Abstract
A high incidence of somatically acquired point mutations in the AML1/RUNX1 gene has been reported in poorly differentiated acute myeloid leukemia (AML, M0) and in radiation-associated and therapy-related myelodysplastic syndrome (MDS) or AML. The vast majority of AML1 mutations identified in these diseases were localized in the amino (N)–terminal region, especially in the DNA-binding Runt homology domain. In this report, we show that AML1 point mutations were found in 26 (23.6%) of 110 patients with refractory anemia with excess blasts (RAEB), RAEB in transformation (RAEBt), and AML following MDS (defined these 3 disease categories as MDS/AML). Among them, 9 (8.2%) mutations occurred in the carboxy (C)–terminal region, which were exclusively found in MDS/AML and were strongly correlated with sporadic MDS/AML. All patients with MDS/AML with an AML1 mutation expressed wild-type AML1 protein and had a significantly worse prognosis than those without AML1 mutations. Most AML1 mutants lost trans-activation potential, regardless of their DNA binding potential. These data suggested that AML1 point mutation is one of the major driving forces of MDS/AML, and these mutations may represent a distinct clinicopathologic-genetic entity.
Introduction
Somatically acquired point mutations of critical genes have been demonstrated to contribute to the development of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Genes encoding key regulatory factors for cell division, differentiation, or cell survival of hematopoietic progenitors, such as Ras, receptors for stem cell factor (c-Kit) and Flt-3 ligand, and transcription factors are frequent mutation targets. However, there is no strong correlation between point mutations of these genes and morphology, drug sensitivity, or prognosis. This is in sharp contrast with certain chimeric oncoproteins that result from recurrent chromosomal translocations and that form distinct clinicopathologic-genetic entities.1 For instance, a majority of leukemias associated with AML1-ETO (MTG8) chimera are de novo AML with maturation, and it is relatively easy to obtain complete remission in these cases by standard chemotherapy, and these patients have favorable prognoses.2 Thus, the AML1-ETO chimera is considered to be the decision factor of the biologic features of leukemia, whereas the point mutations mentioned earlier might merely play a complementary role that contributes to the progression of disease.
The AML1 gene was also found to be altered by point mutations in AML and MDS. Beginning with the first report by Osato et al,3 the unique features of this mutation have been revealed by several studies. First, although the frequency of AML1 mutations in de novo AML is low (< 5%), they have been detected at a substantially higher frequency in a specific subtype of AML, poorly differentiated AML M0 (12%-33%).3-6 We also reported a high frequency (42%) of AML1 mutations among radiation-associated and therapy-related MDS and AML.7 These results suggest that AML1 mutations might play, in certain situations, critical roles in developing hematopoietic malignancy. Second, germ line mutations of AML1 have been shown to occur in a rare autosomal dominant disorder, familial platelet disorder with predisposition to AML (FPD/AML).8-10 All mutations with one exception identified in patients with AML and FPD/AML are in the amino (N)–terminal region of this transcription factor, especially in the Runt homology domain (RHD), which mediates its ability to bind to DNA and core binding factor β (CBFβ). Nearly half of them are missense mutations that replace amino acid residues directly contacting DNA, as shown by analysis of the crystal structure of the RHD–CBFβ–DNA ternary complex.11-14 Most of the other mutations are frame shift or nonsense mutations that abolish the function of the RHD.3,7 The majority of AML1 point mutations abrogate its DNA-binding potential, suggesting that loss of function is the main mechanism through which mutated AML1 contributes to the malignant transformation of hematopoietic progenitors.
Germ line mutations of the RUNX2 gene, which encodes another member of the Runx family of transcription factors that contains the RHD, have been detected in patients with Cleidocranial dysplasia (CCD), an autosomal dominant disorder characterized by skeletal anomalies (reviewed in Otto et al15 ). Although more than 70% of mutations detected in CCD are in the RHD, around one fourth of them are in the carboxy (C)–terminal region. These data prompted us to test whether AML1 point mutations in the C-terminal region occur in hematopoietic malignancy, especially in MDS, because only 6 patients with MDS were so far tested for the C-terminal mutations of AML1.3 Here we report a high frequency of AML1 mutations in the C-terminal, as well as in the N-terminal region of the protein in patients with MDS, especially those with refractory anemia with excess blast (RAEB), RAEB in transformation (RAEBt), and AML following MDS. Our data suggest that AML1 point mutations are strongly associated with these specific types of hematopoietic malignancy.
Patients, materials, and methods
Patients
We examined 160 cases of MDS (46 refractory anemia [RA] and RA with ringed sideroblasts [RARS], 41 RAEB, 35 RAEBt, 34 AML following MDS, 4 chronic myelomonocytic leukemia [CMMoL]) and 115 cases of AML without antecedent MDS, all of whom were diagnosed at Hiroshima University Hospital and its affiliated hospitals between 1995 and 2003. We divided these patients into “sporadic” and “secondary” disease categories according to their past history. The secondary group included (1) atomic-bomb survivors in Hiroshima, who were exposed within 3 Km of the hypocenter7 ; (2) patients who developed disease after radiotherapy and/or chemotherapy for malignancy or myeloproliferative disorder (MPD); and (3) an individual who was occupationally exposed to mustard gas. We also examined 51 cases of MPD in the chronic phase (15 myelofibrosis [MF], 21 essential thrombocythemia [ET], 13 polycythemia vera [PV], and 2 atypical MPD), 23 cases of chronic myeloid leukemia (CML; 20 in the chronic phase and 3 in blast crisis), and 28 cases of acute lymphoid leukemia (ALL).
Diagnosis was based on morphologic and immunophenotypic studies according to the French-American-British (FAB) classification.16,17 Most of the patients in this study were treated in Hiroshima University Hospital or Hiroshima Red Cross Hospital and Atomic Bomb Survivors Hospital with a protocol containing intensive chemotherapy and bone marrow transplantation. Cytogenetic analyses using standard procedures were performed according to the International System of Human Cytogenetic Nomenclature (1995).18 Patient samples were taken after obtaining informed consent and approval from the institutional review board at Hiroshima University. Mononuclear cells were isolated from bone marrow or peripheral blood samples by Ficoll-Conray density gradient centrifugation. Genomic DNA was extracted with a Puregene Kit (Gentra, Minneapolis, MN), and total RNA was extracted using a TRIzol Kit (Gibco Life Technologies, Rockville, MD), according to the manufacturers' instructions.
Identification of AML1 mutations
Polymerase chain reaction–single-strand conformation polymorphism (PCR-SSCP) was performed as follows: 100 ng genomic DNA was amplified by PCR in a total volume of 20 μL containing 1 × PCR buffer (Perkin-Elmer, Foster City, CA), 0.2 mM dNTP (deoxynucleotide triphosphate; Perkin-Elmer), 0.2 μM of each primer, and 0.5 U AmpliTaq (Perkin-Elmer). PCR of exons 3 through 8 of the AML1 gene was performed by using the following flanking intronic, forward/reverse primers: 5′-AGCTGTTTGCAGGGTCCTAA-3′/5′-GTCCTCCCACCACCCTCT-3′ for exon 3, 5′-CATTGCTATTCCTCTGCAACC-3′/5′-CCATGAAACGTGTTTCAAGC-3′ for exon 4, 5′-CCACCAACCTCATTCTGTTT-3′/5′-AGACATGGTCCCTGAGTATA-3′ for exon 5, 5′-GGGGGCCCATTCTGCTGAGAGG-3′/5′-GAGCATCAAGGGGAAACCCC-3′ for exon 6, 5′-AATCCCACCCCACTTTACAT-3′/5′-CTCAGCTGCAAAGAATGTGT-3′ for exon 7b, and 5′-TCCGCTCCGTTCTCTTGC-3′/5′-GCTTGTCGCGAACAGGAG-3′ for exon 8. To identify AML1 mutations, SSCP analysis was performed on a GenePhor system (Amersham Pharmacia Biotech, Buckinghamshire, England) using 12.5% GeneGel Excel (Amersham Pharmacia Biotech). After electrophoresis, gels were silver-stained to visualize the bands. All PCR products with abnormal SSCP bands were confirmed by an independent amplification and SSCP analysis.
PCR products that showed abnormal bands were subcloned into a pCR2.1 vector (Invitrogen, Carlsbad, CA), and 8 independent clones were sequenced in both directions using a BigDye Terminator Cycle sequencing kit (Perkin-Elmer) and were analyzed on an ABI Prism 310 Genetic Analyzer (Perkin-Elmer). To confirm the mutations, PCR products from cDNA were also sequenced. First-strand cDNA was synthesized by using total RNA and random hexamers with SuperScript II reverse transcriptase (Gibco). The cDNA products were amplified with the following primers: 5′-GCAGGGTCCTAACTCAATCG-3′/5′-GCTCGGAAAAGGACAAGCTC-3′ for the N-terminal region (corresponding to exons 3 through 5), 5′-CTACCGCAGCCATGAAGAAC-3′/5′-TTCTGCAGAGAGGGTTGTCA-3′ (corresponding to exons 4 to 7b) and 5′-CCAATACCTGGGATCCATTG-3′/5′-CCTCAGTAGGGCCTCCACAC-3′ (corresponding to exons 7b to 8) for the C-terminal region. Subcloned PCR products were sequenced as described earlier.
Cell culture and transfection
The cell lines Cos-7 and HeLa were cultured in Dulbecco modified Eagle medium (DMEM; Gibco), and U937 cells in RPMI1640 (Gibco) supplemented with 10% fetal calf serum, 2 mM glutamine at 37°C in a humidified atmosphere with 5% CO2. Cos-7 cells were transfected by using SuperFect (Qiagen, Hilden, Germany) according to the manufacturer's instructions. For the reporter assay, HeLa cells were transfected by the calcium phosphate precipitation method, and U937 cells were transfected by Effectene (Qiagen).
A cDNA encoding CBFβ-MYH11 (kindly provided by Dr T. Watanabe, Tohoku University)19 and PCR-generated fragments encoding AML1, AML1 mutants, or CBFβ with or without an N-terminus FLAG (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys) epitope were also subcloned into the pcDNA3.1 expression vector (Invitrogen). The integrity of the amplified sequence was confirmed by DNA sequencing. A reporter plasmid containing a macrophage colony-stimulating factor receptor (M-CSFR) promoter (pM-CSF-R-luc) was kindly provided by Dr D. Zhang (Beth Israel Hospital and Harvard Medical School).20
Immunoprecipitations and immunoblot analysis
Cos-7 cells were transfected by using Superfect (Qiagen) with 5 μg pcDNA3.1-CBFβ and 5 μg FLAG-tagged AML1 or AML1 mutant expression plasmid. After 24 hours, the cells were lysed in the lysis buffer (20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, pH 7.5; 150 mM NaCl; 1% Nonidet P40; 1 mM phenylmethlsulfonyl fluoride [PMSF]; 1 μg/mL leupeptin). The lysates were sonicated and then incubated with protein G (Amersham Pharmacia Biotech) to block nonspecific binding of proteins. A portion of each lysate was removed for immunoblot analysis. A 20 μL volume of a 50% slurry of anti-FLAG M2 beads (Sigma, St Louis, MO) was added to the lysates, incubated for 4 hours at 4°C, and washed 3 times with lysis buffer. FLAG beads were blocked in phosphate-buffered saline (PBS) containing 1% bovine serum albumin prior to addition to the lysates. Immunoblot analysis was performed as reported previously.7 The primary antibodies used in this study were anti-M2 antibody (Sigma) and anti-CBFβ polyclonal antibody (Oncogene Research Products, Boston, MA). Bound antibodies were detected by enhanced chemiluminescence (ECL) using a Western blotting kit (Amersham Pharmacia Biotech).
Electrophoretic mobility shift assay (EMSA) and transcriptional assay
Nuclear extracts from Cos-7 cells that were transiently transfected with the corresponding expression plasmid were prepared as described previously.21 Protein concentrations were determined with Bradford reagents (Bio-Rad, Hercules, CA). EMSA was performed as described previously.22 For supershift analysis, 1 μL rabbit AML1 antiserum that was raised against a polypeptide (Arg-Ile-Pro-Val-Asp-Ala-Ser-Thr-Ser-Arg-Arg-Phe-Thr-Pro-Pro-Ser, corresponding to the N-terminal region of AML1 of human and mouse origin) was used. Transcription assays using HeLa and U937 cells were performed by the procedure described elesewhere.7
Statistical methods
Kaplan-Meyer analysis was used to estimate survival of patients with RAEB, RAEBt, and AML following MDS with or without AML1 point mutations.23 The log-rank test was used to compare the probabilities of survival for both groups.
Results
High frequency of AML1 mutations in the N-terminal region in MDS and AML
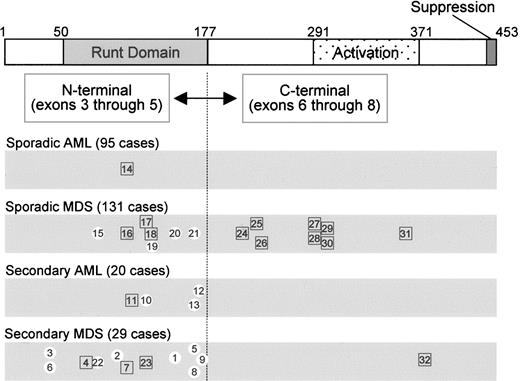
We previously reported 11 cases of secondary MDS or AML (definition of “secondary” given in “Patients, materials, and methods”) and 2 cases of sporadic MDS that harbor somatic mutations of the AML1 gene in exons 3 through 5 (corresponding to amino acids 1-177, including the Runt homology domain, Table 1; Figure 1).7 After publication of this report, one of the sporadic MDS patients (case 8) was found to have a history of receiving chemotherapy for AML (M3). Continuous complete remission (CCR) was achieved for 3 years and then she developed MDS (RAEBt). Because no mutation in the AML1 gene was detected before development of MDS, we reclassified her as secondary MDS. The other sporadic MDS patient (case 7) had a history of manufacturing poison gas (mustard gas) during World War II. Thus, we categorized all of the 13 patients with AML1 mutations as secondary MDS or AML. We extended the N-terminal mutation analysis by PCR-SSCP assay by using genomic DNA extracted from mononuclear cells in the bone marrow of patients. Mutations were further confirmed by sequence analysis of reverse trasnscriptase (RT)–PCR products, and we thereby found 10 new cases (cases 14-23). One patient with sporadic AML (M0) had a frame-shift mutation (case 14), 4 patients with sporadic MDS had silent and missense mutations (cases 15, 19, 20, and 21), 3 patients with sporadic MDS had a frame-shift mutation (cases 16-18), and 2 patients with secondary MDS had a mutation corresponding to an amino acid insertion (cases 22 and 23). The clinical findings of these patients are summarized in Table 1 (cases 14-23). The overall incidence of N-terminal mutations in sporadic and secondary RAEB, RAEBt, and AML following MDS was 8% (7 of 88) and 45.4% (10 of 22), respectively (Table 2). We found only 1 patient (1.1%) with an N-terminal AML1 mutation among 95 patients with sporadic AML without antecedent MDS, and no N-terminal AML1 mutations were found in the patients with ALL, CML, or MPD. These data confirmed findings previously reported by us and others that AML1 mutations in the N-terminal region are not associated with ALL, MPD, or CML, but they are implicated in sporadic AML (except M0) at low frequency, and they are frequently found in secondary AML and MDS (Figure 1; Table 2).3,4,7,8,24
Clinical features and mutation characteristics of the patients with AML1 mutations
. | . | . | . | . | AML1 mutation . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Case no. . | Age, y/sex . | Category and diagnosis . | History* . | Chromosome . | Genome . | cDNA . | Protein . | ||
| 1 | 66/F | Secondary MDS, RA | A-bomb (2.5 km) | 46, XX | 471G>T | 471G>T | Pro157syn | ||
| 2 | 81/F | Secondary MDS, RAEBt | A-bomb (2.0 km) | 45, XX, –5 | 303T>C | 303T>C | Thr101syn | ||
| 3 | 69/M | Secondary MDS, RAEBt | A-bomb (2.7 km) | 46, XY, del(12)(p12), der(14)t(1;14)(p10;p10) | 124G>C | 124G>C | Gly42Arg | ||
| 4 | 59/F | Secondary MDS, RAEBt | A-bomb (1.7 km) | 46, XX, del(3)(p14) | 211delC† | 211delC† | Leu71fsTer94† | ||
| 5 | 68/M | Secondary MDS, RAEBt | A-bomb (0.8 km) | 47, XY, t(3:5)(q27;p13), +4, +5, –7 | 511G>A | 511G>A | Asp171Asn | ||
| 6 | 80/F | Secondary MDS, AML following MDS | A-bomb (2.5 km) | 47, XX, +8, t(9;11)(q22;q23) | 124G>C | 124G>C | Gly42Arg | ||
| 7 | 80/M | Secondary MDS, RAEBt | Manufacturing mustard gas | 46, XY | 316_338dup | 316_338dup | Tyr113Ter | ||
| 8 | 43/F | Secondary MDS, RAEBt | AML(M3)>CCR>RAEBt | 45, XX, –7 | 511G>A | 511G>A | Asp171Asn | ||
| 9 | 39/F | Secondary MDS, RAEBt | Astrocytoma; Rad/Chem Tx | 46, XX | 530G>A | 530G>A | Arg177Gln | ||
| 10 | 62/M | Secondary AML, M0 | Lymphoma; Rad/Chem Tx | 46, XY | 413_414ins CGGGGG | 413_414ins CGGGGG | Gly138_Arg139 insGlyGly | ||
| 11 | 61/F | Secondary AML, M1 | Myelofibrosis; Rad/Chem Tx | 46, XX | 343_364dup | 343_364dup | Ala122fsTer123 | ||
| 12 | 57/F | Secondary AML, M4 | ET; Chem Tx | 45, XX, –18 | 517C>T | 517C>T | Pro173Ser | ||
| 13 | 64/M | Secondary AML, M1 | Polycythemia vera; Chem Tx | 46, XY | 511G>A | 511G>A | Asp171Asn | ||
| 14 | 29/M | Sporadic AML, M0 | — | 46, XY | 314_342insAGGC | 341_342 insAGGC | Ser114fsTer117 | ||
| 15 | 80/M | Sporadic MDS, RAEB | — | 47, XY, +8 | 261C>A | 261C>A | Ile87syn | ||
| 16 | 74/M | Sporadic MDS, AML following MDS | — | 46, XY | 342G>CCTTCC | 342G>CCTTCC | Ser114fsTer119 | ||
| 17 | 65/M | Sporadic MDS, RAEB | — | 47, XY, +8 | 388delA | 388delA | Arg130fsTer148 | ||
| 18 | 47/F | Sporadic MDS, RAEBt | — | 46, XX | 416_423dup | 416_423dup | Arg142fsTer151 | ||
| 19 | 79/M | Sporadic MDS, RAEBt | — | 46, XY | 422_427 + 3dup | 427_428ins GTAGAAGAG | Gly143_Lys144 insArgArgGly | ||
| 20 | 64/F | Sporadic MDS, RAEB | — | 46, XX | 497T>C | 497T>C | Ile166Thr | ||
| 21 | 75/M | Sporadic MDS, RAEBt | — | 45, XY, add(3)(q?13.2), –7 | 512A>G | 512A>G | Asp171Gly | ||
| 22 | 41/M | Secondary MDS, RAEB | AML(M5b)>CCR>RAEB | 46, XY, add(6)(p21.3), del(20)(q11.2q13.3) | 253_254insCCC | 253_254 insCCC | Thr84_Leu85insPro | ||
| 23 | 62/M | Secondary MDS, RAEBt | Esophageal carcinoma; Rad Tx. | 46, XY, add(17)(p11.2) | 411C>AGGA | 411C>AGGA | Val137_Gly138 insGly | ||
| 24 | 62/M | Sporadic MDS, AML following MDS | — | 46, XY | 669_670ins ACCGT | 669_670ins ACCGT | Ala224fsTer228 | ||
| 25 | 75/M | Sporadic MDS, RAEBt | — | 46, XY | 695_708del | 695_708del | Phe232fsTer567 | ||
| 26 | 73/M | Sporadic MDS, RAEBt | — | 46, XY | Intron 7a 725-13T>A | 724_725ins ATTCTCTTCAG | Asp242fsTer287 | ||
| 27 | 55/F | Sporadic MDS, AML following MDS | — | 46, XX | 871delT | 871delT | Ser291fsTer300 | ||
| 28 | 75/M | Sporadic MDS, AML following MDS | — | 46, XX | 878G>AGGGCCCA | 878G>AGGGCCCA | Arg292fsTer574 | ||
| 29 | 70/F | Sporadic MDS, RAEBt | — | 46, XY | 876_886 + 3dup | 886_887ins GTATCGACTCTCAA | Thr296fsTer305 | ||
| 30 | 54/M | Sporadic MDS, RAEB | — | 46, XY | Intron 7b 886 + 1_4del | 886_887ins (208 bp) 886_887ins (108 bp) | Thr296fsTer338 Thr296Ser ins(36aa) | ||
| 31 | 64/M | Sporadic MDS, RAEB | — | 47, XY, +1, der(1;7)(q10;p10), +8 | 1091_1095del | 1091_1095del | Ala364fsTer570 | ||
| 32 | 87/F | Secondary MDS, RAEBt | A-bomb (2.0 km) | 46, XX | 1130_1133dup | 1130_1133dup | Leu378fsTer573 | ||
. | . | . | . | . | AML1 mutation . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Case no. . | Age, y/sex . | Category and diagnosis . | History* . | Chromosome . | Genome . | cDNA . | Protein . | ||
| 1 | 66/F | Secondary MDS, RA | A-bomb (2.5 km) | 46, XX | 471G>T | 471G>T | Pro157syn | ||
| 2 | 81/F | Secondary MDS, RAEBt | A-bomb (2.0 km) | 45, XX, –5 | 303T>C | 303T>C | Thr101syn | ||
| 3 | 69/M | Secondary MDS, RAEBt | A-bomb (2.7 km) | 46, XY, del(12)(p12), der(14)t(1;14)(p10;p10) | 124G>C | 124G>C | Gly42Arg | ||
| 4 | 59/F | Secondary MDS, RAEBt | A-bomb (1.7 km) | 46, XX, del(3)(p14) | 211delC† | 211delC† | Leu71fsTer94† | ||
| 5 | 68/M | Secondary MDS, RAEBt | A-bomb (0.8 km) | 47, XY, t(3:5)(q27;p13), +4, +5, –7 | 511G>A | 511G>A | Asp171Asn | ||
| 6 | 80/F | Secondary MDS, AML following MDS | A-bomb (2.5 km) | 47, XX, +8, t(9;11)(q22;q23) | 124G>C | 124G>C | Gly42Arg | ||
| 7 | 80/M | Secondary MDS, RAEBt | Manufacturing mustard gas | 46, XY | 316_338dup | 316_338dup | Tyr113Ter | ||
| 8 | 43/F | Secondary MDS, RAEBt | AML(M3)>CCR>RAEBt | 45, XX, –7 | 511G>A | 511G>A | Asp171Asn | ||
| 9 | 39/F | Secondary MDS, RAEBt | Astrocytoma; Rad/Chem Tx | 46, XX | 530G>A | 530G>A | Arg177Gln | ||
| 10 | 62/M | Secondary AML, M0 | Lymphoma; Rad/Chem Tx | 46, XY | 413_414ins CGGGGG | 413_414ins CGGGGG | Gly138_Arg139 insGlyGly | ||
| 11 | 61/F | Secondary AML, M1 | Myelofibrosis; Rad/Chem Tx | 46, XX | 343_364dup | 343_364dup | Ala122fsTer123 | ||
| 12 | 57/F | Secondary AML, M4 | ET; Chem Tx | 45, XX, –18 | 517C>T | 517C>T | Pro173Ser | ||
| 13 | 64/M | Secondary AML, M1 | Polycythemia vera; Chem Tx | 46, XY | 511G>A | 511G>A | Asp171Asn | ||
| 14 | 29/M | Sporadic AML, M0 | — | 46, XY | 314_342insAGGC | 341_342 insAGGC | Ser114fsTer117 | ||
| 15 | 80/M | Sporadic MDS, RAEB | — | 47, XY, +8 | 261C>A | 261C>A | Ile87syn | ||
| 16 | 74/M | Sporadic MDS, AML following MDS | — | 46, XY | 342G>CCTTCC | 342G>CCTTCC | Ser114fsTer119 | ||
| 17 | 65/M | Sporadic MDS, RAEB | — | 47, XY, +8 | 388delA | 388delA | Arg130fsTer148 | ||
| 18 | 47/F | Sporadic MDS, RAEBt | — | 46, XX | 416_423dup | 416_423dup | Arg142fsTer151 | ||
| 19 | 79/M | Sporadic MDS, RAEBt | — | 46, XY | 422_427 + 3dup | 427_428ins GTAGAAGAG | Gly143_Lys144 insArgArgGly | ||
| 20 | 64/F | Sporadic MDS, RAEB | — | 46, XX | 497T>C | 497T>C | Ile166Thr | ||
| 21 | 75/M | Sporadic MDS, RAEBt | — | 45, XY, add(3)(q?13.2), –7 | 512A>G | 512A>G | Asp171Gly | ||
| 22 | 41/M | Secondary MDS, RAEB | AML(M5b)>CCR>RAEB | 46, XY, add(6)(p21.3), del(20)(q11.2q13.3) | 253_254insCCC | 253_254 insCCC | Thr84_Leu85insPro | ||
| 23 | 62/M | Secondary MDS, RAEBt | Esophageal carcinoma; Rad Tx. | 46, XY, add(17)(p11.2) | 411C>AGGA | 411C>AGGA | Val137_Gly138 insGly | ||
| 24 | 62/M | Sporadic MDS, AML following MDS | — | 46, XY | 669_670ins ACCGT | 669_670ins ACCGT | Ala224fsTer228 | ||
| 25 | 75/M | Sporadic MDS, RAEBt | — | 46, XY | 695_708del | 695_708del | Phe232fsTer567 | ||
| 26 | 73/M | Sporadic MDS, RAEBt | — | 46, XY | Intron 7a 725-13T>A | 724_725ins ATTCTCTTCAG | Asp242fsTer287 | ||
| 27 | 55/F | Sporadic MDS, AML following MDS | — | 46, XX | 871delT | 871delT | Ser291fsTer300 | ||
| 28 | 75/M | Sporadic MDS, AML following MDS | — | 46, XX | 878G>AGGGCCCA | 878G>AGGGCCCA | Arg292fsTer574 | ||
| 29 | 70/F | Sporadic MDS, RAEBt | — | 46, XY | 876_886 + 3dup | 886_887ins GTATCGACTCTCAA | Thr296fsTer305 | ||
| 30 | 54/M | Sporadic MDS, RAEB | — | 46, XY | Intron 7b 886 + 1_4del | 886_887ins (208 bp) 886_887ins (108 bp) | Thr296fsTer338 Thr296Ser ins(36aa) | ||
| 31 | 64/M | Sporadic MDS, RAEB | — | 47, XY, +1, der(1;7)(q10;p10), +8 | 1091_1095del | 1091_1095del | Ala364fsTer570 | ||
| 32 | 87/F | Secondary MDS, RAEBt | A-bomb (2.0 km) | 46, XX | 1130_1133dup | 1130_1133dup | Leu378fsTer573 | ||
Cases 1 through 13 were reported previously.7 CCR indicates continuous complete remission; Rad Tx, radiation therapy; Chem Tx, chemotherapy; ET, essential thrombocythemia; and —, no history that belongs to the “secondary” categories described in “Patients, materials, and methods.”
Distances in parentheses indicate how far from the center of the explosion the patient was
The previous description (208delC; Ser70fsTer93)7 was incorrect
AML1 point mutations identified in AML and MDS. Horizontal bars show the AML1 protein diagrammatically (453 aa), indicating the Runt domain (50-177), the trans-activation domain (291-371), and the suppression domain (446-453). AML1 is divided into N-terminal and C-terminal regions. Case numbers (Table 1) with AML1 mutations are indicated according to disease categories (sporadic AML, sporadic MDS, secondary AML, and secondary MDS). Numbers in white circles indicate missense/silent mutations, and numbers in squares indicate frame-shift/nonsense mutations.
AML1 point mutations identified in AML and MDS. Horizontal bars show the AML1 protein diagrammatically (453 aa), indicating the Runt domain (50-177), the trans-activation domain (291-371), and the suppression domain (446-453). AML1 is divided into N-terminal and C-terminal regions. Case numbers (Table 1) with AML1 mutations are indicated according to disease categories (sporadic AML, sporadic MDS, secondary AML, and secondary MDS). Numbers in white circles indicate missense/silent mutations, and numbers in squares indicate frame-shift/nonsense mutations.
Frequency of AML 1 mutations in the specific types of hematopoietic disease
. | . | . | AML 1 mutation . | . | . | ||
|---|---|---|---|---|---|---|---|
| Diagnosis . | History . | No. cases . | Total, no. (%) . | N-terminal, no. (%) . | C-terminal, no. (%) . | ||
| RAEB, RAEBt, and AML following MDS (MDS/AML) | |||||||
| RAEB | |||||||
| Sporadic | 36 | 5 (13.9) | 3 (8.3) | 2 (5.6) | |||
| Secondary | 5 | 1 (20.0) | 1 (20.0) | 0 (0.0) | |||
| Total | 41 | 6 (14.6) | 4 (9.8) | 2 (4.9) | |||
| RAEBt | |||||||
| Sporadic | 23 | 6 (26.1) | 3 (13.0) | 3 (13.0) | |||
| Secondary | 12 | 9 (75.0) | 8 (66.7) | 1 (8.3) | |||
| Total | 35 | 15 (42.9) | 11 (31.4) | 4 (11.4) | |||
| AML following MDS | |||||||
| Sporadic | 29 | 4 (13.8) | 1 (3.4) | 3 (10.3) | |||
| Secondary | 5 | 1 (20.0) | 1 (20.0) | 0 (0.0) | |||
| Total | 34 | 5 (14.7) | 2 (5.8) | 3 (8.8) | |||
| Total MDS/AML | |||||||
| Sporadic | 88 | 15 (17.0) | 7 (8.0) | 8 (9.1) | |||
| Secondary | 22 | 11 (50.0) | 10 (45.5) | 1 (4.5) | |||
| Total | 110 | 26 (23.6) | 17 (15.4) | 9 (8.2) | |||
| AML without antecedent MDS (de novo AML) | |||||||
| AML without antecedent MDS | |||||||
| Sporadic | 95 | 1 (1.1) | 1 (1.1) | 0 (0.0) | |||
| Secondary | 20 | 4 (20.0) | 4 (20.0) | 0 (0.0) | |||
| Total | 115 | 5 (4.3) | 5 (4.3) | 0 (0.0) | |||
| Others | |||||||
| RA/RARS | Total | 45 | 1 (2.2) | 1 (2.2) | 0 (0.0) | ||
| CMMoL | Total | 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| MPD | Total | 51 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| ALL | Total | 28 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| CML/CML-BC | Total | 23 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
. | . | . | AML 1 mutation . | . | . | ||
|---|---|---|---|---|---|---|---|
| Diagnosis . | History . | No. cases . | Total, no. (%) . | N-terminal, no. (%) . | C-terminal, no. (%) . | ||
| RAEB, RAEBt, and AML following MDS (MDS/AML) | |||||||
| RAEB | |||||||
| Sporadic | 36 | 5 (13.9) | 3 (8.3) | 2 (5.6) | |||
| Secondary | 5 | 1 (20.0) | 1 (20.0) | 0 (0.0) | |||
| Total | 41 | 6 (14.6) | 4 (9.8) | 2 (4.9) | |||
| RAEBt | |||||||
| Sporadic | 23 | 6 (26.1) | 3 (13.0) | 3 (13.0) | |||
| Secondary | 12 | 9 (75.0) | 8 (66.7) | 1 (8.3) | |||
| Total | 35 | 15 (42.9) | 11 (31.4) | 4 (11.4) | |||
| AML following MDS | |||||||
| Sporadic | 29 | 4 (13.8) | 1 (3.4) | 3 (10.3) | |||
| Secondary | 5 | 1 (20.0) | 1 (20.0) | 0 (0.0) | |||
| Total | 34 | 5 (14.7) | 2 (5.8) | 3 (8.8) | |||
| Total MDS/AML | |||||||
| Sporadic | 88 | 15 (17.0) | 7 (8.0) | 8 (9.1) | |||
| Secondary | 22 | 11 (50.0) | 10 (45.5) | 1 (4.5) | |||
| Total | 110 | 26 (23.6) | 17 (15.4) | 9 (8.2) | |||
| AML without antecedent MDS (de novo AML) | |||||||
| AML without antecedent MDS | |||||||
| Sporadic | 95 | 1 (1.1) | 1 (1.1) | 0 (0.0) | |||
| Secondary | 20 | 4 (20.0) | 4 (20.0) | 0 (0.0) | |||
| Total | 115 | 5 (4.3) | 5 (4.3) | 0 (0.0) | |||
| Others | |||||||
| RA/RARS | Total | 45 | 1 (2.2) | 1 (2.2) | 0 (0.0) | ||
| CMMoL | Total | 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| MPD | Total | 51 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| ALL | Total | 28 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| CML/CML-BC | Total | 23 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
Frequent occurrence of C-terminal mutations of the AML1 gene in MDS
To investigate the AML1 mutations in the C-terminal portion of AML1, we analyzed exons 6 through 8. Point mutations were detected in patients with MDS and were found preferentially in sporadic MDS. Nine of 110 patients (8.2%) with RAEB, RAEBt, and AML following MDS had C-terminal AML1 mutations, whereas no mutation was detected in the following patients: 115 AML without antecedent MDS, 45 RA/RARS, 28 ALL, 23 CML, and 51 MPD in accordance with previous studies.3,8 The clinical findings of these patients are summarized in Table 1 (cases 24-32). All but one patient (case 32, an atomic-bomb survivor) had sporadic MDS. Eight of 15 (53%) patients with sporadic MDS had an AML1 point mutation that occurred in the C-terminal region. By contrast, only 1 of 12 (8%) patients with secondary MDS with AML1 point mutations had mutations occurring in the C-terminal region; this difference was statistically significant (P = .013; chi-square test).
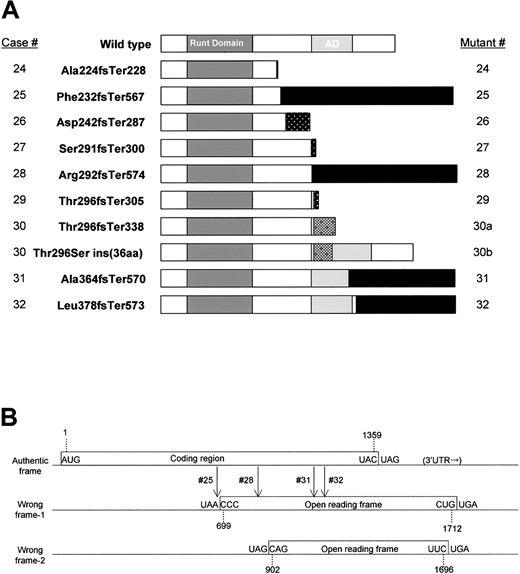
All of the C-terminal mutations (cases 24-32) resulted in frame shifts similar to the Runx2 mutations identified in CCD.15 The consequences of the C-terminal mutations were unusual (Figure 2A). Usually, frame-shift mutations are created by insertions or deletions of nucleotides in exons or by loss of splice donor or acceptor sites, and they result in truncation of the authentic protein followed by a relatively short additional stretch of amino acid residues originating from the wrong reading frame or from intronic sequences. Among the 9 C-terminal AML1 mutations found, only 3 cases (cases 24, 27, and 29) obeyed this general rule. In 4 cases (cases 25, 28, 31, and 32), small nucleotide insertions or deletions (4-14 base pair [bp]) occurred in exons, but the stretches of additional amino acids resulting from the wrong reading frame were 195 to 335 residues in length (Table 1), because the wrong frame contained an in-frame termination codon 353 bp downstream (3′) of the authentic termination codon (Figure 2B). Thus, the mutated AML1 proteins in these 4 cases were even longer than wild-type AML1 and appeared to be fusion proteins rather than truncations of AML1 (Figure 2A).
AML1 mutations in the C-terminal region. (A) Horizontal bars show the wild-type (453 aa) and mutant AML1 sequences, indicating the Runt domain (50-177), the trans-activation domain (291-371), and the C-terminal mutants. The numbers in the left-hand column indicate the case numbers (24 through 32) described in Table 1. Patient 30 expressed 2 types of mutated AML1 mRNA and protein that originated from one genomic mutation (described in “Results”). Cases 25, 28, 31, and 32 expressed extraordinary long stretches originating from a wrong reading frame (indicated by black bars). (B) Open reading frames (ORFs) of the human AML1 gene. Boxes showed ORFs including the authentic frame that encodes the wild-type AML1 protein. Vertical arrows show locations of frame shifts in cases 25, 28, 31, and 32. Only relevant ORFs are shown.
AML1 mutations in the C-terminal region. (A) Horizontal bars show the wild-type (453 aa) and mutant AML1 sequences, indicating the Runt domain (50-177), the trans-activation domain (291-371), and the C-terminal mutants. The numbers in the left-hand column indicate the case numbers (24 through 32) described in Table 1. Patient 30 expressed 2 types of mutated AML1 mRNA and protein that originated from one genomic mutation (described in “Results”). Cases 25, 28, 31, and 32 expressed extraordinary long stretches originating from a wrong reading frame (indicated by black bars). (B) Open reading frames (ORFs) of the human AML1 gene. Boxes showed ORFs including the authentic frame that encodes the wild-type AML1 protein. Vertical arrows show locations of frame shifts in cases 25, 28, 31, and 32. Only relevant ORFs are shown.
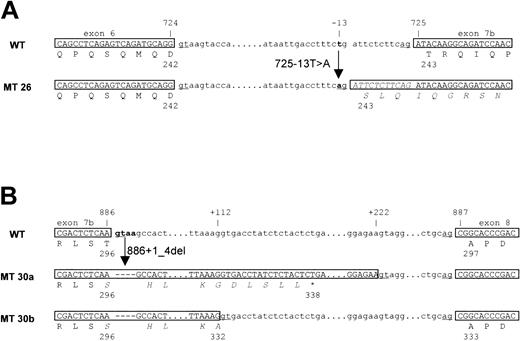
The other 2 cases (cases 26 and 30) had mutations within introns. Case 26 had a single nucleotide replacement (T > A) in the intron between exons 6 and 7b that formed a new splice acceptor, resulting in the insertion of 11 nucleotides in the mRNA, thus creating a frame-shift mutation (Figure 3A). Case 30 had a deletion of the splice donor signal adjacent to the end of exon 7b (Figure 3B). As a result, 2 types of mRNA were created from the single mutation. One mRNA, designated 30a, was made by a cryptic splice donor site (212 bp downstream from the authentic splice donor signal), resulting in the insertion of an intronic sequence (208 bp) that contained an in-frame termination codon. The predicted mutated protein was a truncation of the wild-type AML1 protein at Thr296 with 42 additional amino acid residues that originated from the intronic sequence. The other message, designated 30b, was created by another cryptic splice donor site (112 bp downstream from the authentic site), resulting in the insertion of an intronic sequence (108 bp), but no frame shift in exon 8. Consequently, the mutated AML1 protein had an insertion of 36 amino acid residues that originated from intronic sequences in the middle of the trans-activation domain of AML1.
Introduction of alternative splice sites in cases 26 and 30. (A) Case 26, the T>A replacement in the intron results in a frame shift that makes a new splice acceptor 11 bp upstream (5′) of the authentic splice acceptor. (B) Case 30, a 4-bp deletion, including splice donor sites, resulted in use of 2 cryptic splice donor sites 208 bp and 108 bp downstream (3′) of the authentic splice donor site. WT indicates wild-type; MT, mutants.
Introduction of alternative splice sites in cases 26 and 30. (A) Case 26, the T>A replacement in the intron results in a frame shift that makes a new splice acceptor 11 bp upstream (5′) of the authentic splice acceptor. (B) Case 30, a 4-bp deletion, including splice donor sites, resulted in use of 2 cryptic splice donor sites 208 bp and 108 bp downstream (3′) of the authentic splice donor site. WT indicates wild-type; MT, mutants.
We observed virtually equal intensities of normal and shifted bands in the PCR-SSCP analysis of all the samples with AML1 mutations, including case 30, and obtained comparable frequencies of normal and mutated sequences by the sequence analysis. In addition, germ line genomic DNA sequences were examined in specimens obtained from nonleukemic organs in 6 cases (cases 4, 5, 6, 9, 10, and 12), and these were found to be normal (data not shown), suggesting that mutations of the AML1 gene were monoallelic at the somatic level.
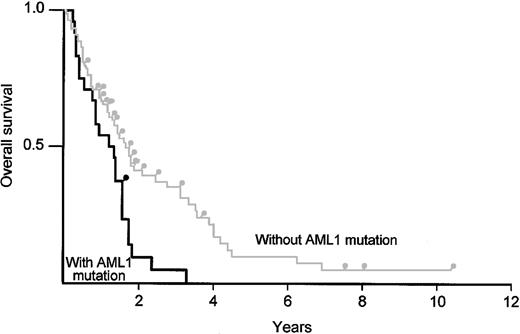
To test whether AML1 point mutations affect the prognosis of MDS, we tracked the overall survival of patients with RAEB, RAEBt, and AML following MDS, comparing survival of those associated with AML1 mutations (n = 26) with that of patients without the mutations (n = 81) (Figure 4). There was no significant difference in the distribution of age (mean of 66.2 years, range 39-87, versus mean of 66.7 years, range 17-82) and sex (16 male to 10 female, versus 52 male to 29 female) between the 2 groups. AML1 point mutations were proven to be a risk factor by a log-rank test (P < .01).
Kaplan-Meier analysis comparing overall survival of patients with MDS/AML (RAEB, RAEBt, and AML following MDS) and AML1 point mutations with the survival of patients without an AML1 mutation. The black line indicates patients with an AML1 point mutation; the gray line indicates patients without an AML1 mutation.
Kaplan-Meier analysis comparing overall survival of patients with MDS/AML (RAEB, RAEBt, and AML following MDS) and AML1 point mutations with the survival of patients without an AML1 mutation. The black line indicates patients with an AML1 point mutation; the gray line indicates patients without an AML1 mutation.
Abilities of AML1 mutants with C-terminal region mutations to bind to DNA and to heterodimerize with CBFβ
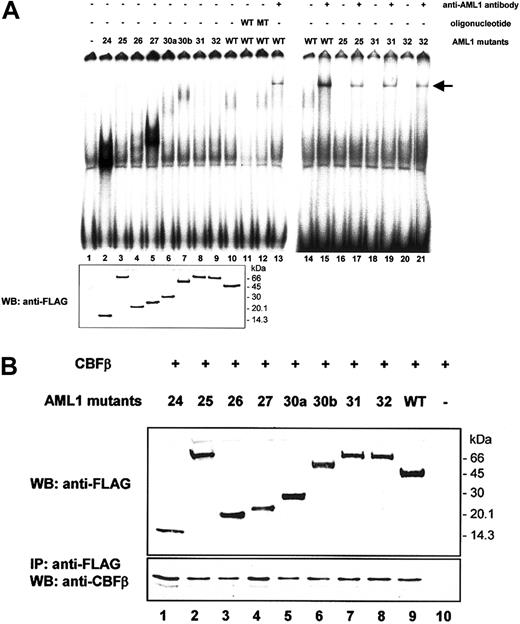
To obtain insight into molecular mechanisms through which the newly discovered C-terminal mutations of AML1 contribute to malignant transformation of hematopoietic progenitors, we analyzed the DNA binding potential of the mutant proteins. A radiolabeled oligonucleotide probe containing the consensus binding sequence for AML1 and nuclear extracts from Cos-7 cells transfected with AML1 mutants were used in EMSA. A DNA/protein complex was detected when using nuclear extract from a transfectant expressing FLAG-tagged wild-type AML1 (Figure 5A, lane 10) that was not observed when using an extract from the mock transfectant (lane 1). This complex was competed for by the nonradiolabeled oligonucleotide containing the AML1 binding site (lane 11) but was not competed for by those containing a mutated AML1 binding site (lane 12). Moreover, this complex was super-shifted by a specific serum against AML1 (lanes 13 and 15), indicating that the complex contains AML1. Among C-terminal AML1 mutants we tested, mutants 24 and 27 had a markedly enhanced DNA-binding potential compared with that of wild-type AML1 (lanes 2 and 5). Mutants 26 and 30b also showed a substantially higher DNA binding potential (lanes 4 and 7). By contrast, mutants 25, 31, and 32, which had long stretches of additional amino acid residues originating from the wrong reading frame, bound to the probe, but only weakly (lanes 3, 8, 9, 17, 19, and 21). These findings were not due to uneven expression levels of AML1 mutants, because an approximately equal amount of protein was detected by immunoblot analysis (lower panel).
Abilities of C-terminal AML1 mutant proteins to bind DNA and to heterodimerize with CBFβ. (A) DNA binding potential of AML1 mutants was analyzed by EMSA using nuclear extract from Cos-7 cells transfected with wild-type AML1 or mutated AML1 expression plasmid vectors. The oligonucleotides used as competitors were as follows: W, containing one wild-type AML1 binding site (CGAGTATTGTGGTTAATACG); and M, containing one mutated AML1 binding site (CGAGTATTGTTAGTAATACG). Equal expression of each mutant was demonstrated by immunoblot analysis (WB) using a FLAG antibody, as shown in the lower panel. An arrow indicates supershifted bands. (B) Heterodimerization ability of AML1 mutants with CBFβ. Cos-7 cells were cotransfected with an expression vector containing CBFβ cDNA and with vectors containing either wild-type AML1 or mutated AML1 cDNA. The expression levels of AML1 in total cell lysates were detected by immunoblot analysis with an anti-FLAG antibody (upper panel). Cell lysates were immunoprecipitated (IP) with an anti-FLAG antibody, and then proteins were detected by immunoblot analysis (WB) using an anti-CBFβ antibody (lower panel).
Abilities of C-terminal AML1 mutant proteins to bind DNA and to heterodimerize with CBFβ. (A) DNA binding potential of AML1 mutants was analyzed by EMSA using nuclear extract from Cos-7 cells transfected with wild-type AML1 or mutated AML1 expression plasmid vectors. The oligonucleotides used as competitors were as follows: W, containing one wild-type AML1 binding site (CGAGTATTGTGGTTAATACG); and M, containing one mutated AML1 binding site (CGAGTATTGTTAGTAATACG). Equal expression of each mutant was demonstrated by immunoblot analysis (WB) using a FLAG antibody, as shown in the lower panel. An arrow indicates supershifted bands. (B) Heterodimerization ability of AML1 mutants with CBFβ. Cos-7 cells were cotransfected with an expression vector containing CBFβ cDNA and with vectors containing either wild-type AML1 or mutated AML1 cDNA. The expression levels of AML1 in total cell lysates were detected by immunoblot analysis with an anti-FLAG antibody (upper panel). Cell lysates were immunoprecipitated (IP) with an anti-FLAG antibody, and then proteins were detected by immunoblot analysis (WB) using an anti-CBFβ antibody (lower panel).
To test whether AML1 mutants are able to interact with CBFβ, we performed immunoblot analysis after immunoprecipitation. Cos-7 cells were cotransfected with the FLAG-tagged wild-type or mutated AML1 (Figure 5B, upper panel), together with CBFβ. CBFβ was coimmunoprecipitated with FLAG-tagged wild-type AML1 by beads coated with anti-FLAG antibody (lower panel, lane 9). CBFβ was also coimmunoprecipitated with AML1 proteins having mutations in the C-terminal domain (lanes 1-8), indicating that these mutants interacted with CBFβ. Taking account of expression levels of mutant proteins, the binding potential of mutants 24 and 27 seemed to be increased.
Transcriptional potential of C-terminal AML1 mutants
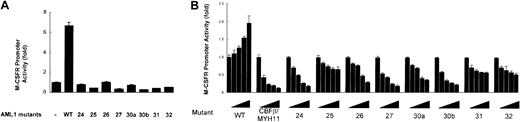
To investigate the transcriptional activities of the C-terminal AML1 mutants, reporter experiments were performed by using the promoter of M-CSFR, which is known to be transcriptionally regulated by AML1.20 When wild-type AML1 and CBFβ were cotransfected in HeLa cells, the promoter activity was induced 7-fold compared with transfection with CBFβ alone (Figure 6A). In contrast, none of the C-terminal mutants induced significant trans-activation, regardless of their DNA-binding potentials (Figure 5A). To examine whether AML1 mutants act as dominant negative inhibitors of wild-type AML1, we performed the same reporter assay by using U937 monocytic cells, in which the activity of the M-CSFR promoter was trans-activated by transfecting AML1 alone in a dose-dependent manner (Figure 6B). As a positive control, we used CBFβ-MYH11, which is formed as a result of inv(16) and is a well-established negative regulator of AML1.25 All mutants suppressed the trans-activation activity of wild-type AML1 in a dose-dependent fashion. Inhibition of the trans-activation seemed to be related to DNA- and CBFβ-binding potential (Figure 5A-B). Thus, the C-terminal AML1 mutants identified in this study lacked trans-activation potential and could act as dominant negative inhibitors of wild-type AML1.
Transcriptional potential of AML1 mutants. (A) Transcriptional activities of the AML1 mutants in HeLa cells. Cells were transfected with 5 μg pM-CSF-R-luc, 3 μg FLAG-tagged AML1 or AML1 mutant expression vector, 1 μg CBFβ expression vector, and 0.25 μg pRL-tk as an internal control to normalize luciferase activities for transfection efficiency. (B) Transcriptional activities of the AML1 mutants in U937 cells. Cells were transfected with 2 μg pM-CSF-R-luc reporter plasmid, the indicated amounts of AML1 expression constructs, and 0.2 μg pRL-tk as an internal control to normalize luciferase activities for evaluation of transfection efficiency. The expression vector containing wild-type AML1 (0.2 μg) was cotransfected with increasing doses (0, 0.1, 0.2, 0.4, and 0.6 μg) of expression vectors containing CBFβ-MYH11 or various AML1 mutations. Each value represents the mean of 3 independent experiments. The error bars indicate the mean ± standard deviation.
Transcriptional potential of AML1 mutants. (A) Transcriptional activities of the AML1 mutants in HeLa cells. Cells were transfected with 5 μg pM-CSF-R-luc, 3 μg FLAG-tagged AML1 or AML1 mutant expression vector, 1 μg CBFβ expression vector, and 0.25 μg pRL-tk as an internal control to normalize luciferase activities for transfection efficiency. (B) Transcriptional activities of the AML1 mutants in U937 cells. Cells were transfected with 2 μg pM-CSF-R-luc reporter plasmid, the indicated amounts of AML1 expression constructs, and 0.2 μg pRL-tk as an internal control to normalize luciferase activities for evaluation of transfection efficiency. The expression vector containing wild-type AML1 (0.2 μg) was cotransfected with increasing doses (0, 0.1, 0.2, 0.4, and 0.6 μg) of expression vectors containing CBFβ-MYH11 or various AML1 mutations. Each value represents the mean of 3 independent experiments. The error bars indicate the mean ± standard deviation.
Discussion
In this study, we established strong correlations between point mutations of the AML1 gene and subgroups of MDS and AML (ie, RAEB, RAEBt, and AML) following MDS. Here we define these 3 disease categories as MDS/AML. Of the 110 patients with MDS/AML who were tested, 26 (23.6%) had an AML1 mutation (Table 2). Conversely, of 32 patients with AML1 point mutation, 26 (81%) belonged to this category (Table 1). Moreover, the prognosis for the patients with AML1 mutations was significantly worse than for patients without AML1 mutations (Figure 4).
The MDS/AML category we defined here is characterized by (1) relatively a low blast percentage in the bone marrow, (2) multilineage dysplasia, (3) resistance to chemotherapy, and (4) poor prognosis. In the FAB classification, this disease entity was designated as MDS, and a substantial number of patients are classified into the RAEBt subgroup.17 In the World Health Organization (WHO) classification proposed in 2001,26 the RAEBt category was eliminated, and it was designated as AML. It is generally accepted that leukemogenesis of MDS/AML is different from that of de novo AML without significant myelodysplastic features. De novo AML is often associated with oncogenic chimeras, which are considered as the major cause of malignant transformation of hematopoietic progenitors. In this subgroup, point mutations are likely a complement for chimeras, as in recent reports that c-Kit mutations are often associated with AML1-ETO or Flt-3 mutations with MLL chimeras.27-29 By contrast, MDS/AML, which is rarely associated with chimeras, develops as a result of accumulation of gene deletions and point mutations.30,31 AML1 is the first gene that was demonstrated to have a high frequency of mutations in patients with MDS/AML. Moreover, AML1 point mutations are rarely detected in other types of hematologic malignancies or MPD, except secondary AML and AML (M0). Furthermore, patients with MDS/AML with AML1 point mutations had a significantly worse prognosis than patients without such mutations, suggesting that this is one of the major decision factors of the biologic features of myeloid malignancy. Thus, “low blast percentage myeloid leukemia with AML1 point mutation” may represent a new subgroup of AML, which would be considered for inclusion in the recurrent genetic abnormalities in the WHO classification.
This report also provides the first demonstration that AML1 point mutations in the C-terminal region correlate with MDS/AML (overall 9 of 110, 8.2%; Table 2). By contrast, of 115 cases of de novo AML (including 13 M0 cases), we found no AML1 mutations in the C-terminal region. These data were supported by Osato et al,3 who tested 109 cases of de novo AML including 9 patients with AML (M0) and did not find mutations in the C-terminal region. Thus, we believe that C-terminal AML1 mutations are rare, if they occur at all, in overall de novo AML. However, there remains a possibility that they are involved in AML (M0) at a relatively low frequency, because, although we and others tested a total of 121 patients with AML (M0) for AML1 point mutations and found 22 (18%) patients with N-terminal mutations,3-6 only 22 patients have been tested so far for C-terminal mutations. Only 1 of 12 mutations identified in secondary MDS/AML was in the C-terminal region. By contrast, AML1 mutations in sporadic MDS/AML were distributed throughout the gene (Figure 1). A simple hypothesis to interpret these data are that ionizing radiation (IR) or alkylating agents preferentially damage the N-terminal region of AML1, whereas there is a source of DNA damage other than IR or alkylating agents that can injure the C-terminal region and ultimately lead to development of sporadic MDS/AML. Alternatively, gene abnormalities frequently induced by radiation or alkylating agents somehow contribute to development of MDS/AML in cooperation with N-terminal AML1 mutants but not with C-terminal ones.
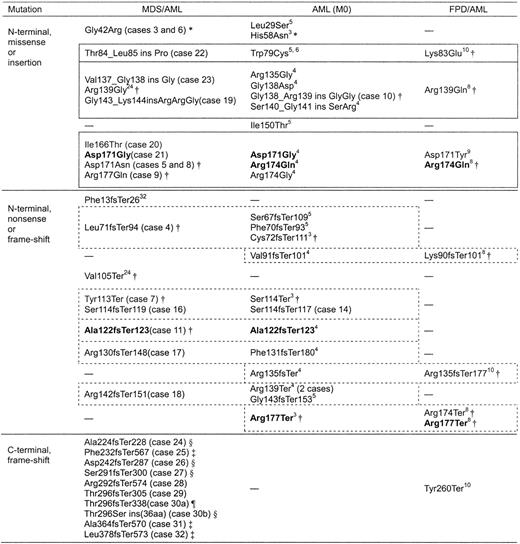
Although the contribution of AML1 point mutations to the development of AML (M0) and MDS/AML is convincing, the following question may be raised: Why do mutations in one gene result in myeloid malignancies with such totally different phenotypes? One possibility is that residual wild-type AML1 contributes to the differentiation of leukemic blasts. Of 22 patients with AML (M0) harboring AML1 gene mutations so far reported, including 2 patients (cases 10 and 14) in this study, 13 (60%) lost the wild-type AML1 gene. In addition, 2 patients with M0 were reported to have a total homozygous deletion of AML1.6 By contrast, all 26 patients with MDS/AML with AML1 mutations in this study and an additional 3 patients reported by others expressed wild-type AML1,3,24 suggesting that this transcription factor differentiates not only normal hematopoietic progenitors in the process of development, but also pathologic blasts in leukemogenesis. This hypothesis is supported by the fact that wild-type AML1 is usually expressed in leukemic blasts harboring AML1-ETO chimeras, which cause AML with maturation (M2). Another possibility is that different AML1 mutations result in different phenotypes. Indeed, C-terminal mutations we found so far only in MDS/AML, suggesting that this type of mutation may induce MDS/AML but not de novo AML. However, there seemed to be little difference in N-terminal mutations between MDS/AML, AML (M0), and FPD/AML (Figure 7). A limited number of amino acid residues were replaced by missense/insertion mutations, and these types of mutations were found in each of the 3 disease categories. Nonsense/frame-shift mutations in each disease category also resulted in similar, or even identical, truncated forms. This finding suggested that, in the case of N-terminal AML1 mutations, the phenotype of the resulting leukemia would be determined by the presence of wild-type AML1 or other genetic abnormalities that contributed to leukemogenesis in cooperation with the AML1 mutation.
AML1 mutations in MDS/AML, AML (M0), and FPD/AML. Items in bold show the identical mutation. Boxes outlined with solid lines indicate N-terminal missense/insertion mutations that replace amino acid residues in three loops directly contacting DNA. Boxes outlined with dashed lines indicate N-terminal nonsense/frame-shift mutants resulting in similar truncated forms of the protein. — indicates previously not reported. * indicates mutant belonging to Type b (described in “Discussion”); †, mutant belonging to Type a-1 (described in “Discussion”); ‡, mutant belonging to Type a-2 (described in “Discussion”); §, mutant belonging to Type a-3 (described in “Discussion”); and ¶, mutant belonging to Type a-4 (described in “Discussion”).
AML1 mutations in MDS/AML, AML (M0), and FPD/AML. Items in bold show the identical mutation. Boxes outlined with solid lines indicate N-terminal missense/insertion mutations that replace amino acid residues in three loops directly contacting DNA. Boxes outlined with dashed lines indicate N-terminal nonsense/frame-shift mutants resulting in similar truncated forms of the protein. — indicates previously not reported. * indicates mutant belonging to Type b (described in “Discussion”); †, mutant belonging to Type a-1 (described in “Discussion”); ‡, mutant belonging to Type a-2 (described in “Discussion”); §, mutant belonging to Type a-3 (described in “Discussion”); and ¶, mutant belonging to Type a-4 (described in “Discussion”).
Most amino acid residues replaced by missense or insertion type mutations in the N-terminal region of AML1 were located in 3 protein loops, called loop(βA′-B), loop(βE-F), and βG′tail, which mediate the DNA-binding potential, as demonstrated by analysis of the crystal structure of the RHD-CBFβ-DNA ternary complex (Figure 7).11-14 Nonsense and frame-shift mutations in the N-terminal region result in partial deletion of the Runt homology domain. Thus, these mutants are predicted to lose DNA-binding ability and trans-activating potential (we designate this type of mutation as Type a-1), and indeed, we and others have confirmed this type for 18 mutants (Figure 7).3,7,10,24 However, there are 4 missense mutations (Leu29Ser, Gly42Arg, His58Asn, and Ile150Thr) that replace amino acids out of the 3 loops. The Gly42Arg and His58Asn mutant proteins were shown to bind to DNA more avidly than wild-type AML1 and to have enhanced trans-activation potential (Type b).3,7 Analysis of C-terminal mutants provided 3 additional types of mutation: attenuated DNA-binding potential without trans-activation potential (Type a-2, mutants 25, 31, and 32), enhanced DNA-binding ability but no trans-activation potential (Type a-3, mutants 24, 26, 27, and 30b), and DNA-binding ability equivalent to wild-type AML1 but no trans-activation potential (Type a-4, mutant 30a). Mutants belonging to Type a-3 have a similar structure to that of an alternative splice variant, AML1a, which also binds to DNA more avidly than wild-type AML1b but lacks trans-activation potential.33 Currently, we can offer no adequate interpretation for the question as to why mutations resulting in such divergent biochemical features contribute equally to the same type of myeloid malignancy. However, we can say that, with a few exceptions such as Gly42Arg or His58Asn, AML1 mutants lose their trans-activation potential, suggesting a loss of function mechanism. We believe that further careful analysis of these AML1 mutants will reveal the key molecular system that induces myeloid malignancy.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-09-3074.
Supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ryoko Yamaguchi for excellent technical support in carrying out these experiments.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal