Abstract
There is little information available regarding the outcome of unrelated bone marrow transplantation (BMT) for non-Hodgkin lymphoma (NHL). Therefore, we retrospectively analyzed the data of 124 patients who underwent unrelated BMT through the Japan Marrow Donor Program (JMDP) between July 1992 and August 2001. The overall survival (OS), progression-free survival (PFS), cumulative incidences of disease progression, and nonprogression mortality at 3 years after BMT were 49.7%, 42.6%, 24.5%, and 32.9%, respectively, with a median follow-up duration of 565 days among survivors. The incidence of grades II-IV acute graft-versus-host disease (GVHD) was 40.9%. Recipient age, previous history of autologous transplantation, and chemosensitivity at transplantation were independent prognostic factors for OS and PFS. The development of grades II-IV acute GVHD was associated with lower incidence of disease progression after transplantation, which suggested the existence of a graft versus lymphoma effect. Unrelated BMT should be considered as a treatment option for patients with high-risk NHL without an HLA-matched related donor.
Introduction
Hematopoietic stem cell transplantation for non-Hodgkin lymphoma (NHL) has been mainly performed using an autologous graft, because the incidence of treatment-related mortality after allogeneic transplantation is as high as 57%.1 However, relapse is a frequent cause of treatment failure after autologous transplantation.2,3 The lower relapse rate after allogeneic transplantation and the recent development of supportive treatments to decrease the risk of treatment-related mortality have facilitated the use of allogeneic transplantation for NHL.1 However, an HLA-matched sibling is available for less than half of the patients. Transplantation from an unrelated donor is a possible alternative for patients who do not have a suitable related donor. To date, however, little information is available regarding the outcome of allogeneic transplantation from an unrelated donor for NHL. Therefore, we retrospectively analyzed the outcome of unrelated bone marrow transplantation for NHL using the database of the Japan Marrow Donor Program (JMDP). The purpose of this study was to elucidate the feasibility of unrelated bone marrow transplantation for NHL and to evaluate the impact of a potential graft-versus-lymphoma effect.
Patients and methods
Patients and transplantation procedure
From July 1992 to August 2001, 124 patients with non-Hodgkin lymphoma (NHL) underwent bone marrow transplantation from a serologically HLA-A, -B, and -DR matched unrelated donor identified through the Japan Marrow Donor Program (JMDP). The application of unrelated transplantation was decided at each center. Fourteen of 19 patients who underwent unrelated transplantation in the first complete remission (CR1) had high-grade lymphoma.
Transplantation was performed according to the protocol of each center, and therefore the conditioning regimen and graft-versus-host disease (GVHD) prophylaxis varied among patients (Table 1). However, 90% of the patients received a total body irradiation (TBI)–containing conditioning regimen. Prophylaxis against GVHD was performed with cyclosporine A or tacrolimus combined with methotrexate with or without corticosteroid in all but one patient. At transplantation, 60 patients were in complete remission (CR) and 60 were not (non-CR). Among the 43 patients whose CR status was reported in detail, 19, 18, 5, and 1 were in CR1, CR2, CR3, and CR4, respectively. Seventy-six patients had chemosensitive disease at transplantation, whereas 33 patients had chemoresistant disease. In this study we defined patients who achieved CR or partial remission (PR) before transplantation as chemosensitive, and patients with responses less than PR were defined as chemoresistant in the same way as previous studies.4,5 Before unrelated donor transplantation, 18 of 101 patients had undergone high-dose therapy and autologous stem cell transplantation (HDT/ASCT). Information regarding previous treatments except for HDT/ASCT or the results of genomic typing were not available in the dataset.
Patient characteristics
Characteristic . | Value . |
|---|---|
| Sex, n, M/F | 78/46 |
| Median age at transplantation, y (range) | 29 (1-59) |
| Median interval from diagnosis to transplantation, d (range) | 470 (183-2329) |
| Histology, n | |
| Low-grade | 10 |
| Follicular lymphoma | 9 |
| Small lymphocytic lymphoma | 1 |
| Intermediate-grade | 42 |
| Peripheral T-cell lymphoma, unspecified | 14 |
| NK-cell lymphoma | 12 |
| Anaplastic large cell lymphoma | 6 |
| Diffuse large B-cell lymphoma | 5 |
| Angioimmunoblastic lymphoma | 1 |
| Mantle cell lymphoma | 1 |
| High-grade | 60 |
| Lymphoblastic lymphoma | 39 |
| Adult T-cell leukemia/lymphoma | 15 |
| Burkitt lymphoma | 5 |
| Unclassified | 12 |
| Previous history of HDT/ASCT, n | |
| Yes | 18 |
| No | 83 |
| ND | 23 |
| Disease status at transplantation, n | |
| CR | 60 (CR1 19, CR2 18, CR3 5, CR4 1) |
| Non-CR | 60 |
| ND | 4 |
| Chemosensitivity at transplantation, n | |
| Sensitive | 76 |
| Resistant | 33 |
| ND | 15 |
| Conditioning regimen, n | |
| TBI-containing regimen | 111 |
| Non-TBI regimen | 13 |
| GVHD prophylaxis, n | |
| CsA ± MTX ± steroid | 76 |
| TCR ± MTX ± steroid | 44 |
| CsA + TCR ± MTX ± steroid | 3 |
| MTX alone | 1 |
Characteristic . | Value . |
|---|---|
| Sex, n, M/F | 78/46 |
| Median age at transplantation, y (range) | 29 (1-59) |
| Median interval from diagnosis to transplantation, d (range) | 470 (183-2329) |
| Histology, n | |
| Low-grade | 10 |
| Follicular lymphoma | 9 |
| Small lymphocytic lymphoma | 1 |
| Intermediate-grade | 42 |
| Peripheral T-cell lymphoma, unspecified | 14 |
| NK-cell lymphoma | 12 |
| Anaplastic large cell lymphoma | 6 |
| Diffuse large B-cell lymphoma | 5 |
| Angioimmunoblastic lymphoma | 1 |
| Mantle cell lymphoma | 1 |
| High-grade | 60 |
| Lymphoblastic lymphoma | 39 |
| Adult T-cell leukemia/lymphoma | 15 |
| Burkitt lymphoma | 5 |
| Unclassified | 12 |
| Previous history of HDT/ASCT, n | |
| Yes | 18 |
| No | 83 |
| ND | 23 |
| Disease status at transplantation, n | |
| CR | 60 (CR1 19, CR2 18, CR3 5, CR4 1) |
| Non-CR | 60 |
| ND | 4 |
| Chemosensitivity at transplantation, n | |
| Sensitive | 76 |
| Resistant | 33 |
| ND | 15 |
| Conditioning regimen, n | |
| TBI-containing regimen | 111 |
| Non-TBI regimen | 13 |
| GVHD prophylaxis, n | |
| CsA ± MTX ± steroid | 76 |
| TCR ± MTX ± steroid | 44 |
| CsA + TCR ± MTX ± steroid | 3 |
| MTX alone | 1 |
HDT/ASCT indicates high-dose therapy and autologous stem cell transplantation; CR, complete remission (CR1, CR2, CR3, CR4; the first, second, third, and fourth CR, respectively); ND, not described; TBI, total body irradiation; GVHD, graft-versus-host disease; CsA, cyclosporine A; MTX, methotrexate; TCR, tacrolimus.
Histology
The JMDP requested the histologic subtype of NHL according to a unique classification system that was a slight modification of the Working Formulation.6 However, the respective centers used different classification systems, such as the Working Formulation, Kiel,7 Lymphoma Study Group (LSG),8 Revised European-American Classification of lymphoid neoplasms (REAL),9 and World Health Organization (WHO) systems.10 In this study, we grouped the histology into low grade, intermediate grade, and high grade as usually accepted in daily practice. There were 10, 42, and 60 patients with low-, intermediate-, and high-grade lymphoma, respectively. Histologic subtypes in detail are described in Table 1. The histologic subtype or grade was unclassified in 12 cases. Transplantation for lymphoblastic lymphoma (LBL) and adult T-cell leukemia/lymphoma (ATLL) was included as in other studies focusing on allogeneic transplantation for NHL.11,12
Data management and statistical considerations
Data were collected by the JMDP using a standardized report form. Follow-up reports were submitted at 100 days, 1 year, and annually after transplantation. Overall survival (OS) was defined as days from transplantation to death from any cause. Progression-free survival (PFS) was defined as days from transplantation to disease progression or death from any cause. Nonprogression mortality was defined as death without disease progression. Patients who were alive at the last follow-up date were censored. Survival was calculated using the Kaplan-Meier method. To evaluate the influence of confounding factors for survival, the log-rank test was used for univariate analyses and proportional hazard modeling was used for multivariate analyses. Cumulative incidences of acute GVHD and disease progression were calculated using the Gray method,13 considering death without acute GVHD and death without disease progression as respective competing risks. The effects of acute and chronic GVHD on survival and disease progression were analyzed among patients who survived without disease progression at 60 and 150 days after transplantation, respectively.14,15 This landmark method was used to exclude bias that may arise from including patients who died too early to develop GVHD in the group without GVHD.
Results
Survival and disease progression
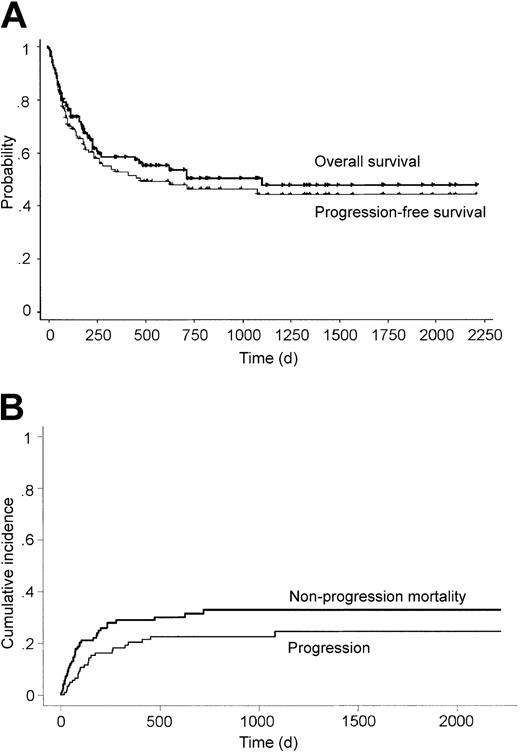
Of the 124 patients, 69 were alive with a median follow-up duration of 565 days (range, 82 to 2217 days) after transplantation (Table 2). The overall 3-year OS and PFS were 49.7% and 42.6%, respectively (Figure 1A). Cumulative incidences of disease progression and nonprogression mortality at 3 years were 24.5% and 32.9%, respectively (Figure 1B). Disease progression was observed in 26 patients, and the median time from transplantation to disease progression was 109 days (range, 0 to 1079 days). Notably, only 1 patient developed disease progression more than 500 days after transplantation. The cause of death was related to disease progression in 17, whereas 36 died without disease progression (Table 2). The major cause of transplantation-related death within 100 days after transplantation was GVHD in 8, infection in 5, venoocclusive disease in 3, acute respiratory distress syndrome in 2, interstitial pneumonitis in 2, renal failure in 2, and other causes in 2.
Transplantation outcome
. | Value . |
|---|---|
| Alive/dead, n | 69/55 |
| Median follow-up for survivors, d (range) | 565 (82-2217) |
| Cause of death | |
| Progression, n | 17 |
| Median days after transplantation (range) | 165 (2-1106) |
| Death without progression, n | 36 |
| Median days after transplantation (range) | 72 (8-718) |
| GVHD, n | 10 |
| Infection, n | 9 |
| IP, n | 6 |
| VOD, n | 3 |
| Renal failure, n | 2 |
| ARDS, n | 2 |
| Others: pericarditis, hemorrhage, cerebral infarction, RRT, n | 4 |
| Not described, n | 2 |
| Disease progression, n | 26 |
| Median days after transplantation (range) | 109 (0-1079) |
| Engraftment, n | |
| Engraftment | 115 |
| Rejection | 2 |
| Death within 20 days | 7 |
| Acute GVHD, n* | |
| Grade 0 | 31 |
| Grade I | 37 |
| Grade II | 30 |
| Grade III | 7 |
| Grade IV | 10 |
| Chronic GVHD, n† | |
| None | 47 |
| Limited | 17 |
| Extensive | 24 |
| Not described | 5 |
. | Value . |
|---|---|
| Alive/dead, n | 69/55 |
| Median follow-up for survivors, d (range) | 565 (82-2217) |
| Cause of death | |
| Progression, n | 17 |
| Median days after transplantation (range) | 165 (2-1106) |
| Death without progression, n | 36 |
| Median days after transplantation (range) | 72 (8-718) |
| GVHD, n | 10 |
| Infection, n | 9 |
| IP, n | 6 |
| VOD, n | 3 |
| Renal failure, n | 2 |
| ARDS, n | 2 |
| Others: pericarditis, hemorrhage, cerebral infarction, RRT, n | 4 |
| Not described, n | 2 |
| Disease progression, n | 26 |
| Median days after transplantation (range) | 109 (0-1079) |
| Engraftment, n | |
| Engraftment | 115 |
| Rejection | 2 |
| Death within 20 days | 7 |
| Acute GVHD, n* | |
| Grade 0 | 31 |
| Grade I | 37 |
| Grade II | 30 |
| Grade III | 7 |
| Grade IV | 10 |
| Chronic GVHD, n† | |
| None | 47 |
| Limited | 17 |
| Extensive | 24 |
| Not described | 5 |
GVHD indicates graft-versus-host disease; IP, interstitial pneumonitis; VOD, venoocclusive disease; ARDS, acute respiratory distress syndrome; RRT, regimenrelated toxicity.
Acute GVHD was evaluated among patients who achieved engraftment and survived more than 20 days after transplantation.
Chronic GVHD was evaluated among patients who survived more than 100 days after transplantation.
Survival, progression, and nonprogression mortality after transplantation. Overall survival, progression-free survival (A), and cumulative incidences of disease progression and nonprogression mortality (B) after unrelated bone marrow transplantation for non-Hodgkin lymphoma.
Survival, progression, and nonprogression mortality after transplantation. Overall survival, progression-free survival (A), and cumulative incidences of disease progression and nonprogression mortality (B) after unrelated bone marrow transplantation for non-Hodgkin lymphoma.
Engraftment and GVHD
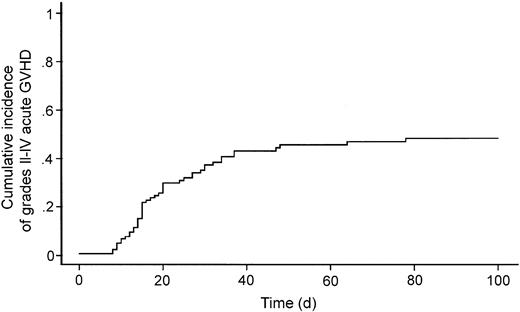
Seven patients died within 20 days after transplantation, and therefore engraftment could not be evaluated. In the others, 2 rejected the graft and 115 achieved engraftment. Among the latter 115 patients, 47 developed grades II-IV acute GVHD (Table 2) with a cumulative incidence of 47.5% (Figure 2). Seven and 10 patients experienced grade III and IV acute GVHD, respectively. Among the 93 patients who were alive at 100 days after transplantation, 17 and 24 developed limited and extensive chronic GVHD, respectively.
Cumulative incidence of grades II-IV acute graft-versus-host disease.
Influence of pretransplantation factors
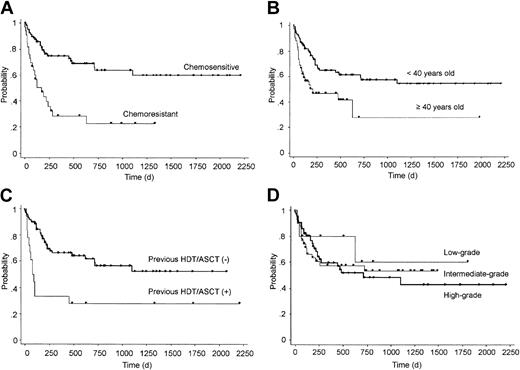
We evaluated the effects of pretransplantation factors on OS after transplantation and identified 3 independent significant risk factors: chemosensitivity before transplantation (chemosensitive versus chemoresistant, relative risk 0.28, 95% confidence interval [CI] 0.15-0.52, P < .0001); previous history of HDT/ASCT (yes versus no, relative risk 0.40, 95% CI 0.20-0.79, P = .0087); and patient age (less than 40 years versus 40 years or more, relative risk 0.42, 95% CI 0.22-0.81, P = .0092) at transplantation (Tables 3 and 4; Figure 3A-C). These 3 factors were also identified as independent risk factors for PFS (data not shown), probably because only a few patients survived after disease progression and the OS and PFS curves were almost superimposed. We further analyzed the impact of disease status among patients who had chemosensitive disease at bone marrow transplantation; 19 were in first CR, 24 were in a later CR, and 16 were in PR. However, there was no significant difference in OS or PFS among them (data not shown). Among 13 deaths in patients with previous history of HDT/ASCT, 11 were from transplantation-related causes before day 100 (median, 56; range, 13 to 97 days after transplantation). Two patients died from disease progression on day 48 and 458, respectively. However, 5 of 6 patients who survived more than 100 days after transplantation were progression free at a median follow-up of 1339 days (range, 493 to 2217 days) after transplantation. Furthermore, we evaluated the impact of histologic grade on outcome. However, there was no significant difference in OS, PFS, or cumulative incidence of disease progression among these histologic grades (Figure 3D and data not shown).
Prognostic factors in univariate analyses
. | 3-year OS, % . | P . |
|---|---|---|
| Sex | .70 | |
| Male | 47.7 | |
| Female | 53.1 | |
| Age at transplantation | .0036 | |
| Less than 40 y | 57.1 | |
| 40 y or more | 28.7 | |
| Histology | .80 | |
| Low grade | 60.0 | |
| Intermediate grade | 53.2 | |
| High grade | 47.6 | |
| Previous HDT/ASCT | .0006 | |
| Yes | 27.8 | |
| No | 56.3 | |
| Chemosensitivity | < .0001 | |
| Chemosensitive | 63.2 | |
| Chemoresistant | 22.8 | |
| Disease status | .0011 | |
| CR | 61.4 | |
| Non-CR | 32.0 | |
| Preparative regimen | .29 | |
| TBI-containing | 50.8 | |
| Non-TBI | 40.0 | |
| Days from diagnosis to transplantation | .53 | |
| Less than 365 d | 44.8 | |
| 365 d or more | 52.1 |
. | 3-year OS, % . | P . |
|---|---|---|
| Sex | .70 | |
| Male | 47.7 | |
| Female | 53.1 | |
| Age at transplantation | .0036 | |
| Less than 40 y | 57.1 | |
| 40 y or more | 28.7 | |
| Histology | .80 | |
| Low grade | 60.0 | |
| Intermediate grade | 53.2 | |
| High grade | 47.6 | |
| Previous HDT/ASCT | .0006 | |
| Yes | 27.8 | |
| No | 56.3 | |
| Chemosensitivity | < .0001 | |
| Chemosensitive | 63.2 | |
| Chemoresistant | 22.8 | |
| Disease status | .0011 | |
| CR | 61.4 | |
| Non-CR | 32.0 | |
| Preparative regimen | .29 | |
| TBI-containing | 50.8 | |
| Non-TBI | 40.0 | |
| Days from diagnosis to transplantation | .53 | |
| Less than 365 d | 44.8 | |
| 365 d or more | 52.1 |
OS indicates overall survival; HDT/ASCT, high-dose therapy and autologous stem cell transplantation; CR, complete remission; TBI, total body irradiation.
Prognostic factors in multivariate analysis
. | Relative risk . | 95% CI . | P . |
|---|---|---|---|
| Age less than 40 y | 0.42 | 0.22-0.81 | .0092 |
| No previous HDT/ASCT | 0.40 | 0.20-0.79 | .0087 |
| Chemosensitive disease | 0.28 | 0.15-0.52 | <.0001 |
. | Relative risk . | 95% CI . | P . |
|---|---|---|---|
| Age less than 40 y | 0.42 | 0.22-0.81 | .0092 |
| No previous HDT/ASCT | 0.40 | 0.20-0.79 | .0087 |
| Chemosensitive disease | 0.28 | 0.15-0.52 | <.0001 |
CI indicates confidence interval.
Overall survival according to pretransplantation factors. OS grouped by chemosensitivity at transplantation (A), age (B), previous history of autologous transplantation (HDT/ASCT) (C), and histologic grade (D).
Overall survival according to pretransplantation factors. OS grouped by chemosensitivity at transplantation (A), age (B), previous history of autologous transplantation (HDT/ASCT) (C), and histologic grade (D).
Influence of acute and chronic GVHD
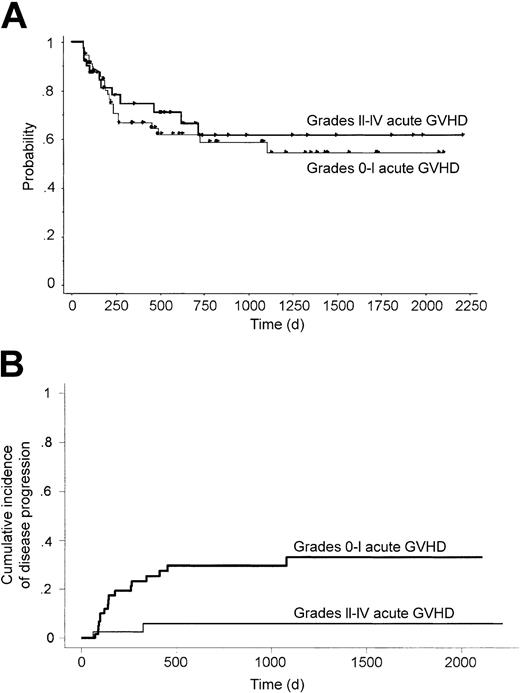
We analyzed the relationship between the development of acute GVHD and the transplantation outcome. In this study, all but 2 patients developed acute GVHD before day 60. Thus, we defined day 60 as a landmark for this analysis. The cumulative incidence of disease progression at 3 years after transplantation was significantly lower in patients who developed grades II-IV acute GVHD (5.9% versus 33.2%, P = .0053; Figure 4B). This effect was preserved even when it was adjusted for the chemosensitivity before transplantation using proportional hazard modeling (relative risk 0.15, 95% CI 0.03-0.65, P = .012). This inverse correlation between the development of acute GVHD and disease progression suggested the existence of a graft-versus-lymphoma (GVL) effect. However, there was no significant difference in 3-year OS (61.4% versus 58.8%, P = .63; Figure 4A) or PFS (58.9% versus 48.9%, P = .28) between patients with and without grades II-IV acute GVHD. When we classified patients into those who developed grades III-IV acute GVHD and those who did not, there was a trend for lower incidence of disease progression (P = .13) but worse OS (P = .075) in patients with acute GVHD. The influence of chronic GVHD was evaluated similarly, with day 150 after transplantation defined as a landmark. However, the cumulative incidence of disease progression at 3 years after transplantation was not different between those with and without chronic GVHD (12.3% versus 14.5%, P = .80).
Survival and progression according to development of acute GVHD. OS (A) and cumulative incidence of disease progression (B) grouped by the development of grades II-IV acute graft-versus-host disease among patients who were alive without disease progression at 60 days after transplantation.
Survival and progression according to development of acute GVHD. OS (A) and cumulative incidence of disease progression (B) grouped by the development of grades II-IV acute graft-versus-host disease among patients who were alive without disease progression at 60 days after transplantation.
Results in specific histologic subtypes
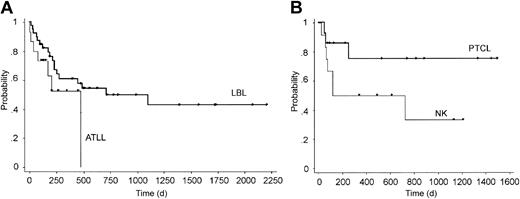
Kaplan-Meier estimates of OS of patients with peripheral T-cell lymphoma (n = 14), natural killer (NK)–cell lymphoma (n = 12), LBL (n = 39), and ATLL (n = 15), which were the 4 major histologic subtypes in this study, are shown in Figure 5. OS of patients with peripheral T-cell lymphoma, which is considered to be associated with poor prognosis,16-18 appeared to be favorable after unrelated allogeneic transplantation with several long-term survivors (3-year OS, 75.0%), although this study contained only a small number of patients. In contrast, the result for ATLL was poor, and there were no survivors beyond 500 days after transplantation.
Overall survival of specific histologic subtypes. (A) Adult T-cell leukemia/lymphoma (ATLL) and lymphoblastic lymphoma (LBL); (B) peripheral T-cell lymphoma (PTCL) and NK-cell lymphoma (NK).
Overall survival of specific histologic subtypes. (A) Adult T-cell leukemia/lymphoma (ATLL) and lymphoblastic lymphoma (LBL); (B) peripheral T-cell lymphoma (PTCL) and NK-cell lymphoma (NK).
Discussion
In this study, we analyzed the outcome of bone marrow transplantation from an unrelated donor for NHL performed through the JMDP. In a similar study from the National Marrow Donor Program (NMDP),19 both OS and PFS were estimated to be 30% at 2 years. The outcome in the present study appeared to be more favorable than that in the NMDP study, which could be attributed to the lower incidence of grades III-IV acute GVHD in the present study (15% versus 30%). This observation is compatible with previous studies showing lower incidence of acute GVHD among Japanese than among whites, which might reflect less diverse genetic background in Japan.20,21
The association between the development of GVHD and reduced disease progression rate has been inconsistent among previous studies.4,11,22 In this study, the development of grades II-IV acute GVHD was associated with a lower incidence of disease progression after transplantation. This result supports the existence of a potential GVL effect. Because the previous studies nearly exclusively included transplantation from an HLA-matched sibling, the use of unrelated donor might have facilitated the GVL effect. Nevertheless, there was no difference in OS or PFS between patients with and without grades II-IV acute GVHD, because the decreased incidence of disease progression was counterbalanced by the increased incidence of transplantation-related mortality. An association between the development of chronic GVHD and the incidence of disease progression was not observed. We suppose that the main reason we failed to observe this association is insufficient statistical power due to the paucity of disease progression (only 9 patients) beyond day 150, a landmark for the analysis.
Chemosensitivity at transplantation was identified as a major prognostic factor for OS. Patients with chemosensitive disease at transplantation were associated with lower nonprogression mortality, a lower incidence of disease progression, and better survival. These results may raise the question whether the effect of allogeneic transplantation was from the GVL effect or from high-dose therapy before transplantation. However, this study strongly suggested the existence of the GVL effect, because the incidence of disease progression was significantly lower in patients who developed acute GVHD, even after adjusted for chemosensitivity before transplantation. Previous history of HDT/ASCT was also a strong prognostic factor. Because the median survival for patients with NHL who had a relapse after HDT/ASCT is extremely short (less than 12 months),23 allogeneic transplantation using conventional or reduced-intensity conditioning is being evaluated in this population. In this study, 11 patients (61%) with a previous history of HDT/ASCT died within 100 days after transplantation from transplantation-related causes. On the other hand, most of the patients who survived beyond day 100 were progression free with long follow-up, suggesting a benefit of allogeneic transplantation to suppress disease progression. Therefore, strategies to decrease transplantation-related mortality are important, especially for patients after HDT/ASCT failure. Allogeneic transplantation with reduced-intensity conditioning is an option that deserves further evaluation.24
LBL, ATLL, peripheral T-cell lymphoma, and NK-cell lymphoma were the 4 major histologic subtypes in this population. The composition of the histologic subtypes in this study was different from that of NHL in general and that in previous studies focusing on allogeneic transplantation for NHL.4,12 Higher ratios of peripheral T-cell lymphoma, NK-cell lymphoma, and ATLL would, at least in part, be a reflection of the histologic population of NHL in Japan.25 Because the long-term results with conventional therapy and/or HDT/ASCT for ATLL had been always dismal,26 allogeneic transplantation even for patients in CR is being tested in a clinical trial in several centers in Japan.27 The long-term results for peripheral T-cell lymphoma and NK-cell lymphoma with conventional therapy, especially in patients with advanced or relapsed disease, were also poor.16-18,28 The high ratio of LBL might be a reflection of Japanese physicians' preference to perform allogeneic transplantation for LBL in CR1.
Transplantation outcome in each histologic subtype should be evaluated further to select patients who will benefit from unrelated transplantation. In this study, there was no difference in OS or PFS among the 3 grades. Although no patient with low-grade lymphoma had disease progression at a median follow-up of 513 days, the number of patients was too small and follow-up period was too short to draw a definite conclusion. Based on the results of this study, unrelated donor bone marrow transplantation deserves to be evaluated in patients with peripheral T-cell lymphoma and NK-cell lymphoma, considering the poor results after conventional chemotherapy for these subtypes.16-18,28 Finally, although the outcome of patients with ATLL was poor in this study, this treatment strategy should not be abandoned because both the number of the patients and the follow-up duration were not enough.
In conclusion, allogeneic bone marrow transplantation from an unrelated donor appeared to be a feasible treatment option for patients with high-risk NHL. Further study is required to determine detailed indications for unrelated transplantation for NHL, including histologic subtype and disease status.
Appendix
The following centers in Japan participated in the bone marrow transplantations for NHL facilitated by the JMDP: Hokkaido University Hospital, Sapporo Hokuyu Hospital, Japanese Red Cross Asahikawa Hospital, Iwate Medical University Hospital, Tohoku University Hospital, Yamagata University Hospital, National Cancer Center Central Hospital, Tokyo Metropolitan Komagome Hospital, Nihon University Itabashi Hospital, Jikei University Hospital, Keio University Hospital, University of Tokyo Hospital, National Tokyo Medical Center, Kanagawa Children's Medical Center, Kanagawa Cancer Center, Tokai University Hospital, Chiba University Hospital, Saitama Cancer Center Hospital, Saitama Medical School Hospital, Jichi Medical School Hospital, Saiseikai Maebashi Hospital, Gunma University Hospital, Niigata Cancer Center Hospital, Saku Central Hospital, Japanese Red Cross Nagoya First Hospital, Nagoya Daini Red Cross Hospital, Meitetsu Hospital, Nagoya University Hospital, Aichi Cancer Center, Showa Hospital, Kanazawa University Hospital, Kinki University Hospital, Osaka University Hospital, Osaka Medical Center and Research Institute for Maternal and Child Health, Matsushita Memorial Hospital, Kansai Medical University Hospital, Hyogo College of Medicine Hospital, Hyogo Medical Center for Adults, Kyoto University Hospital, Tottori University Hospital, Hiroshima Red Cross Hospital and Atomic-Bomb Survivors Hospital, Ehime Prefectural Central Hospital, National Okayama Medical Center, Kyushu University Hospital, Harasanshin General Hospital, Hamanomachi General Hospital, National Kyushu Cancer Center, St Mary's Hospital, Saga Prefectural Hospital, Nagasaki University Hospital, Kumamoto National Hospital, and Oita Medical University Hospital.
Prepublished online as Blood First Edition Paper, November 6, 2003; DOI 10.1182/blood-2003-03-0937.
A complete list of the centers in Japan that participated in the bone marrow transplantations for non-Hodgkin lymphoma facilitated by the Japan Marrow Donor Program (JMDP) appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal