Abstract
The causes of thrombosis and pregnancy loss in antiphospholipid syndrome (APS) are still unknown, although several hypotheses have been proposed and hypofibrinolysis has been implicated. Anti–tissue-type plasminogen activator (tPA) antibodies may induce fibrinolytic defects and preliminary data indicate an association with thrombosis in APS. We measured plasma anti-tPA antibody levels in 91 consecutive patients with APS, 91 healthy controls, 40 patients with antiphospholipid antibodies without APS symptoms, and 23 patients with systemic lupus erythematosus (SLE) without antiphospholipid antibodies and APS symptoms. Patients with APS had anti-tPA antibody levels higher than controls (P = .0001), patients with SLE (P = .0001), and asymptomatic antiphospholipid patients (P = .05). A subgroup of 53 patients had plasma levels of tPA antigen higher (P = .0001) and tPA activity lower (P = .05) than controls, with an inverse correlation (r = –0.454; P = .003) between anti-tPA antibody levels and tPA activity and no correlation with tPA antigen. The 2 patients with the highest antibody levels had tPA activity below the normal range. Their antibodies were, respectively, IgG1 and IgG3; both recognized human tPA, recombinant tPA, and the catalytic domain of tPA, but not β2-glycoprotein I, prothrombin, or plasminogen. Our data indicate that anti-tPA antibodies specifically interacting with the catalytic domain of tPA can be found in patients with APS, representing a possible cause of hypofibrinolysis.
Introduction
The antiphospholipid syndrome (APS) is characterized by the occurrence of events such as arterial or venous thrombosis and/or recurrent pregnancy loss, associated with positivity for lupus anticoagulant (LA) or anticardiolipin antibodies (ACLAs), or both. The syndrome may be primary or secondary to autoimmune or other diseases.1 The etiopathogenesis of thrombosis in APS is still unknown, but a number of hypotheses have been proposed to explain the mechanisms by which antiphospholipid antibodies may promote thrombotic events. These include the activation of endothelial cells, oxidant-mediated vascular endothelium lesions, interference with or modulation of the phospholipid-binding proteins regulating hemostasis, and mechanisms similar to heparin-induced thrombocytopenia.1 Reduced fibrinolytic activity has also been described in patients with APS, and may be responsible for thrombotic events,2-5 and antibodies directed against tissue-type plasminogen activator (tPA) might lead to a hypofibrinolytic state. Our previous studies have shown that an association between anti-tPA antibodies and thrombosis seems to be a peculiarity of APS because normal plasma anti-tPA antibody levels were found in 100 patients with a history of deep vein thrombosis (DVT) without APS,6 but high levels in 3 of 39 patients with primary APS who had a history of thrombosis.7 Furthermore, the patients who had experienced a previous stroke had higher plasma IgG anti-tPA levels than the patients without a history of thrombosis.7
The goal of this study was to measure the plasma levels of anti-tPA antibodies in 91 patients with primary or secondary APS who have developed a thrombotic event or recurrent pregnancy loss, in 91 healthy controls, in 40 patients with antiphospholipid antibodies without history of APS symptoms, and in 23 patients with systemic lupus erythematosus (SLE) without antiphospholipid antibodies and history of APS symptoms. We also evaluated the correlations between the presence of anti-tPA antibodies and the patients' clinical and laboratory data, particularly the type and site of thrombosis and tPA activity and antigen. Finally, in 2 patients with high anti-tPA antibodies, we isolated the IgG fraction and studied antibody interactions with the different types of tPA and its catalytic domain obtained by recombinant DNA technologies.
Patients, materials, and methods
Patients
We tested the plasma of 91 consecutive patients with APS regularly attending 3 different Italian centers (Department of Hematology of Bergamo, Istituto Auxologico Italiano, and Hemophilia and Thrombosis Center of Milano). Twenty-six were men and 65 women with a mean age of 47 years (range, 19-79 years); 68 had primary disease according to the Sapporo criteria,8 and 23 had secondary disease associated with an autoimmune disease (18 had SLE according to the American Rheumatism Association [ARA] criteria, 2 lupuslike syndrome, one rheumatoid arthritis, one Sjögren syndrome, and one polymyalgia rheumatica). All the patients had a history of episodes of thrombosis or pregnancy loss according to the Sapporo criteria.8 Twenty-four patients had had previous arterial thrombosis, 48 venous thrombosis, and 8 both. DVT and ischemic stroke were diagnosed by Doppler ultrasonography and computed tomography; respectively; the other cases were diagnosed by angiography. Eight of the patients with a history of thrombosis had also experienced a previous episode of pregnancy loss. The remaining 11 patients had never suffered from thrombosis but had a history of pregnancy loss (one or more unexplained deaths of a morphologically normal fetus beyond the tenth week of gestation or 3 or more unexplained consecutive spontaneous abortions before the tenth week of gestation). The time since the last episode of thrombosis ranged between 6 months and 5 years. The control group consisted of 91 age- and sex-matched healthy subjects (26 men and 65 women with a median age of 46 years; range, 19-79 years). We also studied 40 patients with persistently elevated antiphospholipid antibody levels without history of thrombosis or pregnancy loss (11 men and 29 women; age range, 21-72 years) and 23 patients with SLE without antiphospholipid antibodies, history of thrombosis, or pregnancy loss (all women; age range, 20-65 years).
Blood samples were collected in the morning by clean puncture of an antecubital vein with minimal stasis using sodium citrate as anticoagulant. The anti-tPA antibody levels were measured in plasma obtained after centrifuging the blood samples at 2500g for 20 minutes, and the plasma samples were frozen in small aliquots and stored at –80°C until testing. Two patients with high antibody levels were further studied to characterize the antibodies: a 37-year-old woman (patient no. 1) with lupuslike syndrome and a previous history of 3 spontaneous pregnancy losses and a 46-year-old woman (patient no. 2) with a previous ischemic stroke. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki, and all of the subjects gave their informed consent before participation.
Diagnosis of lupus anticoagulants
Lupus anticoagulants were diagnosed according to the revised criteria proposed by the Subcommittee for the Standardization of Lupus Anticoagulants9 using a battery of assays consisting of activated partial thromboplastin time (aPTT; Thrombofax, Ortho, Raritan, NJ; or automated aPTT reagent, Organon Teknika, Durham, NC), kaolin clotting time according to Exner et al,10 dilute Russel viper venom time according to Thiagarajan et al,11 and colloidal silica clotting time at low and high bovine phospholipid concentrations (Ortho) according to Chantarangkul et al.12 For patients taking oral anticoagulants, the textarin/ecarin test was also used.13
Measurement of ACLAs
IgG and IgM ACLAs were measured using enzyme-linked immunosorbent assay (ELISA) “in-house” techniques as described by Loizou et al.14 The results were expressed as GPL or MPL units according to Harris et al,15 with 1 unit being equivalent to 1 μg affinity-purified ACLA/mL plasma. Values of more than 21 GPL or MPL units were considered elevated.
Assays for anti-tPA antibodies
To detect anti-tPA antibodies, recombinant tPA (rtPA,Actilyse; Boehringer Ingelheim, Ingelheim, Germany; 10 μg/mL in phosphate-buffered saline [PBS], pH 7.4) was coated overnight onto ELISA microtitration plates (Maxisorp; Nunc, Roskilde, Denmark). After 3 washes with 250 μL 0.1% PBS Tween 20, the wells were coated with 250 μL β2-glycoprotein I (β2-GPI)–free bovine albumin (Sigma Chemical, St Louis, MO; product no. A3803; 10 mg/mL in PBS Tween 20) and then incubated for 2 hours at 37°C to avoid aspecific binding. After repeated washes, the plasma samples (starting from a 1:20 dilution in PBS containing 0.1% Tween 20) were placed into the coated wells and incubated for 45 minutes at room temperature. The 1:20 dilution was chosen to avoid false-positive and nonspecific interference due to total immunoglobulin level.16 After repeated washes, the rtPA-bound immunoglobulins were identified by class-specific mouse monoclonal antibodies (50 μL diluted 1:1000 in washing buffer; Sigma Chemical), which were in turn detected by means of peroxidase-conjugated antimouse immunoglobulin antibodies (50 μL diluted 1:2000 in washing buffer; Sigma Chemical). The bound peroxidase was revealed by ortho-phenylene-diamine (OPD, 0.5 mg/mL in citrated buffer containing H2O2), and absorbance (OD) was read at 492 nm. The results were expressed as units per milliliter (U/mL), referred to an internal standard (plasma collected from a patient with a high anti-tPA antibody titer) arbitrarily fixed at 100 U/mL. This reference plasma was stored in aliquots at –80°C. The standard curve was linear until 0.78 U/mL (r = 0.999) and the same linearity was obtained in plasma samples from patients and controls. Within-assay and between-assay coefficients of variation were lower than 15%.
Characterization of anti-tPA antibodies
The immunoassay was identical to that described except for the fact that the first antibody was an immunoglobulin antihuman immunoglobulin with subclass specificity for IgG1, IgG2, IgG3, or IgG4 (Sigma Chemical).
IgG fraction isolation
The plasma of each patient (2 mL) was added to protein G column (Immunopure Immobilized Protein G; Pierce, Rockford, IL) capable of binding immunoglobulin of the IgG class and equilibrated in PBS, pH 7.4. After washing with 40 mL PBS, the immunoglobulin fractions specifically bound to protein G were eluted in glycine buffer 0.1 M at pH 2.5, and 66 μL Tris (tris(hydroxymethyl)aminomethane) buffer (pH 9.5) was added to every eluted milliliter. After repeated washing of the column, sodium azide was added to avoid the growth of molds. The eluted fractions were collected, concentrated in Minicon B 15 (Amicon, Beverly, MA), and stored at –80°C until testing. Protein recovery was evaluated by measuring absorbance at 280 nm. The recovery of anti-tPA antibodies was evaluated by ELISA and ranged from 50% to 75%. The same method was applied to the plasma of a healthy individual taken as control.
Production of the catalytic domain of tPA
The recombinant molecule consisting of the catalytic domain of the enzyme was obtained by inserting the cDNA coding for the catalytic domain and the first 3 N-terminal residuals of human tPA, tPA del (Val 14-Cys 261), in the vector pET11c, which was then transfected into Escherichia coli cells. It has been demonstrated that the molecule expressed in this way has the same activity as whole tPA in a dog model of coronary thrombosis.17
Evaluation of purified IgG binding to different tPA forms immobilized on microplates
The microplates (Maxisorp; Nunc) were coated overnight (4°C) with 50 μL rtPA (Actilyse; Boehringer Ingelheim), or tPA obtained from human melanoma cells (mtPA, single-chain tPA, Biopool, Umea, Sweden), or the recombinant molecule constituted by the catalytic domain of tPA at a 10-μg/mL concentration in PBS, pH 7.4. After 3 washes with 250 μL 0.1% PBS Tween 20, the wells were coated with 250 μL bovine albumin (10 mg/mL in PBS Tween 20) and then incubated for 2 hours at 37°C. After repeated washes with PBS Tween 20, increasing amounts of IgG purified from the plasma of the patients and the healthy control were placed in the wells starting from a concentration of 16 μg/mL to 250 μg/mL and then incubated for 45 minutes at room temperature. The immunoglobulins specifically bound to the tPA forms were detected by ELISA. The absorbance (OD) was read at 492 nm.
Interactions between the anti-tPA antibodies and the different tPA forms in fluid phase
After the patients' plasma had been incubated for 1 hour at 37°C with increasing concentrations of rtPA, human tPA, or the catalytic domain of tPA (0-0.5 mg/mL), we evaluated the free anti-tPA antibodies still capable of binding to the microplate-immobilized rtPA (ELISA).
Interactions between the anti-tPA antibodies and β2-GPI, prothrombin, or plasminogen in fluid phase
After the patients' plasma had been incubated for 1 hour at 37°C with increasing concentrations of β2-GPI (0-9 mg/mL; a kind gift of Dr E. M. Bevers, Cardiovascular Research Institute Maastricht, The Netherlands), prothrombin (0-4.5 mg/mL; Diagnostica Stago, Asnieres, France), or plasminogen (0-5 mg/mL, Hyphen BioMed, Andresy, France), we evaluated the free anti-tPA antibodies still capable of binding to the microplate-immobilized rtPA (ELISA).
Measurements of tPA and plasminogen activator inhibitor (PAI-1)
Plasma levels of tPA antigen were measured by means of a commercial immunoenzymatic method (Imulyse tPA kit; Biopool).
Plasma activity of tPA was measured using a chromogenic method (Spectrolyse [fibrin] tPA assay kit; American Diagnostica, New York, NY).
Plasma plasminogen activator inhibitor 1 (PAI-1) activity was measured using a commercial immunoassay (Chromolize PAI-1; Biopool).
Statistical analysis
Because the tPA and anti-tPA data were skewed, they were log-transformed before analysis. The results are reported as antilog values of means with SD. The data were statistically analyzed using the Student test for unpaired values. The significance level was set at P less than .05. The Spearman correlation coefficient was calculated to assess relationships between the variables. The associations between abnormally high anti-tPA antibody levels and APS, thrombosis or pregnancy loss, and primary or secondary syndrome were evaluated by logistic regression. Odds ratios and 95% confidence intervals were reported.
Results
The anti-tPA antibody levels of the patients with APS (geometric mean values ± SD, 6.7 ± 2.1) were significantly higher (P = .0001) than those of healthy controls (3.5 ± 2.5 U/mL) and those of patients with SLE without APS symptoms (4.1 ± 1.7 U/mL). There was no significant difference between healthy controls and patients with SLE without APS symptoms and between patients with primary (6.9 ± 1.9 U/mL) or secondary syndrome (5.8 ± 2.6 U/mL). Anti-tPA levels in asymptomatic patients with antiphospholipid antibodies (5.24 ± 1.84 U/mL) were slightly lower than levels in symptomatic APS patients (P = .05) and higher than in healthy controls (P = .01). Three of them were above the 95th percentile of the controls. Fourteen of the APS patients had anti-tPA antibody levels above the 95th percentile of the controls (Figure 1). In patients with APS, the association with abnormally high anti-tPA antibodies was higher than in healthy controls (odds ratio, 3.955; 95% CI, 1.249-12.523; P = .01) and tended to be higher than in asymptomatic patients with antiphospholipid antibodies (odds ratio, 2.242; 95% CI, 0.607-8.287; P = .171).
Plasma levels of anti-tPA antibodies in patients with APS and controls. Anti-tPA antibody levels are expressed in units per milliliter; the horizontal lines represent geometric means. The results show that 91 APS patients had anti-tPA antibody levels higher than those in 91 healthy controls (P = .0001), 23 patients with SLE without antiphospholipid antibodies and APS symptoms (P = .0001), and 40 patients with antiphospholipid antibodies without APS symptoms (P = .05). Fourteen APS patients and 3 patients with antiphospholipid antibodies without APS symptoms had anti-tPA antibody levels higher than the 95th percentile of healthy controls (indicated by the dashed line).
Plasma levels of anti-tPA antibodies in patients with APS and controls. Anti-tPA antibody levels are expressed in units per milliliter; the horizontal lines represent geometric means. The results show that 91 APS patients had anti-tPA antibody levels higher than those in 91 healthy controls (P = .0001), 23 patients with SLE without antiphospholipid antibodies and APS symptoms (P = .0001), and 40 patients with antiphospholipid antibodies without APS symptoms (P = .05). Fourteen APS patients and 3 patients with antiphospholipid antibodies without APS symptoms had anti-tPA antibody levels higher than the 95th percentile of healthy controls (indicated by the dashed line).
Of the 91 patients, 8 had ACLAs alone, 24 had lupus anticoagulant alone, and 59 had both. There was no relationship between anti-tPA antibody levels and patient age or sex, ACLA levels, or the presence of lupus anticoagulants. No significant differences in anti-tPA levels were observed among patients with venous thrombosis (6.38 ± 1.99), arterial thrombosis (7.87 ± 1.97), or a history of pregnancy loss (6.25 ± 3.41), but all the groups had levels significantly higher than healthy controls. Patients with arterial thrombosis and patients with venous thrombosis were not significantly different in the association with abnormally high anti-tPA antibodies (odds ratio, 1.171; 95% CI, 0.307-4.473) and no difference was found between patients with thrombosis and patients with pregnancy loss (odds ratio, 0.794; 95% CI, 0.152-4.137) or primary and secondary syndrome (odds ratio, 0.777; 95% CI, 0.197-3.073), or between male and female patients (odds ratio, 1.562; 95% CI, 0.398-6.126).
Due to limited sample supply we could measure tPA antigen and activity only in a subgroup of 53 APS patients (10 men and 43 women; age range, 19-78 years; 40 primary and 13 secondary). In these patients we found plasma levels of tPA antigen (9.8 ± 1.6 ng/mL) significantly higher than in 53 of the 91 healthy subjects, matched for age and sex with the patients (6.4 ± 1.3 ng/mL; P = .0001). Plasma levels of tPA activity were lower in patients (0.51 ± 1.71 IU/mL) than in healthy controls (0.66 ± 1.40 IU/mL; P = .05) and were even lower in the 9 patients who had anti-tPA levels above the 95th percentile of healthy subjects (0.42 ± 2.00 IU/mL; P = .01). The 2 female patients with the highest anti-tPA antibody levels (130 U/mL and 100 U/mL) had plasma levels of tPA activity below the normal range (0.12 and 0.10 IU/mL, respectively; normal range, 0.20-2.00 IU/mL). An inverse correlation was found between anti tPAplasma levels and tPAactivity (r =–0.454; P = .003). No significant correlation was found between plasma levels of tPA antigen and anti tPA antibodies or tPA activity.
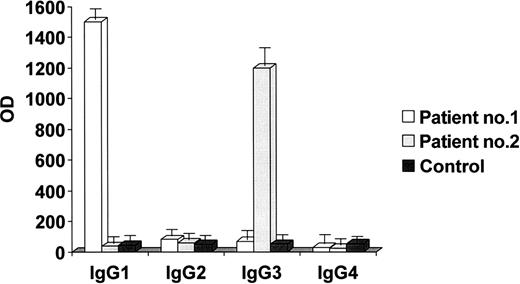
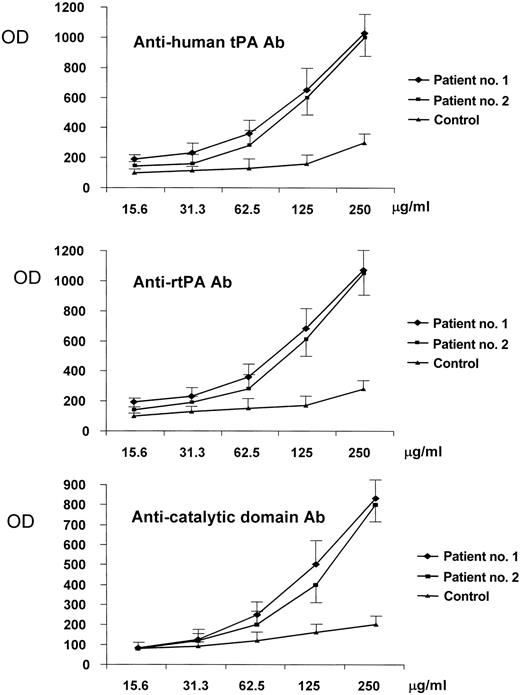
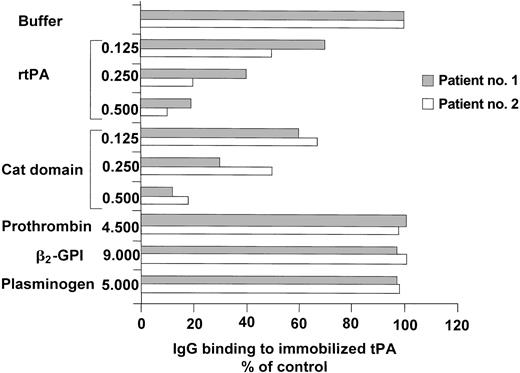
The 2 patients with the highest anti-tPA antibody levels underwent further studies: their main clinical and laboratory data are shown in Table 1. The IgG fractions were isolated from their plasma. The anti-tPA antibodies were IgG1 in one and IgG3 in the other (Figure 2), whereas the antiprothrombin antibodies and anti–β2-GPI antibodies were IgG2 in both, as demonstrated by the IgG binding to immobilized β2-GPI and prothrombin. The IgGs of the 2 patients bound to rtPA, the catalytic domain of the tPA molecule, and to human tPA immobilized on microplates in a concentration-dependent manner. The immunoglobulins isolated from a healthy subject did not bind to rtPA, the tPA catalytic domain, or human tPA (Figure 3). When added to the patients' plasma at concentrations of up to 0.5 mg/mL, both rtPA and the catalytic domain of tPA inhibited the binding of anti-tPA antibodies to the recombinant tPA immobilized on microplates in a concentration-dependent manner, but the addition of prothrombin (at concentrations of up to 50 times higher than physiologic level), β2-GPI (at concentrations of up to 45 times higher than physiologic levels and 50 times higher than those used in vitro to obtain an inhibition of anti–β2-GPI activity in fluid phase18 ), or plasminogen (at concentrations of up to 25 times higher than physiologic levels) did not lead to any such inhibition (Figure 4).
Main clinical and laboratory data of the 2 patients with the highest anti-tPA antibody levels
Characteristics . | Patient no. 1 . | Patient no. 2 . |
|---|---|---|
| Sex | Female | Female |
| Age, y | 37 | 46 |
| Clinical history | 3 pregnancy losses | Ischemic stroke |
| Lupus anticoagulant | + | + |
| ACLAs, GPL units | 13.5 | 180 |
| ACLAs, MPL units | 3.8 | NA |
| Anti-tPA antibodies, U/mL | 130 | 100 |
| tPA antigen, ng/mL | 17.2 | 7 |
| tPA activity, IU/mL | 0.12 | 0.10 |
| PAI activity, IU/mL | 0.1 | 0.2 |
Characteristics . | Patient no. 1 . | Patient no. 2 . |
|---|---|---|
| Sex | Female | Female |
| Age, y | 37 | 46 |
| Clinical history | 3 pregnancy losses | Ischemic stroke |
| Lupus anticoagulant | + | + |
| ACLAs, GPL units | 13.5 | 180 |
| ACLAs, MPL units | 3.8 | NA |
| Anti-tPA antibodies, U/mL | 130 | 100 |
| tPA antigen, ng/mL | 17.2 | 7 |
| tPA activity, IU/mL | 0.12 | 0.10 |
| PAI activity, IU/mL | 0.1 | 0.2 |
Normal ranges: anticardiolipin antibodies, GPL less than 21, MPL less than 21; anti-tPA antibodies, less than 11 U/mL; tPA antigen, 1.7 to 12.3 ng/mL; tPA activity, 0.20-2.00 IU/mL; PAI activity levels, 0.1 to 18.4 IU/mL.
NA indicates not available.
Subclass characterization of anti-tPA immunoglobulins. The immunoglobulins bound to immobilized tPA were revealed using mouse subclass–specific monoclonal antibodies against human immunoglobulins in an ELISA system. The results are expressed as absorbance at 492 nm (mean values ± SE of 3 experiments) and indicate that the anti-tPA antibodies of patient no. 1 are IgG1, whereas those of patient no. 2 are IgG3. No significant signal was obtained from the immunoglobulins of a healthy control. OD indicates absorbance.
Subclass characterization of anti-tPA immunoglobulins. The immunoglobulins bound to immobilized tPA were revealed using mouse subclass–specific monoclonal antibodies against human immunoglobulins in an ELISA system. The results are expressed as absorbance at 492 nm (mean values ± SE of 3 experiments) and indicate that the anti-tPA antibodies of patient no. 1 are IgG1, whereas those of patient no. 2 are IgG3. No significant signal was obtained from the immunoglobulins of a healthy control. OD indicates absorbance.
Binding of immunoglobulins purified from the plasma of 2 patients and a healthy control to different tPA forms immobilized on microplates. The binding of purified IgG to human tPA (top panel), rtPA (middle panel), and the catalytic domain of tPA (bottom panel) was evaluated in patient no. 1 ( ), patient no. 2 (▪), and a healthy control (▴). The horizontal axis indicates the concentrations of purified immunoglobulins. The results are expressed as absorbance (OD) at 492 nm (mean values ± SE of 3 experiments) and indicate that both patients have immunoglobulins directed against the different tPA forms; no significant binding was observed in the healthy control.
), patient no. 2 (▪), and a healthy control (▴). The horizontal axis indicates the concentrations of purified immunoglobulins. The results are expressed as absorbance (OD) at 492 nm (mean values ± SE of 3 experiments) and indicate that both patients have immunoglobulins directed against the different tPA forms; no significant binding was observed in the healthy control.
Binding of immunoglobulins purified from the plasma of 2 patients and a healthy control to different tPA forms immobilized on microplates. The binding of purified IgG to human tPA (top panel), rtPA (middle panel), and the catalytic domain of tPA (bottom panel) was evaluated in patient no. 1 ( ), patient no. 2 (▪), and a healthy control (▴). The horizontal axis indicates the concentrations of purified immunoglobulins. The results are expressed as absorbance (OD) at 492 nm (mean values ± SE of 3 experiments) and indicate that both patients have immunoglobulins directed against the different tPA forms; no significant binding was observed in the healthy control.
), patient no. 2 (▪), and a healthy control (▴). The horizontal axis indicates the concentrations of purified immunoglobulins. The results are expressed as absorbance (OD) at 492 nm (mean values ± SE of 3 experiments) and indicate that both patients have immunoglobulins directed against the different tPA forms; no significant binding was observed in the healthy control.
Inhibition of IgG binding to insolubilized rtPA by soluble tPA forms, prothrombin, β2-GPI, and plasminogen. Plasma from patients no. 1 and 2 was incubated for 1 hour with rtPA or the catalytic (cat) domain of tPA at concentrations of 0.500 mg/mL, 0.250 mg/mL, 0.125 mg/mL, 0 mg/mL, with prothrombin up to a concentration of 4.500 mg/mL, with β2-GPI up to a concentration of 9.000 mg/mL or plasminogen up to a concentration of 5.000 mg/mL, and then tested using the solid-phase immunoassay as described in “Patients, materials, and methods.” The results are expressed as the percentage binding recorded in the absence of added soluble proteins (buffer). When added to the patients' immunoglobulins, both rtPA and the catalytic domain of tPA inhibited the binding of anti-tPA antibodies to the rtPA immobilized on microplates, but prothrombin, β2-GPI, and plasminogen did not inhibit the binding.
Inhibition of IgG binding to insolubilized rtPA by soluble tPA forms, prothrombin, β2-GPI, and plasminogen. Plasma from patients no. 1 and 2 was incubated for 1 hour with rtPA or the catalytic (cat) domain of tPA at concentrations of 0.500 mg/mL, 0.250 mg/mL, 0.125 mg/mL, 0 mg/mL, with prothrombin up to a concentration of 4.500 mg/mL, with β2-GPI up to a concentration of 9.000 mg/mL or plasminogen up to a concentration of 5.000 mg/mL, and then tested using the solid-phase immunoassay as described in “Patients, materials, and methods.” The results are expressed as the percentage binding recorded in the absence of added soluble proteins (buffer). When added to the patients' immunoglobulins, both rtPA and the catalytic domain of tPA inhibited the binding of anti-tPA antibodies to the rtPA immobilized on microplates, but prothrombin, β2-GPI, and plasminogen did not inhibit the binding.
The isolated immunoglobulins from the 2 patients were tested for in vitro inhibition of tPA activity by adding increasing amount of immunoglobulins to plasma from a healthy control and measuring tPA activity after 1 hour of incubation at 37°C (Figure 5). We observed a slight inhibition of tPA activity with both preparations. Immunoglobulins purified from a healthy subject served as negative control and goat anti-tPA antibodies (American Diagnostica, Greenwich, CT) served as positive control.
Inhibition of plasma tPA activity by immunoglobulins purified from the plasma of 2 patients. The tPA activity was measured in a healthy control's plasma after 1 hour of incubation at 37°C with increasing amounts of immunoglobulins purified from the plasma of patients no. 1 and 2. Immunoglobulins purified from a healthy subject served as negative control and goat anti-tPA antibodies served as positive control. Results are expressed as IU/mL tPA activity.
Inhibition of plasma tPA activity by immunoglobulins purified from the plasma of 2 patients. The tPA activity was measured in a healthy control's plasma after 1 hour of incubation at 37°C with increasing amounts of immunoglobulins purified from the plasma of patients no. 1 and 2. Immunoglobulins purified from a healthy subject served as negative control and goat anti-tPA antibodies served as positive control. Results are expressed as IU/mL tPA activity.
Discussion
Previous studies have demonstrated that the plasma of patients with antiphospholipid antibodies also contain antibodies directed against different plasma proteins involved in hemostasis, such as prothrombin, protein C, protein S, factor XII, and annexin V.2,19-21 Antibodies interfering with the “plasminogen/tissue-type plasminogen activator” system have also been described in patients with APS.7,22 In a previous study of 39 patients with antiphospholipid antibodies, we found that 3 had antibodies against tPA and 4 had antibodies against fibrin-bound tPA; all the patients with antibodies against tPA had a history of thrombosis.7 In this study, we analyzed a larger group of patients with APS who had had thrombotic events or recurrent pregnancy loss; their plasma levels of anti-tPA antibody were significantly higher than those of healthy controls who showed a range of antibody levels. This is not surprising because it has been demonstrated that naturally occurring autoantibodies can also be found in healthy subjects23 ; thus tPA can act as described for other autoantigens. Patients with APS had plasma levels of anti-tPA antibody significantly higher than those of patients with antiphospholipid antibodies without history of APS symptoms and SLE patients without antiphospholipid antibodies or history of APS symptoms. No difference was found between primary and secondary syndrome, which indicates that anti-tPA antibodies can be found in patients with APS regardless of any associated autoimmune disease. This is further supported by the observation of normal levels of anti-tPA antibodies in SLE without APS. The presence of anti-tPA antibodies in patients with SLE is associated with a higher frequency of severe Raynaud phenomenon and thrombotic events24 and, among patients with primary APS, the highest antibody levels are found in those with a history of ischemic stroke.7 In this study, anti-tPA antibody levels were similarly high in the patients with a history of vein or arterial thrombosis or recurrent pregnancy loss, although we have no information on their antibody levels at the time of the clinical event or soon afterward. No age-related difference in anti-tPA antibody levels was found, although it is known that ACLA levels increase with age.25 No differences were observed between men and women.
Antiphospholipid antibodies are heterogeneous in relation to their antigen specificity, and previous studies showed a strict correlation between ACLAs and anti–β2-GPI antibodies,26 and cross-reactivity between antiprothrombin and antiplasminogen antibodies.27 Thus we looked for a possible cross-reactivity between anti-tPA and antiphospholipid antibodies. In our group of patients no correlation was found between the plasma levels of anticardiolipin and anti-tPA antibodies, suggesting that they are not the same. In the 2 patients with the highest anti-tPA antibody levels, the antibody subclasses were IgG1 and IgG3, whereas we found that their antiphospholipid antibodies (anti–β2-GPI and antiprothrombin) were IgG2. Studies of patients with SLE and primary APS have also confirmed that the majority of anti–β2-GPI antibodies belong to the IgG2 subclass.26 These data show that anti-tPA antibodies are different from antiphospholipid antibodies and, being IgG1 and IgG3, are probably T lymphocyte–dependent and activate the classical complement pathway.28 Recent studies support an association between the inflammatory state and the clinical manifestations of APS with mechanisms involving complement29 and oxidative processes related to inflammation.30 Complement activation is also important in the fetal loss caused by antiphospholipid antibodies, as shown in an animal model.31 The absence of inhibition of anti-tPA binding to tPA immobilized on microplates after the addition of prothrombin and β2-GPI in fluid phase confirms the absence of cross-reactivity between anti-tPA and antiphospholipid antibodies. However, when an rtPA solution is added under the same conditions, the binding of anti-tPA antibodies to the tPA molecule is remarkably inhibited, thus demonstrating that anti-tPA antibodies bind to the tPA molecule that is not modified by contact with the well (ie, a condition more similar to the in vivo situation). Patients' immunoglobulins can also bind the tPA obtained from human melanoma cells and immobilized on microplates. The rtPA molecule produced from hamster ovarian cells, starting with cDNA derived from the mRNA of human melanoma, has a glycosylation pattern similar but not identical to that of endogenous tPA. In contrast, melanoma tPA is identical to the endogenous molecule, and so the anti-tPA antibodies can also react with human tPA.16 Both tPA and prothrombin molecules have 2 “kringle” domains in their secondary structure.32 The fact that anti-tPA antibodies do not interact with prothrombin suggests that they are not directed against the kringle domains, the only parts that these 2 molecules share.
The catalytic domain of the tPA molecule is completely different from the kringle domains and is located on the C-terminal side; for this reason, we evaluated the interactions between anti-tPA antibodies and the active site of the enzyme using a recombinant molecule obtained by inserting a cDNA coding for the catalytic domain and the first 3 N-terminal residuals of human tPA in a vector.17 The anti-tPA IgGs of the 2 patients investigated interacted with the microplate-immobilized tPA catalytic domain in a dose-dependent manner. We excluded that IgG immunoglobulins bind aspecifically to the catalytic domain by observing that normal IgG did not interact with this domain. When the tPA catalytic domain molecule was added to a solution containing the plasma of the 2 patients, we observed a dose-dependent inhibition of binding between anti-tPA antibodies and microplate-immobilized tPA, thereby showing that the antibodies are mainly directed against the catalytic domain. This result is relevant because it suggests that anti-tPA antibodies may potentially affect enzyme function. Recent studies have demonstrated that IgG purified from the plasma of APS patients can inhibit the degradation of fibrin clots.33 In our 2 patients, the plasma levels of tPA enzymatic activity were lower than those in healthy controls, with normal or slightly increased antigen levels and normal levels of the main tPA inhibitor (PAI-1). The other patients with abnormally high plasma levels of anti-tPA antibodies had lower levels of tPA activity than healthy controls. Because a defect of tPA has been associated with thrombosis,34 APS patients with abnormally high levels of anti-tPA antibodies may have an additional risk for thrombosis. Thus hypofibrinolysis due to anti-tPA antibodies could be added to the list of possible pathologic processes responsible for clinical symptoms in APS. The increased levels of tPA antigen observed in our patients with APS have been already found in a previous study and could be related to an activation of endothelial cells,35 which represent a target for antiphospholipid antibodies.36 The slight inhibition of tPA activity by anti-tPA antibodies we observed in vitro, along with the fact that tPA activity is low in plasma of patients with APS and inversely correlates with anti-tPA levels, supports the hypothesis that anti-tPA antibodies may act in vivo as inhibitors of tPA function.
In conclusion, it is possible to find antibodies specifically recognizing the tPA catalytic domain in the plasma of patients with APS. These antibodies may participate in the hypofibrinolytic state described in APS.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-07-2422.
Supported by grants from Maggiore Hospital of Milan and from MURST, Progetto Biotecnologie, Legge 95/95.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal