-binding capacity through Met2199 and Phe2200.22 No inhibition was found (data not shown), indicating that absence of inhibition of C2 binding to PL was not solely related to absence of the Met2199-Phe2200 residues.
Effect of mAb14C12 on FVIII binding to VWF and PL and clearance rate
. | FVIII + mAb14C12 . | FVIII + mAbBO2C11 . |
|---|---|---|
| FVIII binding to VWF, % | 100 | 1 |
| FVIII binding to PL, % | 96 | 3 |
| Residual FVIII activity, IU/mL | 0.34 | ND |
. | FVIII + mAb14C12 . | FVIII + mAbBO2C11 . |
|---|---|---|
| FVIII binding to VWF, % | 100 | 1 |
| FVIII binding to PL, % | 96 | 3 |
| Residual FVIII activity, IU/mL | 0.34 | ND |
ND indicates not determined.
The capacity of mAb14C12 to interfere with the binding of FVIII to VWF or PL was evaluated in enzyme-linked immunosorbent assay (ELISA). Microtitration plates were coated with VWF or PL as described in “Materials and methods.” FVIII (1 IU/mL) was preincubated with buffer alone, or buffer containing 1.25 μg/mL of either mAb14C12 or mAbBO2C11 as a control, and the mixtures were added to VWF- or PL-coated plates. The residual binding of FVIII was evaluated by sequential addition of a biotin-labeled FVIII heavy chain–specific mAb (mAb15) and avidin-peroxidase. Assays were made in duplicates. Results are shown as percentages of residual FVIII binding, taking preincubation with buffer alone as 100%.
The effect of mAb14C12 on FVIII clearance was measured by injecting 1 IU FVIII intravenously in C57BL/6 FVIII-/- mice, alone or 15 minutes after intravenous injection of a single dose of 10 μg mAb14C12. Bleeding was carried out at 180 minutes, namely the expected FVIII t1/2 in this animal model,17 and the residual FVIII activity was measured in a chromogenic assay (described in “Materials and methods”). The 0.34 IU/mL measured in mice pretreated with mAb14C12 compare with the 0.32 IU/mL measured in mice injected with FVIII alone.
The clearance of FVIII is at least in part due to the binding of the C2 domain to the lipoprotein-receptor-related protein (LRP) receptor23,24 and could, therefore, be reduced by mAb14C12. This was examined in FVIII-/- C57BL/6 mice reconstituted by tail injection of 1 IU human rFVIII. Previous calculation has shown that the average human FVIII t1/2 in this model was 180 minutes.21 The experiment was repeated twice with mice that were first injected with 10 μg mAb14C12, followed 15 minutes later by 1 IU FVIII. No significant difference in FVIII t1/2 was observed in the presence of mAb14C12 after either 180 minutes (Table 1) or 360 minutes. Despite extensive homology between mAb14C12 VH and the C2 domain, the former does not seem to interfere with the physiologic activity and clearance of FVIII.
mAb14C12 binds to unmutated CDR and framework residues of mAbBO2C11
Aligning mAb14C12 VH and VL regions with those of mAbBO2C11 identified a total of 40 putative contact residues between the 2 antibodies (data not shown). Five of mAb14C12 residues are homologous to the C2 domain. Interestingly, only 12 of the 40 putative contact residues are located within CDR of mAb14C12. Residues located in the framework regions of mAb14C12, therefore, contribute significantly to the recognition of mAbBO2C11. Again, mutations contribute very little to this interaction, as only 3 of 40 amino acid residues of mAb14C12 are mutated, suggesting that mAb14C12 reacts in its germ line configuration with mAbBO2C11. However, 5 of the 40 putative contact residues within the variable parts of mAbBO2C11 are involved in the binding to C2. The majority (24 of 40) of such residues in mAbBO2C11 is located in framework regions, and only 6 mutations are observed in putative contact residues. This finding suggests that mAb14C12 reacts with the canonical sequence of the subfamily of Abs to which mAbBO2C11 belongs.
Altogether, the neutralization properties of mAb14C12 on mAbBO2C11 binding to, and inhibition of, FVIII, therefore, result from a combination of an extended homology with the C2 domain and recognition of mAbBO2C11 variable part residues not involved in C2 binding. However, attempts to raise anti-C2 inhibitory Abs by subcutaneous (SC) immunization of BALB/c mice with mAb14C12 in CFA/IFA have failed.
mAb14C12 inhibits the binding to C2 of polyclonal antibodies from unrelated patients
The extended complementarity between variable parts of mAb14C12 and those of mAbBO2C11 in germ line configuration suggests that mAb14C12 could neutralize anti-C2 inhibitory antibodies that carry a canonical sequence identical or close to that of mAbBO2C11. This finding is further stressed by the observation that a major proportion of anti-C2 inhibitory antibodies belong to the DP5 subfamily.14 We, therefore, evaluated the capacity of mAb14C12 to neutralize the inhibitory activity of anti-C2 domain polyclonal antibodies of unrelated patients with hemophilia A. To this end, we used an immunoprecipitation assay in which a radiolabeled C2 domain, obtained by combined transduction-translation with rabbit reticulocytes, was allowed to react in fluid-phase with samples of plasma containing inhibitory Abs to the C2 domain. This assay detects all anti-C2 Abs, irrespective of their capacity to inhibit the function of FVIII.
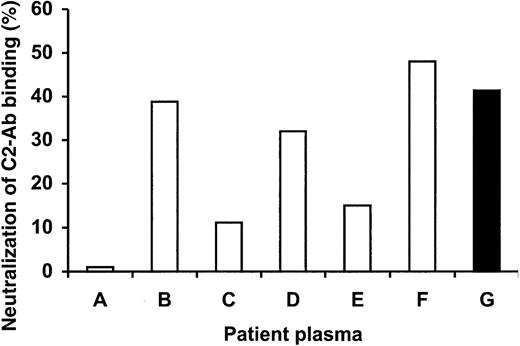
Figure 3 shows that mAb14C12 neutralized up to 50% of the binding capacity of polyclonal anti-C2 Abs purified from the patient from whom mAbBO2C11 was derived (patient G). In such an assay, mAb14C12 also significantly neutralized polyclonal anti-FVIII Abs obtained from 3 of 6 unrelated patients (patients B, D, E). Idiotopes carried by mAbBO2C11 are, therefore, present on a significant proportion of anti-C2 Abs from unrelated patients. However, the capacity of mAb14C12 to neutralize FVIII inhibition was not paralleled by the results obtained in the immunoprecipitation assay (data not shown), indicating that, even in the presence of anti-C2 Abs, the latter are not necessarily the prominent inhibitors.
mAb14C12 blocks C2 domain recognition by inhibitory Abs in plasma samples taken from patients with hemophilia A inhibitor. Plasma samples of unrelated patients with hemophilia A (A-F), or of the patient from whom mAbBO2C11 was cloned (G), were preincubated with 50, 150, or 450 μg/mL mAb14C12 before addition of 35S-methionine–labeled C2 domain, to establish how much mAb14C12 was needed to block the binding of C2 to inhibitory antibodies. IgG Abs from plasma were then precipitated by protein-A Sepharose, and residual radioactivity was counted on the pellet. Maximum blocking of C2 binding (100%) was obtained by adding 450 μg mAb14C12 to the FVIII-mAbBO2C11 mixture. Three of 6 plasma samples (B,D,F) show reactivity with mAb14C12 equivalent to that of the mAbBO2C11 source patient (G). The experiment was run only once and in single determination because of a shortage of plasma material. A control experiment (not shown) was carried out in which human mAbBO2C11 was used instead of polyclonal antibodies, which resulted in 100% neutralization.
mAb14C12 blocks C2 domain recognition by inhibitory Abs in plasma samples taken from patients with hemophilia A inhibitor. Plasma samples of unrelated patients with hemophilia A (A-F), or of the patient from whom mAbBO2C11 was cloned (G), were preincubated with 50, 150, or 450 μg/mL mAb14C12 before addition of 35S-methionine–labeled C2 domain, to establish how much mAb14C12 was needed to block the binding of C2 to inhibitory antibodies. IgG Abs from plasma were then precipitated by protein-A Sepharose, and residual radioactivity was counted on the pellet. Maximum blocking of C2 binding (100%) was obtained by adding 450 μg mAb14C12 to the FVIII-mAbBO2C11 mixture. Three of 6 plasma samples (B,D,F) show reactivity with mAb14C12 equivalent to that of the mAbBO2C11 source patient (G). The experiment was run only once and in single determination because of a shortage of plasma material. A control experiment (not shown) was carried out in which human mAbBO2C11 was used instead of polyclonal antibodies, which resulted in 100% neutralization.
Additional experiments were carried out to determine whether the idiotype recognized by mAb14C12 was also presented on mouse anti-FVIII antibodies. A total of 50 mAbs were assayed in a direct binding ELISA, including 3 inhibitory mAbs with C2 specificity; none of such mAbs was, however, competing with mAbBO2C11. mAb14C12 did not recognize any of these mAbs (data not shown).
mAb14C12 neutralizes mAbBO2C11-mediated inhibiting activity in vivo
To determine whether mAb14C12 had the capacity of neutralizing the inhibitory activity of mAbBO2C11 in vivo, FVIII-/- mice were reconstituted with rFVIII and mAbBO2C11.20 The capacity of mAb14C12 to restore the function of FVIII was examined by using various concentrations of mAb14C12. Preliminary experiments have established that the t1/2 of mAbBO2C11 and mAb14C12 in FVIII-/- mice were of 3 and 5 days, respectively.
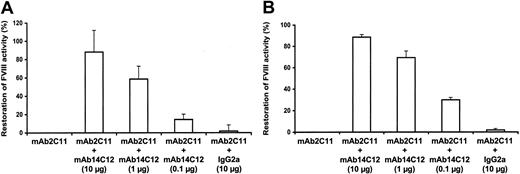
Injection of 1 IU FVIII is sufficient to obtain an average concentration of 0.5 IU/mL. Intravenous administration of 0.5 μg mAbBO2C11 30 minutes before FVIII completely inhibits FVIII activity (Figure 4). The capacity of mAb14C12 to neutralize mAbBO2C11 in vivo and, therefore, to restore FVIII function was evaluated by mixing 10, 1, and 0.1 μg mAb14C12 to 0.5 μg mAbBO2C11 before injection to mice that were injected with 1 IU FVIII 30 minutes later. Figure 4A shows that the mAbBO2C11 inhibitory activity is neutralized in a dose-dependent manner, with 50% neutralization obtained at approximately a one-to-one molar ratio. A maximum of 88.3% (± 23.8%) inhibition was observed at a 50-fold molar excess of mAb14C12.
mAb14C12 capacity to restore normal FVIII function in vivo in the presence of mAbBO2C11. C57BL/6 FVIII-/- mice injected intravenously with 0.5 μg mAbBO2C11 and 1 IU FVIII show complete inhibition of FVIII activity. Injection of complexes made of mAbBO2C11 and various concentrations of mAb14C12 (A) or sequential administration of mAbBO2C11 followed by mAb14C12 (B) show a dose-dependent neutralization of the inhibitory activity of mAbBO2C11 with full FVIII functional activity as measured in a chromogenic assay. Bars show mean of 3 values ± SD. Under both experimental conditions, ± 50% restoration was obtained at an mAbBO2C11/mAb14C12 equimolar ratio. An unrelated IgG2a mAb had no effect.
mAb14C12 capacity to restore normal FVIII function in vivo in the presence of mAbBO2C11. C57BL/6 FVIII-/- mice injected intravenously with 0.5 μg mAbBO2C11 and 1 IU FVIII show complete inhibition of FVIII activity. Injection of complexes made of mAbBO2C11 and various concentrations of mAb14C12 (A) or sequential administration of mAbBO2C11 followed by mAb14C12 (B) show a dose-dependent neutralization of the inhibitory activity of mAbBO2C11 with full FVIII functional activity as measured in a chromogenic assay. Bars show mean of 3 values ± SD. Under both experimental conditions, ± 50% restoration was obtained at an mAbBO2C11/mAb14C12 equimolar ratio. An unrelated IgG2a mAb had no effect.
A second series of experiments was carried out in which FVIII-/- mice were first injected with mAbBO2C11 (0.5 μg) followed 30 minutes later by a mixture of FVIII (1 IU) and various concentrations of mAb14C12. Results similar to the ones reported above with the preincubation of mAb14C12 and mAbBO2C11 were observed with sequential administration of the 2 antibodies (Figure 4B), which indicates that in vivo the affinity of mAb14C12 for mAbBO2C11 is high enough to prevent the binding of mAbBO2C11 to FVIII.
Discussion
Selective neutralization of inhibitor Abs represents one of the hoped-for outcomes of immunotherapy strategies. This outcome could in theory be achieved by using FVIII polypeptides containing Ab-binding sites, as proposed early after the cloning of FVIII was obtained.5,25 However, Ab epitopes are frequently located in regions of FVIII by which it interacts with physiologic partners such as VWF and/or PL. Hence, fragments of FVIII could interfere with such binding and reduce the procoagulant activity of FVIII. To circumvent this risk, alternative strategies have been sought, notably through the use of synthetic peptides mimicking conformational epitopes with a limited number of contact residues.20 Although promising, this approach suffers from the fact that small peptides have a short t1/2 and usually exhibit a low affinity, requiring large doses to achieve a physiologic effect.
A valuable compromise between these 2 possibilities would be to produce large mimotopes, namely FVIII epitopes mimicking natural ones but divergent in amino acid sequence. This would potentially offer better stability and prolonged t1/2 as compared with small peptides. Moreover, such large mimotopes can be altered in such a way as to eliminate the risk of interfering with FVIII function, while maintaining a strong, high-affinity reaction with inhibitor Abs. Obtaining large mimotopes by synthesis is cumbersome and unpractical. However, anti-idiotypic Abs exhibiting extended homology with the antigen could serve as such, or as a template to prepare large mimotopes.
The production of suitable anti-idiotypic Abs for this purpose has been delayed until specific human mAbs with FVIII inhibitory activity were obtained by derivatization of peripheral memory B cells from patients with hemophilia A with inhibitors.7 This, combined to the elucidation of the crystal structure of FVIII domains,8 has opened the possibility of exploring at the clonal level the therapeutic potential of anti-idiotypic Abs for FVIII inhibitors.
The results reported here establish the proof of concept that this strategy is appropriate for designing efficient mimotopes for FVIII inhibitors. Starting from a unique human mAb representative of a major class of inhibitors we have produced an anti-idiotypic Ab that combines several properties: (1) it carries an extensive sequence homology with the C2 domain, spanning more than 90 amino acid residues; (2) it shows an affinity for the variable regions of the anti-C2 Ab comparable to that of the inhibitor Ab for C2; (3) it does not interfere with the binding of FVIII to either VWF or PL, nor does it alter the clearance rate of FVIII; and (4) it fully neutralizes the inhibitory activity of mAbBO2C11 in a reconstituted FVIII-/- mouse model.
The sequence homology between the variable parts of mAb14C12 and the C2 domain of FVIII is primarily carried by the VH region. Remarkably, the latter contains 31 putative surface-exposed residues identical or similar to C2, including one PL binding site (Leu2251-Leu2252). Interestingly, the homology includes a significant number of conserved residues of C2, suggesting significant structural homology between the 2 molecules. This homology is illustrated functionally by the observation that neutralization of mAbBO2C11 inhibiting activity is obtained at molar equivalence between mAbBO2C11 and mAb14C12, both in vitro and in vivo, in keeping with affinity measurement. mAb14C12 can, however, not be considered as carrying a complete internal image of the C2 domain, insofar as it does not bind to VWF or PL, it does not interfere with the binding of FVIII to either VWF or PL and does not elicit anti-C2 Abs by conventional immunization.
An additional reason as to why the homology between the C2 domain and mAb14C12 VH region is interesting is related to the strong participation of residues located in mAb14C12 framework regions (ie, 45%), with only 6 mutated residues over a total of 31 (21%). In other words, the C2 domain presents a sequential and structural homology with some families of Abs in their germ line configuration.26 The C2 domain general organization is made of a barrel of 2 series of 4 beta sheets linked by flexible loops and is reminiscent of the organization of immunoglobulin domains, with loops represented by hypervariable regions, or CDRs, constituting the antigen-binding site.
The results obtained in vivo with FVIII-/- mice reconstituted with human FVIII suggest that mAb14C12 Abs and the like have a therapeutic potential. Full humanization of the Ab would reduce its potential immunogenicity but will also prolong its t1/2 to up to 3 weeks. A single injection could, therefore, exert a significant effect for more than 1 month, which represents a definite advantage over small peptides. The risk of inducing Abs cross-reacting with the C2 domain on administration of mAb14C12 is likely to be remote, because of the use of such a fully humanized Ab and the fact that administration would be made by the intravenous route only. Besides, only a single or a small number of injections would be all that is required. Finally, the anti-idiotypic Ab variable part can be used as a template for the design of molecules, which would be even more adapted to the neutralization of inhibitors, such as multimeric versions of mAb14C12 VH region,27 or combination of V regions from different anti-idiotypic Abs.
The results presented here show that idiotypes recognized by mAb14C12 are expressed by a significant number of patients that have anti-C2 inhibitory Abs. This seemingly reflects the observation that the interaction between anti-C2 inhibitors similar to mAbBO2C11 and mAb14C12 depends on residues present in the Ab germ line sequence. C2 inhibitors are prominent in the production of anti-FVIII inhibitors: mAb14C12 or a derivative of it might, therefore, be helpful for a significant proportion of patients with inhibitor. However, current knowledge suggests that a second C2 epitope is recognized by inhibitory Abs,13 which would mean that full neutralization of C2 inhibitors would require another set of anti-idiotypic Abs. We are currently extending our approach to identify such a second epitope.
Currently, 5 main clusters of B-cell epitopes have been described, although the vast majority of antibodies bind to either the C2 or the A2 domain.28 Efficient use of anti-idiotypic antibodies for patients with inhibitors would, therefore, require in addition to neutralize antibodies toward at least the main A2 domain. Whether anti-A2 antibodies derive from a small number of precursors as do anti-C2 antibodies is not fully understood.29 Obviously, this should be determined through clonal analysis of anti-A2 antibodies derived from patients' memory cell repertoire. Whatever the case, it is likely that efficient neutralization of inhibitors would require a set of anti-idiotypic antibodies or of their derivatives. However, full neutralization might not be an absolute requirement, as a reduction of inhibitor titers to levels of 10 BU/mL or less is sufficient to render patients amenable to tolerance induction using infusions of FVIII.30 Another possible difficulty might emerge from the fact that inhibitor antibodies change specificity over time and could, therefore, “escape” control by anti-idiotypic antibodies. Specificity can change for a number of reasons, associated to the FVIII product used, the conditions under which FVIII is administered (bleeding, infection), but also as the result of an evolving immune response. The latter can be brought about by somatic hypermutation, receptor editing, or epitope spreading.31 The efficacy of anti-idiotypic neutralization of inhibitors will depend on the possibility of generating reagents interacting with inhibitors in germ line configuration, as it is suggested in the present study.
Potential therapeutic applications include the use of antiidiotypic Abs for short-term neutralization of inhibitors, for instance before a surgical procedure. Such Abs have the potential of interacting with B cells carrying the corresponding idiotype. The consequence of this binding can be a functional neutralization of the cell producing the inhibitor or even the translation of a signal leading to target cell apoptosis.32-34 Besides, the heavy chain of the Ab can be engineered in such a way as to favor its interaction with complement or with the Fcγ receptor present on natural killer (NK) cells.35 Therefore, it can be expected that anti-idiotypic Abs would induce a long-lasting elimination of inhibitor production.
Prepublished online as Blood First Edition Paper, December 11, 2003; DOI 10.1182/blood-2003-07-2207.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal