Abstract
How lipopolysaccharide (LPS) signals through toll-like receptors (TLRs) to induce nuclear factor (NF)–κB and inflammatory cytokines in sepsis remains unclear. Major candidates for that process are myeloid differentiation protein 88 (MyD88) and MyD88 adaptor-like/TIR domain-containing adaptor protein (Mal/TIRAP) but their role needs to be further defined. Here, we have examined the role of MyD88 and Mal/TIRAP in primary human cells of nonmyeloid and myeloid origin as physiologically relevant systems. We found that MyD88 and Mal/TIRAP are essential for LPS-induced IκBα phosphorylation, NF-κB activation, and interleukin 6 (IL-6) or IL-8 production in fibroblasts and endothelial cells in a pathway that also requires IKK2. In contrast, in macrophages neither MyD88, Mal/TIRAP, nor IκB kinase 2 (IKK2) are required for NF-κB activation or tumor necrosis factor α (TNFα), IL-6, or IL-8 production, although Mal/TIRAP is still involved in the production of interferon β (IFNβ). Differential usage of TLRs may account for that, as in macrophages but not fibroblasts or endothelial cells, TLR4 is expressed in high levels at the cell surface, and neutralization of TLR4 but not TLR2 blocks LPS signaling. These observations demonstrate for the first time the existence of 2 distinct pathways of LPS-induced NF-κB activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilization of TLR4, MyD88, Mal/TIRAP, and IKK2, and reveal a layer of complexity not previously expected.
Introduction
The development of novel therapies for sepsis depends on the understanding of the basic mechanisms of the disease.1 The principal active agent involved in the pathogenesis of sepsis is bacterial lipopolysaccharide (LPS), an essential component of the surface of gram-negative bacteria. LPS exerts its toxic effects by potently activating macrophages and endothelial cells, and inducing the expression of inflammatory cytokines such as tumor necrosis factor α (TNFα) and interleukin 6 (IL-6).2-5 Thus, elucidating how LPS signals through cell-surface receptors to induce inflammatory gene expression in humans is of major importance.
Central to the recognition of LPS and also many other microbial products by the host is a family of transmembrane proteins that have leucine-rich repeats in their extracellular domains known as the toll-like receptors (TLRs).6 LPS interacts with a heterologous receptor that contains TLR47,8 as well as CD149,10 and MD2.11-13 As CD14 is a glycosyl phosphatidylinositol–anchored protein and MD2 is on the cell surface, transduction of the LPS signal across the membrane is mediated by TLR4. TLR4, as all TLR family members, contains a cytoplasmic domain that is homologous to a cytoplasmic domain found in the IL-1 receptor known as the Toll/IL-1 receptor (IL-1R) homology (TIR) domain that is essential for downstream signaling.14-16
The presence of the TIR domain in both TLR and IL-1 receptor family members suggested that these receptors use an identical framework of signaling molecules to exert their downstream effects. This was supported by subsequent studies in mouse and human cell lines. Thus, IL-1R and TLR4 were shown to engage the TIR-containing cytosolic adaptor molecule myeloid differentiation protein 88 (MyD88) through homotypic interactions,17-19 with subsequent recruitment of IL-1R–associated kinase (IRAK) and IRAK2, IRAK4, and TRAF6.17,18,20,21 TRAF6 is thought to subsequently activate nuclear factor (NF)–κB either through the IκB kinase (IKK) complex and the kinases TAB-1 and TAK-1,22 or through evolutionarily conserved signaling intermediate in Toll pathways (ECSIT) and mitogen-activated protein kinase/ERK kinase kinase 1 (MEKK-1).23
The recent derivation of MyD88-/- mice, however, challenged a universal role for MyD88 in LPS signaling. Although there was the expected complete ablation of IL-1 signaling, LPS still activated NF-κB although the ability to induce TNFα from macrophages was lost.24 In addition, LPS-induced NF-κB activation and up-regulation of costimulatory molecules in bone marrow–derived dendritic cells from these mice was not compromised.25 To account for a MyD88-independent pathway of NF-κB activation, a novel MyD88 homologue termed MyD88 adaptor-like (Mal) protein26 or TIR domain-containing adaptor protein (TIRAP)27 was described. This was shown to act as an adaptor protein specifically involved in TLR4 but not other TLRs or IL-1R–induced NF-κB activation.26,27 As Mal/TIRAP does not contain the death domain (DD) found in MyD88, this was proposed to induce an alternative signaling pathway that also involves RNA-dependent protein kinase (PKR)27 and leads to the induction of IFNβ and interferon-inducible protein-10 (IP-10).28
Despite the studies referenced above, the relevance of MyD88 and Mal/TIRAP to primary human macrophages and other human primary cells has yet to be established. This is of key importance given the major role of LPS in the pathology of sepsis. In this study, we addressed the question of specificity of MyD88 and Mal/TIRAP in TLR signaling by examining the effect of the wild-type (wt) and dominant-negative (dn) forms of MyD88 and Mal/TIRAP on LPS- and IL-1–induced NF-κB activation and cytokine production in primary human cells of nonmyeloid and myeloid origin. Our data show for the first time that the requirement for MyD88 and Mal/TIRAP in LPS signaling is cell-type–dependent. Evidence is provided that in human synovial fibroblasts (SFs) and umbilical vein endothelial cells (HUVECs), MyD88 and/or Mal/TIRAP are used as adaptor molecules for LPS signaling, whereas in human macrophages LPS can use MyD88 and/or Mal/TIRAP-independent alternative pathways to activate NF-κB and induce the expression of inflammatory cytokines. This conclusion of alternative pathways used by LPS is further supported by the cell-type–specific usage of TLR4 and IKK2. In contrast, Mal/TIRAP but not MyD88 is still used in human macrophages for LPS-induced IFNβ production. Our study demonstrates the complexity of the signaling mechanisms used by LPS and reveals a specific role for MyD88 and Mal/TIRAP in NF-κB activation in nonmyeloid cells with important implications in terms of therapies targeted at blocking LPS and IL-1 signaling.
Materials and methods
Reagents
Human recombinant macrophage colony-stimulating factor (M-CSF), IL-1, and TNFα were gifts of Genetics Institute (Cambridge, MA), Roche (Milan, Italy), and the Centre of Molecular and Macromolecular Studies (Lodz, Poland), respectively. Mouse antihuman TLR4 (HTA125) and TLR2 antibodies (N-17) used for blocking studies were purchased from BioCarta (Oxford, United Kingdom) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. The mouse antihuman TLR4 antibody (HTA125) was also used for fluorescence-activated cell sorting (FACS). The mouse IgG1 and IgG2a isotope control antibodies and the secondary phycoerythrin (PE)–conjugated antimouse immunoglobulin (Ig) were purchased from Becton Dickinson (Oxford, United Kingdom).
Isolation of cells and culture
Primary human synovial fibroblasts (SFs), umbilical vein endothelial cells (HUVECs), and peripheral blood monocytes were isolated and cultured as previously described.29-32 Macrophages were derived from monocytes after differentiation for 2 to 3 days with 100 ng/mL M-CSF. The mouse macrophage cell line RAW 264.7 was obtained from ATCC (Middlesex, United Kingdom) and mouse peritoneal macrophages were obtained from DBA/1 mice after intraperitoneal injection of starch.
Adenoviral vectors and their propagation
Recombinant, replication-deficient adenoviral vectors having no insert (Ad0), or encoding β-galactosidase (Adβ-gal) and green fluorescent protein (AdGFP) were provided by Drs A. Byrnes and M. Wood (Oxford, United Kingdom) and Quantum Biotech (Carlsbad, AB, Canada), respectively. The adenovirus encoding wild-type or dominant-negative forms of MyD88 (AdMyD88wt and AdMyD88dn) and Mal/TIRAP (AdMalwt, AdMalP125H, and MalTIR) were generated as previously described.33 The cDNAs used for the construction of MyD88 and Mal/TIRAP recombinant adenoviruses were gifts of Dr K. Burns (University of Lausanne, Switzerland) and Dr J. Sims (Amgen, Seattle, WA), respectively.18,26 Finally, adenoviruses encoding dominant-negative IKK2 (AdIKK2dn) or IκBα were kindly donated by Dr R. de Martin (University of Vienna, Austria) and have been previously used in other studies.34-36 All viruses produced are E1/E3-deleted, belong to the Ad5 serotype, and were grown as previously described.37
Adenoviral infection of cells
HUVECs and macrophages were infected in serum-free medium with adenoviruses at a multiplicity of infection (MOI) of 100. After 2 hours, the adenovirus-containing medium was removed and complete medium was added back. For passaged SF, an MOI of 500 was used as these cells are more resistant to adenoviral infection.38 For the mouse RAW macrophage cell line and primary peritoneal macrophages, an MOI of 500 was also used.
Western blotting and electrophoretic mobility shift assay (EMSA)
Two days after infection, SFs, HUVECs, or macrophages were stimulated with either vehicle control, 20 ng/mL TNFα, 20 ng/mL IL-1, or 10 ng/mL to 100 ng/mL LPS for 45 minutes, and cytosolic and nuclear extracts were prepared as described.39 Cytosolic proteins were subsequently separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% (wt/vol) polyacrylamide gel and transferred onto a polyvinylidene difluoride (PVDF) membrane for Western blotting. Antibodies for IKK2 and IκBα were purchased from Santa Cruz Biotechnology. Nuclear extracts (10 μg) were examined for NF-κB DNA-binding activity by EMSA as previously described.40
Analysis of cytokines
Cells were plated in 96- and 48-well tissue-culture plates (Falcon, Oxford, United Kingdom) and were either left uninfected or infected with adenovirus. Two days after infection, cells were stimulated for 20 hours with 20 ng/mL TNFα, 20 ng/mL IL-1, and various concentrations (0.01 ng/mL-1000 ng/mL) of chloroform-extracted Escherichia coli LPS (Sigma, Poole, United Kingdom). In preliminary experiments, these concentrations induced maximal cytokine production (data not shown). Supernatants were analyzed for TNFα, IL-6, and IL-8 by enzyme-linked immunosorbent assay (ELISA; Becton Dickinson). For TLR-blocking experiments, cells were incubated with neutralizing anti-TLR2 or anti-TLR4 antibodies for 30 minutes prior to addition of LPS. Absorbance was read on a spectrophotometric ELISA plate reader (Labsystems Multiscan Biochromic, Cambridge, United Kingdom) and analyzed using the Ascent software (Thermo Labsystems, Cambridge, United Kingdom). In all cases, viability of the cells was not significantly affected over this time period when examined by the 3-[4,5 dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) assay (Sigma).41
Semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR)
RNA was isolated using the Qiagen RNA Blood isolation kit (Qiagen, Valencia, CA). Total RNA was reverse transcribed with Superscript II Rnase H- reverse transcriptase (Gibco Life Technologies, Carlsbad, CA) and oligo(dT) primer. For mouse IFNβ amplification, the primers 5′-TGAGGACATCTCCCACGTCAA-3′ and 5′-TCCAAGAAAGGACGAACATTCG-3′ were used. For human IFNβ amplification, the primers 5′-GCTACAACTTGCTTGGATTCC-3′ and 5′-CCTTAGGATTTCCACTCTGAC-3′ were used. Amplification was performed in a Primus 96plus PCR machine (MWC-Biotech, Munich, Germany) and the number of PCR cycles used was within the exponential phase of the reaction: that is, where there is a log-linear relationship between the number of PCR cycles and the yield of PCR products.42
Results
Generation of adenoviral constructs expressing MyD88 and Mal/TIRAP
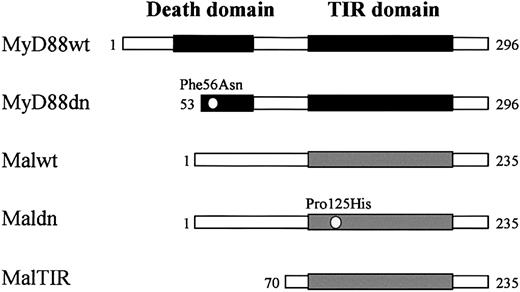
The role of TIR-expressing adaptor molecules (MyD88, Mal/TIRAP) in transducing signals from TLR/IL-1R families has been studied in many systems but not primary human macrophages or other primary human cells. To remedy this, adenoviral constructs were generated expressing both the wild-type and dominant-negative forms of MyD88 and Mal/TIRAP (Figure 1). The MyD88 dominant-negative adenovirus (AdMyD88dn) contained a 52 amino acid deletion as well as a Phe56Asn point mutation in the DD, rendering it incapable of signaling as previously described by Burns and colleagues.18 The Mal/TIRAP dominant-negative cDNAs used were previously described by Fitzgerald et al26 and consisted of a Pro125His mutation that disrupts TIR function (AdMaldn) or a truncated molecule that has the TIR only (AdMalTIR).
Wild-type (wt) and dominant-negative (dn) forms of MyD88 and Mal/TIRAP used in the present study. MyD88wt contains an N-terminal DD and a C-terminal TIR domain, whereas Malwt lacks a DD but still contains a C-terminal TIR domain. MyD88dn has a point mutation (Phe56Asn) and a 52–amino acid deletion spanning its DD that disrupts DD-DD interactions. Maldn has a point mutation (Pro125His) in its TIR domain that disrupts TIR domain interactions. MalTIR consists only of the Mal/TIRAP TIR domain.
Wild-type (wt) and dominant-negative (dn) forms of MyD88 and Mal/TIRAP used in the present study. MyD88wt contains an N-terminal DD and a C-terminal TIR domain, whereas Malwt lacks a DD but still contains a C-terminal TIR domain. MyD88dn has a point mutation (Phe56Asn) and a 52–amino acid deletion spanning its DD that disrupts DD-DD interactions. Maldn has a point mutation (Pro125His) in its TIR domain that disrupts TIR domain interactions. MalTIR consists only of the Mal/TIRAP TIR domain.
MyD88dn inhibits IL-1– but not LPS-induced NF-κB activation and cytokine production in primary human macrophages
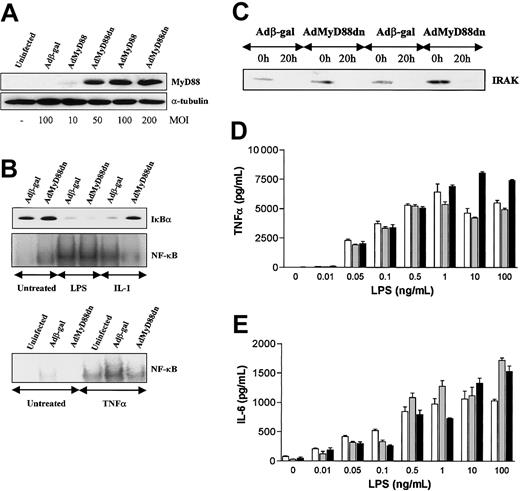
Infection of human macrophages with AdMyD88dn resulted in high levels of MyD88dn at an MOI of more than 50 (Figure 2A). Similar levels of expression were also seen with MyD88wt (data not shown). As expected, MyD88dn inhibited IL-1–induced IκBα degradation and NF-κB activation, whereas it had no effect on TNFα-induced activation of this transcription factor (Figure 2B). With respect to cytokine production, neither IL-1 nor TNFα were able to induce the expression of TNFα, IL-6, or IL-8 in human M-CSF–differentiated macrophages (data not shown) suggesting that in addition to NF-κB activation another signal is also required for this process. However, in contrast to IL-1, MyD88dn had no effect on LPS function as shown by IκBα degradation, NF-κB activation, and IRAK degradation (Figure 2B-C), a consequence of the activation of this kinase, or the production of the NF-κB–dependent cytokines TNFα, IL-6 (Figure 2D-E), and IL-8 (data not shown). This lack of effect of MyD88dn was independent of the LPS concentration used (Figure 2D-E).
MyD88dn inhibits IL-1– but not LPS-induced NF-κB activation or cytokine production (for LPS) in human macrophages. Macrophages were generated from peripheral blood monocytes after 2 days of culture with 100 ng/mL M-CSF. Marcophages were infected for 2 hours with adenoviruses overexpressing β-galactosidase or MyD88dn in serum-free medium. Then cells were cultured in complete medium for another 2 days and treated with 20 ng/mL IL-1, 20 ng/mL TNFα, or 0.01 ng/mL to 100 ng/mL LPS. For Western blot and EMSA, 10 ng/mL LPS was used. (A) The expression of MyD88dn at different MOIs was examined by Western blot. (B) After 45 minutes of treatment, cytosolic and nuclear extracts were collected and assayed for IκBα by Western blot (upper panel) and NF-κB DNA binding by EMSA (lower panels). (C) After 20 hours of treatment with LPS, cytosolic extracts were collected and assayed for IRAK degradation by Western blot. After 20 hours of treatment with LPS, supernatants from uninfected (white bars), Adβ-gal–infected (gray bars), or AdMyD88dn-infected (black bars) cells were collected and assayed by ELISA for TNFα (D) and IL-6 (E). Mean cytokine production (± SD) of triplicate cultures is shown and is representative of 5 independent experiments from unrelated donors.
MyD88dn inhibits IL-1– but not LPS-induced NF-κB activation or cytokine production (for LPS) in human macrophages. Macrophages were generated from peripheral blood monocytes after 2 days of culture with 100 ng/mL M-CSF. Marcophages were infected for 2 hours with adenoviruses overexpressing β-galactosidase or MyD88dn in serum-free medium. Then cells were cultured in complete medium for another 2 days and treated with 20 ng/mL IL-1, 20 ng/mL TNFα, or 0.01 ng/mL to 100 ng/mL LPS. For Western blot and EMSA, 10 ng/mL LPS was used. (A) The expression of MyD88dn at different MOIs was examined by Western blot. (B) After 45 minutes of treatment, cytosolic and nuclear extracts were collected and assayed for IκBα by Western blot (upper panel) and NF-κB DNA binding by EMSA (lower panels). (C) After 20 hours of treatment with LPS, cytosolic extracts were collected and assayed for IRAK degradation by Western blot. After 20 hours of treatment with LPS, supernatants from uninfected (white bars), Adβ-gal–infected (gray bars), or AdMyD88dn-infected (black bars) cells were collected and assayed by ELISA for TNFα (D) and IL-6 (E). Mean cytokine production (± SD) of triplicate cultures is shown and is representative of 5 independent experiments from unrelated donors.
MyD88dn inhibits LPS-induced NF-κB activation and cytokine production in RAW 264.7 cells but not primary mouse peritoneal macrophages
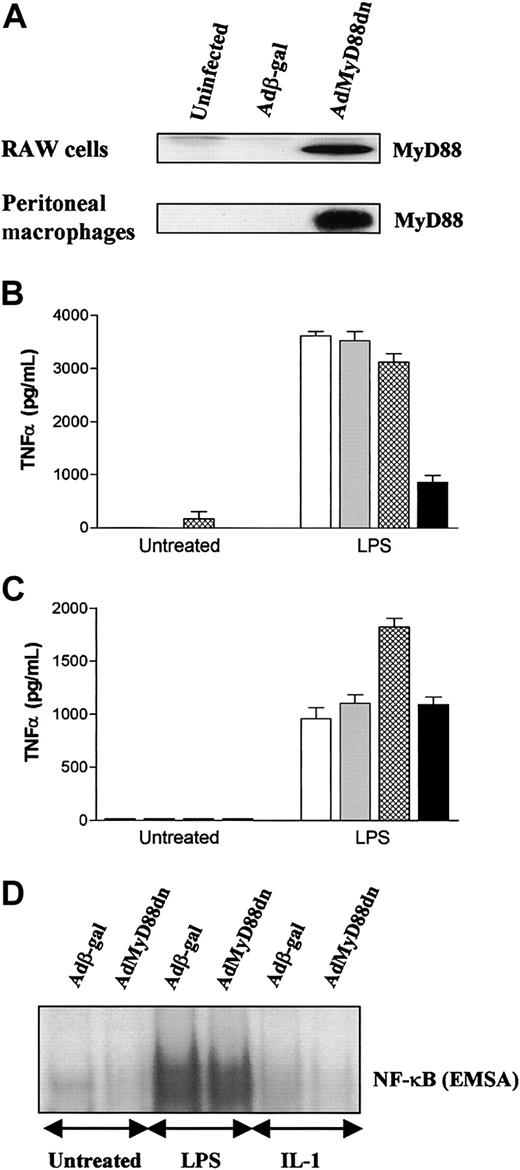
The data in “MyD88dn inhibits IL-1– but not LPS-induced NF-κB activation and cytokine production in primary human macrophages” on LPS did partially agree with data from MyD88-/- mice, suggesting a redundant role for MyD88 in LPS-induced NF-κB activation, but disagreed with data obtained in studies with the RAW 264.7 mouse macrophage cell line or the human THP-1 monocytic line.19,26,27 Because, however, these studies used transfection rather than viral delivery, they were also repeated in RAW 264.7 cells with AdMyD88dn. Adenoviral gene transfer into RAW 264.7 gave high levels of expression of MyD88dn that resulted in inhibition of LPS-induced TNFα production (Figure 3A-B). This result raised the issue of whether the requirement for MyD88 in LPS signaling is an artifact of macrophage cell lines. When these studies were repeated in primary peritoneal macrophages, AdMyD88dn also resulted in high levels of expression of MyD88dn (Figure 3A) but this had no effect on LPS-induced TNFα production or NF-κB activation (Figure 3C-D). In contrast to LPS, IL-1 did not induce NF-κB activation (Figure 3D) or cytokine production (data not shown) above background levels in this cell type.
MyD88dn inhibits LPS-induced TNFα production in mouse RAW cells but not in perinoneal macrophages. RAW cells and murine peritoneal macrophages were left uninfected (white bars) or infected for 2 hours with adenoviruses overexpressing β-galactosidase (gray bars), MyD88wt (cross-hatched bars), or MyD88dn (black bars) in serum-free medium, and then cultured in complete medium for another day. (A) Cells were lysed and cytosolic extracts collected and examined for the expression of MyD88. RAW cells (B) and peritoneal macrophages (C) were treated with 1 μg/mL LPS for an additional 20 hours, and supernatants were assayed by ELISA for TNFα. Mean cytokine production (± SD) of triplicate cultures is shown and is representative of 3 independent experiments. Peritoneal macrophages were also treated with 1 μg/mL LPS or 20 ng/mL IL-1 for 45 minutes and nuclear extracts were collected and assayed for NF-κB activation by EMSA (D).
MyD88dn inhibits LPS-induced TNFα production in mouse RAW cells but not in perinoneal macrophages. RAW cells and murine peritoneal macrophages were left uninfected (white bars) or infected for 2 hours with adenoviruses overexpressing β-galactosidase (gray bars), MyD88wt (cross-hatched bars), or MyD88dn (black bars) in serum-free medium, and then cultured in complete medium for another day. (A) Cells were lysed and cytosolic extracts collected and examined for the expression of MyD88. RAW cells (B) and peritoneal macrophages (C) were treated with 1 μg/mL LPS for an additional 20 hours, and supernatants were assayed by ELISA for TNFα. Mean cytokine production (± SD) of triplicate cultures is shown and is representative of 3 independent experiments. Peritoneal macrophages were also treated with 1 μg/mL LPS or 20 ng/mL IL-1 for 45 minutes and nuclear extracts were collected and assayed for NF-κB activation by EMSA (D).
Maldn does not inhibit either IL-1– or LPS-induced NF-κB activation and cytokine production in primary human macrophages
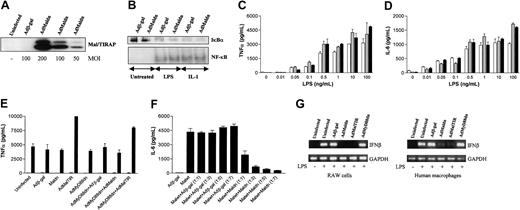
The ability of macrophages from MyD88-/- mice to still induce NF-κB in response to LPS suggested the existence of an alternative adaptor molecule.24 The identification of Mal/TIRAP as a putative TLR4-specific TIR adaptor appeared to fulfill this role although studies were chiefly confined to cell lines.26,27 Infection of human macrophages with AdMaldn produced high levels of expression of the protein over the endogenous levels (Figure 4A) and similar expression was seen with AdMalwt and AdMalTIR (data not shown). As expected, Maldn had no effect on IL-1–induced NF-κB activation (Figure 4B), although there was also no effect on LPS-induced IκBα degradation, NF-κB activation (Figure 4B), or the production of cytokines, TNFα (Figure 4C), IL-6 (Figure 4D), or IL-8 (data not shown). As for MyD88dn, the impotency of Maldn did not depend on the LPS concentration.
Mal/TIRAP is dispensable for IL-1– or LPS-induced NF-κB activation or cytokine production in human macrophages. Human macrophages (A-G) or RAW cells (G) were infected for 2 hours with adenoviruses expressing β-galactosidase, Malwt, Maldn, or MalTIR in serum-free medium. Cells were cultured in complete medium for another 2 days and treated with 20 ng/mL IL-1, 0.01 ng/mL to 100 ng/mL LPS, or 20 ng/mL TNFα. For Western blot and EMSA, 10 ng/mL LPS was used. (A) The expression of MyD88dn at different MOIs was examined by Western blot. (B) After 45 minutes of treatment, cytosolic and nuclear extracts were collected and assayed for IκBα expression by Western blot (upper panel) and NF-κB DNA binding by EMSA (lower panel). After 20 hours of treatment with LPS, supernatants from uninfected (white bars), Adβ-gal–infected (gray bars), or AdMaldn-infected (black bars) cells were collected and assayed by ELISA for TNFα (C) and IL-6 (D). (E) Combinations of AdMyD88dn with Adβ-gal, AdMaldn, or AdMalTIR were also used to infect human macrophages, and their effect on TNFα production after treatment for 20 hours with 10 ng/mL LPS was examined. (F) Combinations of AdMalwt with Adβ-gal or AdMaldn were also used to infect human macrophages and IL-6 production in the absence of further treatment was examined after 20 hours. A 1:1 ratio means that MOI of 100 for each virus was used, whereas 1:3, 1:5, and 1:7 ratios mean that MOIs of 300, 500, and 700, respectively, were used for the higher-ratio virus. Mean cytokine production (± SD) of triplicate cultures is shown and is representative of 5 independent experiments from unrelated donors. Finally, total mRNA levels from RAW cells or human macrophages were extracted, quantified, and equal amounts subjected to RT-PCR for IFNβ and glyceraldehyd-3-phosphate dehydrogenase (GAPDH) amplification (G). A representative of 3 independent experiments is shown.
Mal/TIRAP is dispensable for IL-1– or LPS-induced NF-κB activation or cytokine production in human macrophages. Human macrophages (A-G) or RAW cells (G) were infected for 2 hours with adenoviruses expressing β-galactosidase, Malwt, Maldn, or MalTIR in serum-free medium. Cells were cultured in complete medium for another 2 days and treated with 20 ng/mL IL-1, 0.01 ng/mL to 100 ng/mL LPS, or 20 ng/mL TNFα. For Western blot and EMSA, 10 ng/mL LPS was used. (A) The expression of MyD88dn at different MOIs was examined by Western blot. (B) After 45 minutes of treatment, cytosolic and nuclear extracts were collected and assayed for IκBα expression by Western blot (upper panel) and NF-κB DNA binding by EMSA (lower panel). After 20 hours of treatment with LPS, supernatants from uninfected (white bars), Adβ-gal–infected (gray bars), or AdMaldn-infected (black bars) cells were collected and assayed by ELISA for TNFα (C) and IL-6 (D). (E) Combinations of AdMyD88dn with Adβ-gal, AdMaldn, or AdMalTIR were also used to infect human macrophages, and their effect on TNFα production after treatment for 20 hours with 10 ng/mL LPS was examined. (F) Combinations of AdMalwt with Adβ-gal or AdMaldn were also used to infect human macrophages and IL-6 production in the absence of further treatment was examined after 20 hours. A 1:1 ratio means that MOI of 100 for each virus was used, whereas 1:3, 1:5, and 1:7 ratios mean that MOIs of 300, 500, and 700, respectively, were used for the higher-ratio virus. Mean cytokine production (± SD) of triplicate cultures is shown and is representative of 5 independent experiments from unrelated donors. Finally, total mRNA levels from RAW cells or human macrophages were extracted, quantified, and equal amounts subjected to RT-PCR for IFNβ and glyceraldehyd-3-phosphate dehydrogenase (GAPDH) amplification (G). A representative of 3 independent experiments is shown.
To investigate whether either the failure to identify a functional role for Mal/TIRAP or MyD88 was due to redundancy between these molecules, studies were also performed using a combination of AdMaldn and AdMyd88dn but this again had no effect on LPS-induced TNFα production (Figure 4E). Although the majority of our data were generated with AdMaldn, AdMalTIR was also used. Infection of macrophages with this virus again had no effect and rather enhanced LPS-induced TNFα production (Figure 4E), although the reason for this effect is unclear. To confirm the effectiveness of Maldn as an inhibitor in primary human macrophages we also used AdMalwt. AdMalwt-induced IL-6 production in the absence of LPS stimulation was effectively blocked by the coexpression of Maldn (Figure 4F), indicating that the dominant-negative can act as an inhibitor of Mal signaling in this cell type.
As the data so far suggested that neither Mal/TIRAP nor MyD88 are essential for LPS-induced NF-κB activation in macrophages, we decided to investigate whether Mal/TIRAP and MyD88 are involved in LPS-induced NF-κB–independent pathways such as IFNβ production. We found that AdMaldn and AdMalTIR but not AdMyD88dn inhibited LPS-induced IFNβ mRNA expression in both the mouse RAW cell line and primary human macrophages (Figure 4G). However, this was not due to an obvious role of Mal/TIRAP on IRF3 activation, as neither Maldn nor MalTIR inhibited LPS-induced IRF3 phosphorylation in RAW cells or human macrophages (data not shown), in agreement with previously published studies in Mal/TIRAP-deficient mice that show that this adaptor is not required for this function.43,44 How Maldn and MalTIR control the expression of IFNβ in human macrophages is at present unclear.
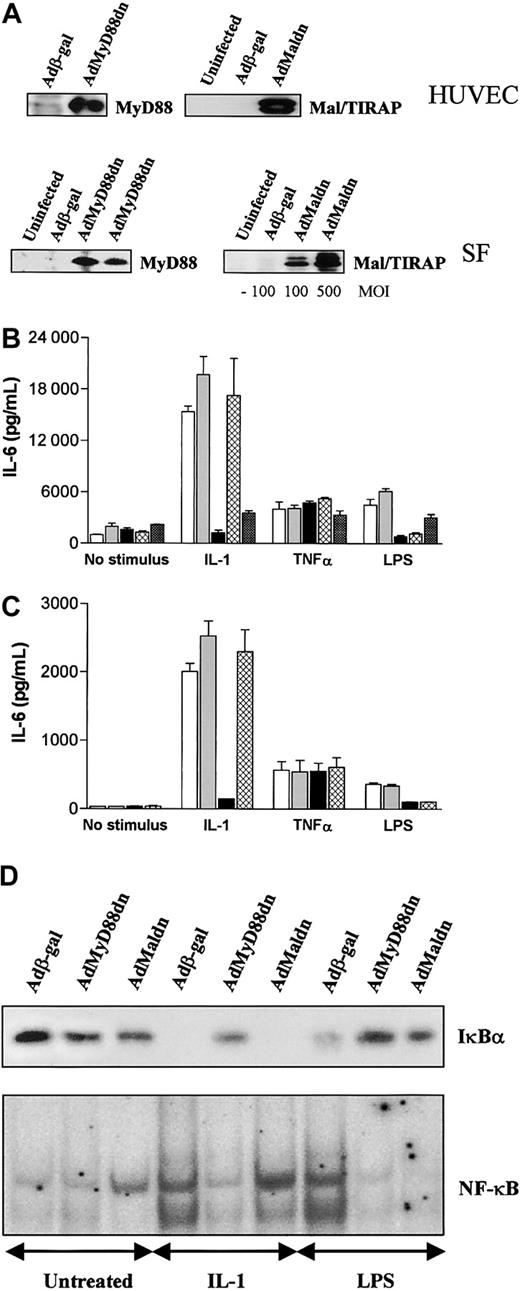
MyD88 or Mal/TIRAP are both required for LPS-induced NF-κB activation and cytokine production in human SFs and HUVECs
LPS can stimulate a number of nonmyeloid cells such as endothelial cells, fibroblasts, and B lymphocytes. In general, signaling in nonmyeloid cells by LPS has been restricted to cell lines often transfected with TLR4/CD14. Since our adenoviral constructs can effectively infect HUVECs and SFs, we examined the role of MyD88 and Mal/TIRAP in these cells. Infection with the appropriate viral constructs resulted in the expression of wild-type or dominant-negative versions of MyD88 and Mal/TIRAP in these cells (Figure 5A). Unlike macrophages, MyD88dn blocked both IL-1– and LPS-induced IL-6 (Figure 5B-C) or IL-8 (data not shown) production in HUVECs and SFs. However, Maldn only blocked LPS-induced signaling in these cells (Figure 5B-C), whereas TNFα-induced cytokine production was unaffected by either construct (Figure 5B-C). MyD88wt and Malwt, on the other hand, had no effect on any stimulus but their expression alone did activate NF-κB and did induce IL-6 and IL-8 production (data not shown). The same differential effects of MyD88dn and Maldn in signaling were also observed at the transcription factor level where MyD88dn inhibited both LPS- and IL-1–induced IκBα degradation and NF-κB activation in SF whereas Maldn only blocked LPS activity (Figure 5D). Similar data were obtained in HUVECs (data not shown).
MyD88dn and Maldn block LPS-induced NF-κB activation and cytokine production in human SFs and HUVECs. SFs and HUVECs were infected with adenoviruses overexpressing β-galactosidase, MyD88dn, Maldn, or MalTIR in serum-free medium. Then cells were cultured in complete medium for another day. (A) Cells were examined for the expression of MyD88dn and Maldn. SFs (B) and HUVECs (C) were treated with 20 ng/mL IL-1, 1 μg/mL LPS, or 20 ng/mL TNFα for 20 hours, and supernatants from uninfected (white bars), Adβ-gal– (gray bars), AdMyD88dn- (black bars), AdMaldn- (cross-hatched bars), or AdMalTIR-infected (checkered bars) cells were assayed by ELISA for IL-6. Mean cytokine production (± SD) of triplicate cultures is shown and is representative of 5 independent experiments from unrelated donors. (D) SFs were stimulated for 45 minutes with 20 ng/mL IL-1, or 1 μg/mL LPS; cytosolic and nuclear extracts were collected and then examined for IκBα expression by Western blot (upper panel) and NF-κB DNA binding by EMSA (lower panel).
MyD88dn and Maldn block LPS-induced NF-κB activation and cytokine production in human SFs and HUVECs. SFs and HUVECs were infected with adenoviruses overexpressing β-galactosidase, MyD88dn, Maldn, or MalTIR in serum-free medium. Then cells were cultured in complete medium for another day. (A) Cells were examined for the expression of MyD88dn and Maldn. SFs (B) and HUVECs (C) were treated with 20 ng/mL IL-1, 1 μg/mL LPS, or 20 ng/mL TNFα for 20 hours, and supernatants from uninfected (white bars), Adβ-gal– (gray bars), AdMyD88dn- (black bars), AdMaldn- (cross-hatched bars), or AdMalTIR-infected (checkered bars) cells were assayed by ELISA for IL-6. Mean cytokine production (± SD) of triplicate cultures is shown and is representative of 5 independent experiments from unrelated donors. (D) SFs were stimulated for 45 minutes with 20 ng/mL IL-1, or 1 μg/mL LPS; cytosolic and nuclear extracts were collected and then examined for IκBα expression by Western blot (upper panel) and NF-κB DNA binding by EMSA (lower panel).
Overexpression of MalTIR domain blocks IL-1–induced cytokine production
The data with Maldn suggest that unlike MyD88, Mal/TIRAP has a selective role in LPS signaling that is utilized by both LPS and IL-1. However, it must be noted that the natures of the dominant-negative forms of MyD88 and Mal/TIRAP are different in so far as MyD88dn has a functioning TIR domain whereas Maldn does not (Figure 1). Therefore, we repeated the studies in SFs and HUVECs with the MalTIR construct. Infection with AdMalTIR inhibited LPS signaling as expected but in addition to that it also inhibited IL-1–induced IL-6 (Figure 5B). These data would suggest that the TIR of Mal/TIRAP is capable of interacting with the IL-1 receptor when overexpressed. The implications of the differences that Maldn and MalTIR have on IL-1 function, and on our understanding of the signaling mechanisms of this cytokine will be addressed in the “Discussion.”
Different requirements for TLR4 and IKK2 in LPS signaling in human macrophages and SFs or HUVECs
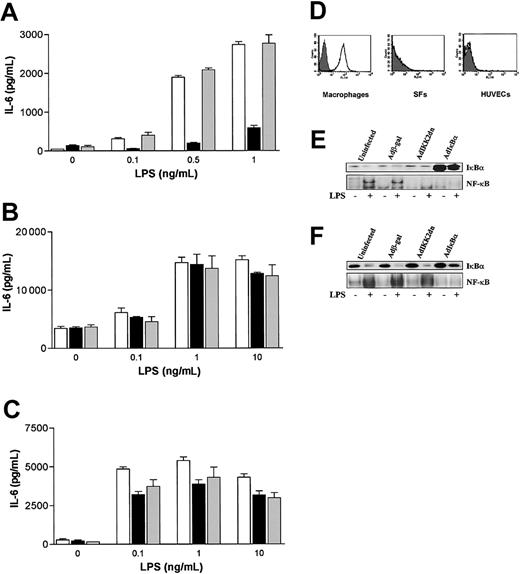
The data obtained so far indicated a gross difference in LPS signaling mechanisms between macrophages on one hand and SFs and HUVECs on the other. This could not be accounted for higher LPS concentrations used with nonmyeloid cells since Maldn and MyD88dn failed to inhibit macrophage activation at any concentration of endotoxin (Figure 2D-E and Figure 4C-D). An obvious putative reason for the data was that LPS is signaling through different receptors and/or pathways. As TLR4 is the most widely accepted, although not the sole LPS receptor, the ability of an anti-TLR4 neutralizing antibody to block LPS-induced cytokine production in SFs, HUVECs, and human macrophages was examined. In macrophages, an anti-TLR4 monoclonal antibody used at 10 μg/mL prevented LPS-induced TNFα and IL-6 production at LPS concentrations of 0.1 ng/mL to 1 ng/ml (Figure 6A) although the effect was lost at higher LPS concentrations (> 10 ng/mL, data not shown). This is likely to be due to increased competition of the higher LPS concentrations with the anti-TLR4 neutralizing antibody for the receptor. In contrast, in SFs and HUVECs this antibody at concentrations of 10 μg/mL (Figure 6B-C) and 50 μg/mL (data not shown) did not inhibit LPS-induced IL-6 (Figure 6B-C) or IL-8 production (data not shown), suggesting that LPS may not require cell surface TLR4 to signal in these cell types. This result was unlikely to be due to contaminants present in the LPS preparation that induce signaling via TLR2 as shown previously45 as the LPS used in all the studies shown here was purified by phenol-chloroform-extraction45 and an anti–TLR2-blocking antibody had no effect in LPS signaling induced by LPS in any cell type (Figure 6). The expression of TLR4 was also examined. Using FACS analysis, TLR4 was detected at high levels in the surface of primary human macrophages. However, on SFs and HUVECs TLR4 was undetectable (Figure 6D).
Different requirements for TLR4 and IKK2 in LPS signaling in human macrophages and SFs or HUVECs. Macrophages (A), SFs (B), or HUVECs (C) were left untreated (white bars) or treated for 30 minutes with 10 μg/mL TLR4 (black bars) or TLR2 (gray bars) neutralizing antibodies prior to addition of graded doses of chloroform-extracted LPS. After 20 hours, supernatants were collected and assayed by ELISA for TNFα and IL-6. Mean cytokine production (± SD) of triplicate cultures is shown and is representative of 5 independent experiments for macrophages and 3 independent experiments for SFs and HUVECs. (D) SFs, HUVECs, and macrophages were analyzed for the expression of TLR4 by FACS staining. A representative of 3 independent experiments is shown. HUVECs (E) and macrophages (F) were infected with adenoviruses overexpressing β-galactosidase, IKK2dn, or IκBα for 2 hours in serum-free medium. Then cells were cultured in complete medium for another day and stimulated with 1 μg/mL and 10 ng/mL LPS, respectively. After 45 minutes, cytosolic and nuclear extracts were collected and assayed for IκBα expression by Western blot (upper panel) and NF-κB DNA binding by EMSA (lower panels). A representative of 3 independent experiments from unrelated donors is shown.
Different requirements for TLR4 and IKK2 in LPS signaling in human macrophages and SFs or HUVECs. Macrophages (A), SFs (B), or HUVECs (C) were left untreated (white bars) or treated for 30 minutes with 10 μg/mL TLR4 (black bars) or TLR2 (gray bars) neutralizing antibodies prior to addition of graded doses of chloroform-extracted LPS. After 20 hours, supernatants were collected and assayed by ELISA for TNFα and IL-6. Mean cytokine production (± SD) of triplicate cultures is shown and is representative of 5 independent experiments for macrophages and 3 independent experiments for SFs and HUVECs. (D) SFs, HUVECs, and macrophages were analyzed for the expression of TLR4 by FACS staining. A representative of 3 independent experiments is shown. HUVECs (E) and macrophages (F) were infected with adenoviruses overexpressing β-galactosidase, IKK2dn, or IκBα for 2 hours in serum-free medium. Then cells were cultured in complete medium for another day and stimulated with 1 μg/mL and 10 ng/mL LPS, respectively. After 45 minutes, cytosolic and nuclear extracts were collected and assayed for IκBα expression by Western blot (upper panel) and NF-κB DNA binding by EMSA (lower panels). A representative of 3 independent experiments from unrelated donors is shown.
These data suggest that there are gross differences in LPS-induced NF-κB activation and signaling in general between macrophages and nonmyeloid cells such as SFs and HUVECs. We investigated this further by analyzing the role of IKK2 as a kinase that has been deemed as essential for NF-κB activation. As expected, expression of IKK2dn in HUVECs prevented LPS-induced NF-κB activation (Figure 6E). However, this was not the case in macrophages where IKK2 had no effect in this process (Figure 6F).
Discussion
This study has produced the novel finding that there are unexpected major differences in the mechanisms of LPS-induced NF-κB activation and cytokine production in primary cells of nonmyeloid and myeloid origin. In SFs and HUVECs, LPS signals via MyD88 or Mal/TIRAP and IKK2 to induce NF-κB activation and cytokine production through a pathway that cannot be shown to require TLR4. In contrast, in primary human macrophages, LPS signals via TLR4 but neither MyD88 nor Mal/TIRAP or IKK2 appear to be essential for NF-κB activation and cytokine production. These observations provide insight into the specificity of the adaptor molecules MyD88 and Mal/TIRAP in LPS signaling and have important implications for therapeutic strategies aimed at inhibiting LPS activity.
The observation that LPS signals via TLR4, a TIR domain–containing receptor, suggested that LPS is actually using similar intracellular signaling mechanisms to those used by IL-1 receptor family members that also contain a TIR domain in their cytoplasmic region. This hypothesis was initially supported by studies in a variety of cell lines that showed that LPS signals via MyD88, IRAK, and TRAF6.19,26,27,46 However, although studies with MyD88-/- mice confirmed the importance of this adaptor to IL-1 receptor function,24 they suggested that at least with respect to NF-κB activation, MyD88 does not have a role in LPS signaling. Our observation that LPS-induced NF-κB activation and cytokine production can occur in the absence of MyD88 in primary macrophages disagrees with previous studies in macrophage cell lines19,26,27 but confirms the finding from MyD88-/- mice that MyD88 is not required for NF-κB activation. The failure to show a role for MyD88 in macrophages was not due to the approach of using an adenovirus delivery system rather than transfection because adenoviral infection of the RAW cell line with AdMyD88dn did inhibit LPS signaling, as expected from transfection studies. Also, the MyD88dn was functional in primary macrophages as shown by the suppression of IL-1 signaling. Therefore, the reason for the differences in the role of MyD88 between primary cells and cell lines may relate more to changes in signaling mechanisms induced by cell transformation. There are antecedents for this as we and others have previously shown that pathways leading to NF-κB activation are aberrant in cell lines, as was observed with NF-κB–inducing kinase (NIK) that has a major role in LPS or cytokine-induced NF-κB activation in cell lines but not primary cells where it appears to be important for lymphotoxin β receptor (LTβR) and B-cell activating factor receptor (BAFF-R) signaling.35,47-49
However, the differences between our data and MyD88-/- mice are more difficult to understand. Thus, in addition to its lack of effect on NF-κB activation, MyD88dn was also unable to block LPS-induced cytokine production. This is in contrast to the original observation that MyD88-/- macrophages do not produce cytokines in response to LPS. Since LPS signaling to NF-κB by the mutant macrophages is largely unaffected, the reason proposed for the loss of cytokine production was that this is due to a slight delay in the kinetics of activation of NF-κB, although this hypothesis to our knowledge has not been confirmed, and indeed the recent study by Hoebe et al (published while the present manuscript was reviewed) showed that MyD88-/- macrophages do produce TNFα in response to LPS, albeit at lower levels.50,51 This suggested that the discrepancy of our data with those in MyD88-/- macrophages may not be as great as first thought. There may be several explanations as to why the MyD88 and Mal/TIRAP role is different with respect to cytokine production between our system and the knockout mice. First, this may reflect human-to-mouse species differences as previously described in other cases such as severe combined immunodeficient (SCID) mice and human SCID patients,52 X-linked immunodeficiency (xid) mice and human X-linked agamma globulinemia (XLA) patients.53 Second, our differences could relate to as-yet-undetected changes in macrophage development that result in a failure of LPS to induce cytokine expression. Such a lesion would not be present in our studies where MyD88 activity is inhibited in already mature macrophages. Alternatively, differences may occur as a result of the expression of mutated or truncated versions of these proteins compared with their complete absence in mouse knockouts, as for example with NIK.34-36,48 Finally, this may be due to a “second signal” provided for example by M-CSF or recombinant adenoviruses that bypasses the requirement of MyD88 and/or Mal/TIRAP for cytokine production. However, M-CSF has also been used to generate bone marrow–derived macrophages from MyD88- and Mal/TIRAP-deficient mice43,44 and adenoviral gene transfer into cells at an MOI of 100 does not induce type I IFN production or activation of signaling pathways such as MAPKs, PKR, interferon regulatory factor 3 (IRF3) (data not shown), or NF-κB. These findings provide evidence against such a possibility although this cannot be unequivocally excluded.
The absence of a role for MyD88 in LPS signaling in macrophages led to the study being extended to the more recently identified TIR-containing adaptor molecule, Mal/TIRAP. Initial studies mainly in cell lines showed that this molecule was a potential TLR4-specific adaptor molecule, thus explaining the data from MyD88-/- macrophages.26,27 However, when we examined the role of Mal/TIRAP in TUR4 signaling in primary human macrophages we found that the mutated versions of Mal/TIRAP do not impair LPS signaling. This does not appear to be due to an inability of Mal/TIRAP dominant-negative to function, as it is still able to inhibit the production of IL-6 by macrophages induced by the simple overexpression of the wild-type protein. Our data are more in agreement with results obtained in macrophages from the very recently derived Mal/TIRAP-/- mice. In these studies, Mal/TIRAP was shown to be involved in TLR1/2, TLR2/6, and TLR4 signaling.43,44 LPS-induced NF-κB activation was unaffected although LPS-induced TNFα production from Mal/TIRAP-/- macrophages was abrogated in a similar manner to that seen with MyD88-/- mice.43,44 Again the differences between Mal/TIRAP-/- and the early studies26,27 could be due to differences between cell lines and primary cells as discussed earlier in this section. In addition to the studies described here on macrophages, we also examined the role of MyD88 and Mal/TIRAP in LPS-induced NF-κB activation and cytokine production in another primary myeloid cell type, human immature dendritic cells, where we obtained very similar results (A.L., E.A., J.G., S.S., M.F., and B.M.F., manuscript in preparation, January 2003).
We also investigated the role of MyD88 and Mal/TIRAP in LPS signaling in primary nonmyeloid cells such as SFs and HUVECs that are also involved in the physiologic response to endotoxin. We found that similarly to studies in cell lines, MyD88 is essential but Mal/TIRAP is not required for IL-1 signaling.19,26,27 This was in contrast to the response to LPS where both MyD88 and Mal/TIRAP were needed for NF-κB activation and cytokine production. The difference in inhibitory activity of Maldn or MyD88dn in the different cell systems could not be trivially explained by the concentration of LPS used or the expression of the transgene. An obvious question that arises from the data is whether the roles of Mal/TIRAP and MyD88 in LPS signaling are totally redundant or display the existence of essential parallel pathways (Figure 7). We believe that the latter is more likely since Mal/TIRAP, unlike MyD88, does not contain a DD and is therefore likely to engage alternative molecules to MyD88. This thesis is supported by the different nature of the MyD88dn and Maldn molecules, as the former binds to the TIR domain of IL-1R/TLRs but fails to engage distal signaling pathways due to the defective DD. In contrast, Maldn has no functional TIR domain and presumably acts as a sink for molecules that bind to the other regions of Mal/TIRAP. The observed lack of effect of Maldn on IL-1 signaling certainly provides further evidence for the idea that Maldn acts as a sink, as one would presume that this cytokine does not require the signaling pathways that Mal/TIRAP engages. It was interesting to note, however, that the overexpression of the MalTIR domain did block IL-1 signaling, an observation that is easily explained by the saturation of the IL-1R TIR domain.
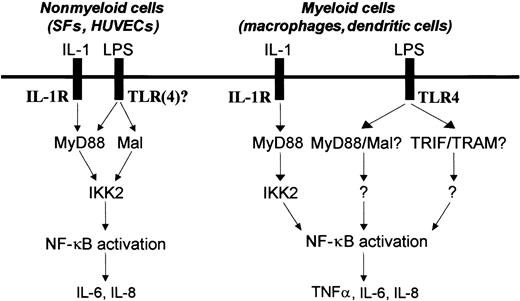
LPS uses a different framework of signaling molecules such as TLR4, MyD88, Mal, and IKK2 to activate NF-κB and induce NF-κB–dependent cytokine production in human nonmyeloid (SFs and HUVECs) and myeloid cells (macrophages and dendritic cells).
LPS uses a different framework of signaling molecules such as TLR4, MyD88, Mal, and IKK2 to activate NF-κB and induce NF-κB–dependent cytokine production in human nonmyeloid (SFs and HUVECs) and myeloid cells (macrophages and dendritic cells).
Another question that immediately arises is what pathways are mediated by Mal/TIRAP as compared with MyD88. The similar inhibitory effects of Mal/TIRAP and MyD88 dominant-negative on NF-κB activation and IκBα degradation in nonmyeloid cells suggests the convergence of signaling pathways on the processes of IκBα phosphorylation, possibly at the level of IKK2, although other signaling pathways could also be distal of Mal/TIRAP. Another possibility, suggested by Fitzgerald et al,26 is that MyD88 is part of the Mal/TIRAP pathway that still uses TRAF6 and IRAK1/2, which would more simply explain our data that both molecules lead to NF-κB activation. What pathways are triggered by Mal/TIRAP in primary human macrophages remain undefined but seem to involve IFNβ production and probably expression of IFNβ-dependent genes as we have found that AdMaldn and AdMalTIR but not AdMyD88dn block LPS-induced IFNβ mRNA expression as previously shown in the RAW cell line. However, the mechanism by which Maldn and MalTIR control the expression of IFNβ in human macrophages is unclear as this is not due to an obvious role of Mal/TIRAP on IRF3 phosphorylation and activation (data not shown), in agreement with studies in Mal/TIRAP-deficient mice.43,44 Previous studies have also shown that PKR and STAT1α/β activation as well as IRF3 activation and IP-10 production are distal of Mal/TIRAP.27,28,54 In our studies, however, we were unable to observe induction of PKR phosphorylation by LPS or show any role for Mal/TIRAP or MyD88 on LPS-induced IP-10 production in primary human macrophages (data not shown).
These data pose a further question as to why Maldn and MyD88dn fail to inhibit LPS signaling in macrophages but do so in other cells. One possibility is that there are other TIR adaptor molecules, and 2 such candidates, TRIF/TICAM-1 and TRAM/TICAM-2, were described while the present manuscript was under review.55-59 The generation of TRIF/TICAM-1–deficient or mutant mice suggested a major role for this adaptor protein in LPS signaling.50,51 LPS-stimulated macrophages from these mice did not produce cytokines, and did not activate IRF3. Interestingly, NF-κB activation in these cells in response to LPS was normal but completely abrogated when MyD88 and TRIF/TICAM-1 double-deficient mice were generated. TRAM/TICAM-2–deficient macrophages had also a defect in NF-κB activation but only at later time points.57 If adaptor molecules such as TRIF/TICAM-1 and TRAM/TICAM-2 are expressed at higher levels in macrophages compared with other cells, it is possible that the overexpressed dominant-negative forms of MyD88 and Mal/TIRAP fail to titrate these other adaptors out. The other main possibility is that LPS uses different receptors and/or signaling pathways in different cell types. Since its discovery, TLR4 has been the major receptor believed to be involved in LPS signaling as well demonstrated in murine cells of the myeloid lineage.7,60,61 This is in agreement with our studies in primary human macrophages showing that neutralizing TLR4 blocks LPS signaling. However, in contrast in SFs and HUVECs, we were unable to show any role for TLR4 in LPS signaling, as neutralizing TLR4 in these cells had no effect. This correlated with the lack of detectable levels of surface TLR4 expression on HUVECs or SFs whereas abundant levels could be detected in primary macrophages. Previous studies have suggested the presence of TLR4 in HUVECs, although this was shown by PCR and not by FACS.62 Even then, the mRNA levels of TLR4 detected in HUVECs were found to be very much lower than the mRNA levels of TLR4 found in myeloid cells.62 The possibility that there are major differences in the signaling pathways used by LPS between cell systems is supported by the studies on IKK2 that appeared to be used only in SFs and HUVECs (Figure 6E-F).63,64 The data would suggest that in SFs and HUVECs LPS signals by an unknown TLR via MyD88, Mal/TIRAP, and IKK2 to activate NF-κB and induce the expression of cytokines, although the possibility that TLR4 is cryptic and cannot be blocked by the antibody still exists. In contrast, in macrophages LPS signals via TLR4 using as-yet-unidentified molecules (Figure 7). This model would require Mal/TIRAP to act as an adaptor molecule for signaling through other TLRs distinct from TLR2 and TLR4. Indeed, we have evidence that Mal/TIRAP is also essential for the response to both TLR3 (polyI:C) and TLR5 ligands (flagellin) in HUVECs (A.L., E.A., J.G., S.S., M.F., and B.M.F., manuscript in preparation, January 2003). The relationship of MyD88 and IKK2 is further strengthened by our observation that IL-1 signaling to NF-κB in SFs, HUVECs, and macrophages always uses both molecules.64 Interestingly, in human immature dendritic cells where LPS signaling is also blocked by an anti-TLR4 neutralizing antibody (data not shown), IKK2 is not required for LPS-induced NF-κB activation and cytokine production,63 further supporting significant differences in LPS signaling between human myeloid and nonmyeloid cells.
In summary, this study has shown major differences in the mechanisms used by LPS to activate primary human myeloid and nonmyeloid cells, and has revealed a layer of complexity not previously expected. As LPS is a major pathogenic factor in sepsis, the observation that the pathway leading to NF-κB activation and cytokine production in response to LPS is different between primary human myeloid and nonmyeloid cells such as macrophages and endothelial cells may have important consequences when the development of therapeutics is being considered.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-04-1356.
Supported by the Arthritis Research Campaign (United Kingdom) and the Wellcome Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Drs J. Sims, T. Bird, and D. Smith (Amgen, Seattle, WA) for providing the Mal/TIRAP constructs used in this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal