Abstract
The clinical, cytogenetic, and molecular findings of 2 Fanconi anemia (FA) subtype D1 kindreds, initially identified through a young child with a solid tumor (medullobastoma, Wilms tumor), are described. Each kindred subsequently had a second affected child; one developed Wilms tumor followed by a medulloblastoma, and the other developed T-lineage acute lymphoblastic leukemia. Cytogenetic studies revealed an unusually high spontaneous chromosome aberration rate, contrasting with other FA subtypes. Molecular analysis revealed biallelic BRCA2/FANCD1 mutations. The patients did not exhibit bone marrow failure. Our studies suggest that the D1 subtype represents a severe end of the cytogenetic spectrum within FA, consistent with a critical downstream role of BRCA2 in the FA pathway. Furthermore, this FA subgroup may be preferentially associated with an increased predisposition to solid tumors in early childhood. Recognition of this constellation of findings has significant implications for medical management and genetic counseling of FA families.
Introduction
Fanconi anemia (FA) is an autosomal recessive disease associated with variable congenital anomalies, bone marrow failure, and predisposition to malignancy.1,2 Bone marrow failure usually precedes the development of myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML), but some patients initially present with MDS or AML. Other tumor types have more rarely been observed.3-5 The median age of onset of hematologic disease in children with FA is 7 years.6
FA is heterogeneous in its clinical manifestations and underlying genetic defects, yet it is unified by its cellular hypersensitivity to DNA cross-linking agents such as mitomycin C (MMC) and diepoxybutane (DEB).7,8 This hypersensitivity to cross-linking agents is the basis of the standard in vitro diagnostic test of FA and has led, in combination with somatic cell fusion assays, to the identification of at least 11 different complementation groups (A, B, C, D1, D2, E, F, G, I, J, and L).2,9,10 Eight FA genes have been cloned.1,2 Although the specific function of these genes has not been elucidated, they cooperate in a common pathway involved in the repair of damaged DNA.1,11,12 Recently, it has been shown that the D1 subgroup results from biallelic mutation of the BRCA2 gene.13 Unlike other FA proteins, BRCA2 appears to play a more direct role in DNA repair through DNA binding,14 RAD51 binding,15,16 and regulation of homologous recombination activity.17
To date, there appears to be little correlation between specific FA subgroups and specific cellular or clinical findings.18 In general, FA-G patients have a more severe clinical course, with earlier onset of bone marrow failure and hematologic malignancies, compared with FA-A patients. Here we describe clinical and cytogenetic findings in 2 kindreds, supporting the hypothesis that the FA-D1 subgroup represents a severe end of the FA spectrum from both the cytogenetic and oncologic perspectives. Our studies reveal that FA-D1 individuals have not only high levels of chromosome damage in response to cross-linking agents but also unusually high baseline (or spontaneous) levels of chromosome abnormalities. These spontaneous rates are higher than those observed in other FA subgroups or in other chromosomal instability syndromes.19 Further, these FA-D1 kindreds were not identified through the hematologic findings of aplastic anemia and bone marrow failure, but rather through a young child presenting with a solid tumor (medulloblastoma, Wilms tumor) who subsequently showed lethal sensitivity to chemotherapy. Each kindred later had a second affected child, one who developed a Wilms tumor followed by a medulloblastoma, and the other who developed T-lineage acute lymphoblastic leukemia (ALL). Such malignancies are rare among patients with FA.4,5,20
Patients, materials, and methods
Patients
All 4 children (kindred 1-sibling 1 and sibling 2; kindred 2-sibling 1 and sibling 2) were cared for at the Children's Hospitals and Clinics (Minneapolis, MN) between March 1988 and the present. Informed consent was obtained for all patient samples in accordance with University of Minnesota Medical School Institutional Review Board requirements. Samples were coded to protect patient confidentiality.
Establishment of cell cultures for cytogenetic analyses
Cell cultures for cytogenetic analyses were established from both siblings from kindred 1 and from the younger sibling (sibling 2) from kindred 2. Peripheral blood lymphocytes (PBLs) or skin fibroblasts were evaluated. PBLs or fibroblasts from a healthy control subject were processed concurrently. Cultures were initiated with phytohemagglutinin (M form; Gibco, Grand Island, NY) in RPMI 1640 media supplemented with 18% fetal calf serum (Hyclone, Logan, UT). Fibroblasts from skin biopsies were initiated in culture in T-25 flasks in Chang Medium D (Irvine Scientific) supplemented with 1% glutamine-penicillin-streptomycin, and early-passage fibroblasts (<10-passage stage) were used for breakage studies.
Evaluation of chromosome abnormalities
Metaphase cells (both PBLs and fibroblasts) were G-banded after brief pretreatment in 25% pancreatin (Gibco), followed by counterstaining with Wright stain. Fifty G-banded metaphase cells from the baseline, MMC, and DEB conditions were evaluated for chromosomal abnormalities including chromatid and chromosome gaps and breaks, deletions, balanced translocations, unbalanced translocations (ie, derivative chromosomes), inversions, rings, and radial figures, as previously described.21
Complementation analysis
Complementation analysis for FA subtypes A, C, and G was performed as previously described22 with retroviral vectors carrying cDNA of FANCA, FANCC, and FANCG genes.
Analysis of monoubiquitination of FANCD2
The status of FANCD2 monoubiquitination was evaluated after lysing cells and immunoblotting with an anti-FANCD2 monoclonal antibody as previously detailed.12 This assay distinguishes the nonubiquitinated FANCD2 (FANCD2-S) from the monoubiquitinated FANCD2 (FANCD2-L) isoform.
BRCA2 protein expression
Expression of BRCA2 was evaluated by immunoblotting with antibodies against BRCA2, as previously described.13
Sequencing of the BRCA2 gene
Sequencing of BRCA2 from gDNA has been described previously.13
Results
Two kindreds with solid tumors of early childhood and severe chemotoxicity
Kindred 1. Sibling 1 was a 27-month-old white boy who presented with gait instability. His past medical history was remarkable for normal birth weight, patent ductus arteriosus, and developmental delay. Blood counts were normal. Computed tomography scan revealed a mass in the right cerebellar hemisphere. Gross total tumor resection was accomplished, and pathology was consistent with medulloblastoma. Chemotherapy (vincristine, etoposide, cisplatin, and cyclophosphamide) was initiated. On day 8, he developed rash, fever, and pancytopenia. He recovered by day 35 and started his second course of chemotherapy, but subsequently developed severe treatment toxicity 9 weeks after initial diagnosis. His clinical and hematologic features are summarized in Table 1.
Clinical and hematologic findings
. | Kindred 1-sibling 1 . | Kindred 1-sibling 2 . | Kindred 2-sibling 1 . | Kindred 2-sibling 2 . |
|---|---|---|---|---|
| Clinical findings | ||||
| Aplastic anemia | No | No | No | No |
| Hyperpigmentation (café-au-lait spots) | Yes | Yes | No | No |
| Thumb and radial anomalies | No | No | No | Bifid thumb |
| Skeletal anomalies | No | No | No | No |
| Congenital kidney defects | No | No | No | No |
| Microcephaly or microphthalmia | Yes | Yes | Yes | Yes |
| Mental retardation or learning disabilities | No | No | No | No |
| Low birth weight | No | No | Yes | Yes |
| Gastrointestinal defects | No | No | No | No |
| Heart defects | Yes | No | No | No |
| Tumor | Medulloblastoma | Wilms tumor; medulloblastoma | Wilms tumor; AML | T-cell ALL |
| Hematologic findings | ||||
| RBC count, × 1012/L | 4.29 (4.6) | 5.34 (4.5) | NA | 4.28 (4.6) |
| Hemoglobin, g/L | 132 (125) | 114 (120) | NA | 126 (125) |
| Hematocrit | 0.384 (0.37) | 0.373 (0.36) | NA | 0.361 (0.37) |
| MCV, fL | 90 (81) | 70 (78) | NA | 84 (81) |
| WBC count, × 109/L | 16.8 (6-17) | 5.4 (6-17.5) | NA | 13.4 (6-17) |
| Platelet count, × 109/L | 522 | 334 | NA | 194 |
. | Kindred 1-sibling 1 . | Kindred 1-sibling 2 . | Kindred 2-sibling 1 . | Kindred 2-sibling 2 . |
|---|---|---|---|---|
| Clinical findings | ||||
| Aplastic anemia | No | No | No | No |
| Hyperpigmentation (café-au-lait spots) | Yes | Yes | No | No |
| Thumb and radial anomalies | No | No | No | Bifid thumb |
| Skeletal anomalies | No | No | No | No |
| Congenital kidney defects | No | No | No | No |
| Microcephaly or microphthalmia | Yes | Yes | Yes | Yes |
| Mental retardation or learning disabilities | No | No | No | No |
| Low birth weight | No | No | Yes | Yes |
| Gastrointestinal defects | No | No | No | No |
| Heart defects | Yes | No | No | No |
| Tumor | Medulloblastoma | Wilms tumor; medulloblastoma | Wilms tumor; AML | T-cell ALL |
| Hematologic findings | ||||
| RBC count, × 1012/L | 4.29 (4.6) | 5.34 (4.5) | NA | 4.28 (4.6) |
| Hemoglobin, g/L | 132 (125) | 114 (120) | NA | 126 (125) |
| Hematocrit | 0.384 (0.37) | 0.373 (0.36) | NA | 0.361 (0.37) |
| MCV, fL | 90 (81) | 70 (78) | NA | 84 (81) |
| WBC count, × 109/L | 16.8 (6-17) | 5.4 (6-17.5) | NA | 13.4 (6-17) |
| Platelet count, × 109/L | 522 | 334 | NA | 194 |
Age-appropriate values are in parentheses.
RBC indicates red blood cell; MCV, mean corpuscular volume; WBC, white blood cell; NA, not available.
Sibling 2 was a full-term boy with a birth weight of 2.7 kg. At 15 months of age, he presented with a large abdominal mass. Physical examination was significant for abnormal facies, epicanthus, clinodactyly, and hypo- and hyperpigmented skin lesions. Following left nephrectomy, a stage III Wilms tumor was diagnosed. Parents refused treatment with chemotherapy or radiation. He remained stable for 36 months, but then developed a large right-sided posterior fossa mass. A gross total resection revealed a high-grade medulloblastoma. Chemotherapy and radiation therapy were declined, and the patient died of progressive disease 8 weeks later.
Kindred 2. Sibling 1 was a 6-month-old white boy with stage II Wilms tumor. His body weight (5 kg) was below the fifth percentile for age. After tumor resection, he received vincristine and dactinomycin therapy. Three months after treatment his tumor recurred in the liver; treatment with ifosfamide and etoposide was initiated. He developed severe pancytopenia and septic shock requiring a prolonged hospital stay (2 months). His treatment was switched to doxorubicin, then ifosfamide without etoposide. Again toxicity was evident, with the development of severe mucositis and pancytopenia. One year following ifosfamide and etoposide treatment, he developed AML. Daunomycin and cytarabine were ineffective, and high-dose cytarabine with mitoxantrone resulted in lethal toxicity.
Sibling 2 had been followed since birth with serial physical examinations and abdominal ultrasound scans because of the clinical findings of her older brother. She had hyperpigmented macules on her extremities and trunk and an elfin facies, with small palpebral fissures. At 4 years 11 months of age, she was diagnosed with T-cell ALL. Remission induction was successful with dexamethasone, vincristine, PEG-l-asparaginase, intrathecal cytarabine, and intrathecal methotrexate. Because of her known cellular hypersensitivity to DNA-damaging agents, she is being treated with antimetabolite-based chemotherapy only, with elimination of doxorubicin and cyclophosphamide. She has shown marked myelosuppression, requiring significant dose reductions of oral 6-mercaptopurine and methotrexate, but remains in remission.
Heightened spontaneous chromosomal instability
Cytogenetic studies were undertaken in kindred 1-sibling 1 at the time of development of the toxic response to chemotherapy. Cytogenetic studies in the younger siblings were initiated at the time of presentation of Wilms tumor (kindred 1) and at birth (kindred 2). All 3 of the patients showed marked chromosome instability in fibroblasts and PBLs that were cultured under routine conditions, in the absence of either MMC or DEB. Interestingly, these rates of spontaneous chromosome instability were high, compared with concurrently processed specimens from a series of healthy controls and a series of 28 other DEB test-confirmed FA patients.
As noted in Table 2, this increase in spontaneous chromosome abnormalities was due to both increased rates of chromosome breaks per cell and to increased rates of rearrangements. Such rearrangements included balanced translocations, derivative chromosomes, inversion, rings, and radials. The rearrangements were seen only at low frequencies in cells from the control FA patients and very rarely in cells from healthy control individuals. The increase in spontaneous chromosome abnormalities of the cells initially suggested a unique genetic syndrome,23 discrete from FA. Bloom syndrome was discounted by the presence of normal sister chromatid exchanges (SCEs) in these patients' cells (data not shown).
Spontaneous chromosome breakage and rearrangement
. | Chromatid gaps and breaks/cell . | Rearrangements/cell . | Total aberrations/cell . | % cells with aberrations . |
|---|---|---|---|---|
| Kindred 1-sibling 1, skin | 1.18 | 0.98 | 2.16 | 94* |
| Kindred 1-sibling 2 | ||||
| Blood | 0.78 | 0.10 | 0.88 | 46* |
| Skin | 0.98 | 0.40 | 1.38 | 68* |
| Kindred 2-sibling 2 | ||||
| Blood | 0.24 | 0.24 | 0.48 | 40* |
| Skin | 0.20 | 0.28 | 0.48 | 44* |
| Healthy controls, blood | 0.024 | 0.004 | 0.025 | 3.59 |
| FA controls, blood | 0.254 | 0.016 | 0.269 | 23.1 |
. | Chromatid gaps and breaks/cell . | Rearrangements/cell . | Total aberrations/cell . | % cells with aberrations . |
|---|---|---|---|---|
| Kindred 1-sibling 1, skin | 1.18 | 0.98 | 2.16 | 94* |
| Kindred 1-sibling 2 | ||||
| Blood | 0.78 | 0.10 | 0.88 | 46* |
| Skin | 0.98 | 0.40 | 1.38 | 68* |
| Kindred 2-sibling 2 | ||||
| Blood | 0.24 | 0.24 | 0.48 | 40* |
| Skin | 0.20 | 0.28 | 0.48 | 44* |
| Healthy controls, blood | 0.024 | 0.004 | 0.025 | 3.59 |
| FA controls, blood | 0.254 | 0.016 | 0.269 | 23.1 |
Chromosome abnormalities were evaluated for the 3 indicated patients. For each sample, 50 G-banded metaphases were analyzed. Breaks and rearrangements were also scored for a control series of 28 DEB-confirmed FA patients and a series of 27 non-FA individuals. The subtype of these control FA patients was unknown. However, because FA-D1 is a rare subtype, accounting for less than 5% of all FA patients, few if any of these control FA patients are likely to be from subtype D1. Rearrangements included balanced translocations, derivative chromosomes, inversions, dicentrics, and rings.
For each of the 3 patients tested, the percentage of cells with aberrations fell above the upper limit of the 95% Cl for the 28 FA controls and the 27 healthy controls
Cellular hypersensitivity to MMC and DEB
We next evaluated MMC and DEB sensitivity of PBLs from the younger siblings in each kindred (Table 3). The rates of DEB- or MMC-induced chromosomal breakage were indicative of hypersensitivity to cross-linking agents, consistent with the diagnosis of FA.
Mutagen-induced chromosome breakage
. | DEB, 0.1 μg/mL . | . | MMC, 20 ng/mL . | . | ||
|---|---|---|---|---|---|---|
| Patient . | Average no. of aberrations/cell . | Cells with aberrations, % . | Average no. of aberrations/cell . | Cells with aberrations, % . | ||
| Kindred 1-sibling 2 | 13.12 | 98 | NE | NE | ||
| Kindred 2-sibling 2 | 2.68* | 71 | 2.90* | 85 | ||
. | DEB, 0.1 μg/mL . | . | MMC, 20 ng/mL . | . | ||
|---|---|---|---|---|---|---|
| Patient . | Average no. of aberrations/cell . | Cells with aberrations, % . | Average no. of aberrations/cell . | Cells with aberrations, % . | ||
| Kindred 1-sibling 2 | 13.12 | 98 | NE | NE | ||
| Kindred 2-sibling 2 | 2.68* | 71 | 2.90* | 85 | ||
For FA, the average number of aberrations/cell after DEB is 0.66 to 11.88 and for MMC, 1.19 to 22.96. For healthy controls, respective values are 0.00 to 0.12 and 0.00 to 0.32.
NE indicates not evaluable.
Specimen obtained after therapy
Assignment of kindreds 1 and 2 to FA subgroup D1
We next used a combination of retroviral transduction and immunoblotting to determine the subtype of these FA patients.12,22 Complementation analysis with retroviral vectors carrying cDNA for FANCA, FANCC, and FANCG genes22 failed to correct the hypersensitivity of cells from either of these siblings, thus ruling out subtypes A, C, or G.
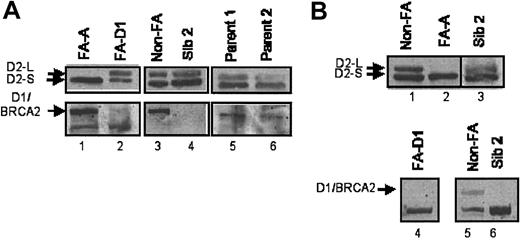
In the FA/BRCA pathway, 6 FA proteins (A, C, E, F, G, L) form a nuclear complex, required for the monoubiquitination of D2.9,11,12 Monoubiquitinated FANCD2 subsequently interacts with the downstream BRCA1 and BRCA2 proteins. As predicted, cells derived from a known FA patient, complementation group A, expressed only the nonubiquitinated isoform of FANCD2 (FANCD2-S, Figure 1A lane 1). In contrast, the 2 patient-derived cell lines (Figure 1A lane 4 and Figure 1B lane 3) expressed both isoforms of FANCD2 (-S and -L), suggesting that these patients are FA subtype BRCA2/D1. Consistent with the assignment of the D1 subtype, cells derived from these FA patients expressed no BRCA2 protein (Figure 1A lanes 2 and 4 and Figure 1B lane 6).
Absence of BRCA2 protein in FA-D1 cells. (A). Kindred 1-sibling 2 does not express BRCA2. Lymphoblast cell lines established from an FA subtype A patient (lane 1), an FA subtype D1 patient (lane 2), a control non-FA patient (lane 3), or member of kindred 1 (lanes 4-6) were lysed and immunoblotted with an antibody against FANCD2 (top panels) or BRCA2 (bottom panels). Cell lysates from sibling 2 contained ubiquitinated FANCD2 but were missing the BRCA2 protein. (B). Kindred 2-sibling 2 does not express the BRCA2 protein. Lymphoblast cell lines established from a control non-FA patient (lanes 1 and 6), an FA subtype A patient (lane 2), an FA subtype D1 patient (lane 5), or kindred 2-sibling 2 (lanes 3, 4, and 7) were lysed and immunoblotted for FANCD2 (top panels, lanes 1-4) or BRCA2 (bottom panels, lanes 5-7). Lanes 3 and 4 represent 2 different lysates from sibling 2. Ubiquitinated FANCD2 was detectable in lysates from sibling 2, but FANCD1/BRCA2 was absent.
Absence of BRCA2 protein in FA-D1 cells. (A). Kindred 1-sibling 2 does not express BRCA2. Lymphoblast cell lines established from an FA subtype A patient (lane 1), an FA subtype D1 patient (lane 2), a control non-FA patient (lane 3), or member of kindred 1 (lanes 4-6) were lysed and immunoblotted with an antibody against FANCD2 (top panels) or BRCA2 (bottom panels). Cell lysates from sibling 2 contained ubiquitinated FANCD2 but were missing the BRCA2 protein. (B). Kindred 2-sibling 2 does not express the BRCA2 protein. Lymphoblast cell lines established from a control non-FA patient (lanes 1 and 6), an FA subtype A patient (lane 2), an FA subtype D1 patient (lane 5), or kindred 2-sibling 2 (lanes 3, 4, and 7) were lysed and immunoblotted for FANCD2 (top panels, lanes 1-4) or BRCA2 (bottom panels, lanes 5-7). Lanes 3 and 4 represent 2 different lysates from sibling 2. Ubiquitinated FANCD2 was detectable in lysates from sibling 2, but FANCD1/BRCA2 was absent.
Biallelic mutations in RCA2
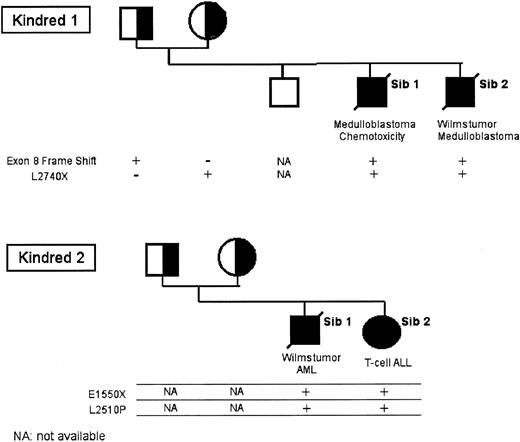
We next sequenced the BRCA2 gene in lymphoblasts derived from members of the 2 kindreds (Figure 2). For kindred 1-sibling 2, sequencing of BRCA2 revealed 2 definitive mutations. The paternal mutant allele has the 886-887delGT mutation (exon 8), resulting in a protein truncation. The maternal mutant allele has the 8447T>A mutation (exon 18), resulting in a L2740X change and a protein truncation. The exon 8 mutation is a known mutation in the Breast Cancer Gene Registry (BIC) registry,24 having been observed in several breast/ovarian cancer kindreds, whereas the exon 18 mutation is novel.
Segregation of mutant BRCA2 alleles in the 2 FA-D1 kindreds. gDNA was prepared from primary cells (lymphocytes or fibroblasts) from the indicated family members, and BRCA2 sequencing was performed.
Segregation of mutant BRCA2 alleles in the 2 FA-D1 kindreds. gDNA was prepared from primary cells (lymphocytes or fibroblasts) from the indicated family members, and BRCA2 sequencing was performed.
For kindred 2-sibling 2, sequencing of BRCA2 also revealed 2 mutations: 4876G>T (E1550X) and 7757T>C (L2510P). The E1550X mutation is a truncating mutation, seen in families with a high cancer risk, whereas the L2510P is novel. Sequencing of BRCA2 in lymphoblasts derived from parents and family members confirmed the (expected) segregation of the 2 BRCA2 mutant alleles.
There is no consanguinity between kindreds 1 and 2. The parents of all 4 children are between 32 and 42 years of age and have negative personal histories for malignancy. In kindred 1, the only malignancies reported were paternal grandmother with unilateral renal cell carcinoma at age 63, paternal-maternal great grandfather with unknown cancer in his 80s, paternal grandfather with skin cancer on his lip in his 50s, paternal-paternal great grandfather with colorectal cancer in his 50s, and paternal-paternal great grandmother with breast carcinoma in her 90s. Maternal history is currently unknown. In kindred 2, the only malignancies reported were paternal grandfather with prostate cancer in his 70s and maternal-maternal great-grandmother with colorectal cancer in her 60s.
Discussion
We have demonstrated that the FA-D1 subtype represents a distinct and severe end of the FA spectrum, with respect to cytogenetic and oncologic findings. Specifically, the cytogenetic manifestations include high rates of spontaneous chromosome instability, unusual for most FA patients. Similarly, the oncologic presentation of solid tumors or T-lineage ALL is uncommon among FA patients. Despite the severity of the cytogenetic and oncologic findings, other clinical findings of FA-D1 patients are consistent with other FA subtypes (Table 1). Although the hematologic parameters in the FA-D1 patients were normal (Table 1), the children in this study were young, ranging from 6 months to 5 years of age. The mean age of bone marrow failure in children with FA is 7 years.
Both kindreds were first described in an abstract submitted to the American Society of Human Genetics in 1999.23 At that time, only 3 FA genes had been cloned, and the relationship of these genes to BRCA2 had not been established. Yet, the combined features of marked spontaneous chromosome instability, solid tumors of very early childhood, in vitro hypersensitivity to cross-linking agents and radiation, and lethal sensitivity to chemotherapy in these kindreds were sufficiently unique to consider the possibility of a new chromosomal instability syndrome, distinct from FA.
The phenotypic manifestations of FA are highly variable, even within specific complementation groups, with respect to somatic, hematologic, and oncologic manifestations.1,18 Spontaneous chromosome instability involving primarily chromatid gaps and breaks and nonhomologous chromosome exchanges was one of the first cellular manifestations to be noted in FA.25 Although, on average, patients have higher spontaneous rates of chromosome damage than non-FA individuals, there is considerable overlap of these ranges, including many FA patients who do not have evidence of increased spontaneous damage.
From the cytogenetic perspective, the FA-D1 subgroup is an exception. These individuals demonstrate marked spontaneous chromosome instability with spontaneous aberration rates exceeding those typical for other FA subtypes. This increased spontaneous instability includes both simple chromatid gaps and breaks as well as more complex rearrangements. The relative sensitivity to MMC and DEB of FA cells is similar or higher than other FA subtypes; however, some of these FA-D1 cells may also show marked sensitivity to ionizing radiation, equivalent in severity to that seen in ataxia telangiectasia (data not shown). Although moderate radiosensitivity has been described for FA, such extreme sensitivity is not generally characteristic.26-28 However, a recent report by Gatti29 describes marked in vitro radiosensitity in 5 FA lymphoblastoid lines each representing a different complementation group. With respect to in vivo sensitivity, marked toxicity in response to radiation therapy has been described for several FA patients.28,30
The more severe cellular and clinical phenotype observed in these FA-D1 kindreds suggests that the BRCA2 gene may be distinct from other FA genes, perhaps functioning in a different cellular pathway. However, recent studies have indicated that BRCA2 has an epistatic relationship with other FA genes and functions downstream in the FA/BRCA pathway.31 Also BRCA2 has been shown to physically interact with the FANCG protein, further suggesting that it functions directly in the pathway.32 Unlike other FA proteins, BRCA2 appears to play a critical downstream function in the pathway, directly involved with RAD51 and DNA repair.14-17 The more severe clinical and cellular phenotype of FA-D1 may therefore result from a more severe defect in DNA repair by homologous recombination.
From the oncology perspective, the FA-D1 subgroup may be associated with a high incidence of solid tumors of early childhood and early age of onset of T-lineage ALL, in the absence of preceding aplastic anemia. Both solid tumors and T-lineage ALL are rare among patients reported in the International Fanconi Anemia Registry (IFAR).20 Also, medulloblastoma has been only rarely reported in FA patients.33,34 We do not know if any of the 8 FA patients with Wilms tumors or medulloblastoma reported in the IFAR have the FA-D1 subtype. A recent report indicates that some BRCA2 kindreds contain adults with breast cancer and children with brain tumors.35 As for the patients described here, there may be no signs of bone marrow dysfunction prior to or concurrent with tumor presentation. This may be due to the earlier age of onset of the solid tumors and does not rule out the possibility that signs of bone marrow dysfunction might develop with time. Importantly, our experience with kindred 1-sibling 2 suggests the possibility of an association with multiple tumor occurrence. It is now recognized that in patients with FA who present with the more classic hematologic manifestations of bone marrow failure, there remains predisposition to solid tumors later in life. Those tumors, for which significant relative risks have been reported, include head and neck squamous cell carcinoma and hepatic, esophageal, and gynecologic malignancies.5,20,36 In general, such tumor development does not occur in FA during childhood but rather after the second decade of life, with increased risk accruing with increasing age. Twenty-one of the 754 registered FA cases were further reported to have multiple malignancies.3,20 Perhaps among the FA-D1 patients who present with solid tumors at a very young age, there is again a shift to an earlier age for the development of a second (nontherapy-related) tumor.
Finally, the therapy-related consequences of kindred 1-sibling 1 and kindred 2-sibling 1 underscore the marked sensitivity of FA-D1 patients to chemotherapy and radiation. In these 2 families one of the children who received chemotherapy succumbed to therapy-associated AML within 1 year of therapy; and the other died from pancytopenia and multiorgan failure related to therapy.
Numerous questions remain. Is the solid tumor predisposition in early childhood specific to Wilms tumor and medulloblastoma, or will other solid tumors be identified in FA-D1 families? How many kindreds with children presenting in early childhood with solid tumors harbor a BRCA2 etiology? How many pediatric cancer patients who have experienced an unexpectedly severe toxic response to chemotherapy harbor BRCA2 mutations? Studies to address these questions are currently being initiated. There is likely to be heterogeneity in the cytogenetic phenotypes, due to heterogeneity in the types of BRCA2 mutations. A recent report confirms that biallelic BRCA2 mutations can result in other hematologic malignancies, including AML.37
It is critical to make the diagnosis of FA in families such as those described in the current study and to consider a diagnosis of FA in young patients who present with medulloblastoma, Wilms tumor, or T-cell leukemia, especially if these patients have any stigmata of FA (eg, café au lait spots, short stature). Once a definitive diagnosis of FA is made, especially the D1 subtype, oncologists should consider further reducing the dose of radiation and chemotherapeutic agents.
Identification of these FA-D1 families will permit BRCA2 carrier detection for extended family members. Women heterozygous for a functionally deleterious BRCA2 mutation face significantly increased risks for the development of breast and ovarian cancers. The risks for breast cancer have ranged from 26% by age 70 to lifetime risks in excess of 80%, and ovarian cancer risks of 10% to 20% by 70 and lifetime risks approaching 30%.38 BRCA2+/- heterozygotes also face an increased risk of pancreatic, stomach, gallbladder, melanoma, prostate, and other cancers, and men who carry such gene alterations face an increased risk for breast cancer (∼6%).24 However, as demonstrated here, it does not necessarily follow that all BRCA2 mutations will be predictive of the occurrence of breast or ovarian cancer.
The absence of an increase in BRCA2-related cancers in the pedigrees of kindreds 1 and 2 (with the caveat that we do not have information from the maternal side of kindred 1) raises an unsettling dilemma for other at-risk family members. Neither kindred would have been ascertained as a BRCA2 family by pedigree analysis, despite the fact that 3 of the 4 BRCA2 mutations identified are documented deleterious mutations. Thus, predictive testing for BRCA2 must be considered cautiously in such families, in conjunction with extensive pedigree analysis and genetic counseling.
Prepublished online as Blood First Edition Paper, December 11, 2003; DOI 10.1182/blood-2003-06-1970.
Supported in part by a grant from the Charles H. Hood Foundation and by a grant from the Doris Duke Charitable Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank E. Fox for genomic BRCA2 sequencing. We gratefully acknowledge the participation of kindreds 1 and 2 in these research investigations. We also wish to acknowledge the nurses of Children's Hospitals and Clinics who provided compassionate care for these children.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal