Abstract
The thrombomodulin–protein C–protein S (TM-PC-PS) pathway exerts anticoagulant and anti-inflammatory effects. We investigated the role of TM in the pulmonary immune response in vivo by the use of mice with a mutation in the TM gene (TMpro/pro) that was earlier found to result in a minimal capacity for activated PC (APC) generation in the circulation. We here demonstrate that TMpro/pro mice also display a strongly reduced capacity to produce APC in the alveolar compartment upon intrapulmonary delivery of PC and thrombin. We monitored procoagulant and inflammatory changes in the lung during Gram-positive (Streptococcus pneumoniae) and Gram-negative (Klebsiella pneumoniae) pneumonia and after local administration of lipopolysaccharide (LPS). Bacterial pneumonia was associated with fibrin(ogen) depositions in the lung that colocalized with inflammatory infiltrates. LPS also induced a rise in thrombin-antithrombin complexes in bronchoalveolar lavage fluid. These pulmonary procoagulant responses were unaltered in TMpro/pro mice, except for enhanced fibrin(ogen) deposition during pneumococcal pneumonia. In addition, TMpro/pro mice displayed unchanged antibacterial defense, neutrophil recruitment, and cytokine/chemokine levels. These data suggest that the capacity of TM to generate APC does not play a role of importance in the pulmonary response to respiratory pathogens or LPS.
Introduction
Thrombomodulin (TM) is a membrane glycoprotein that forms a high-affinity complex with thrombin. The thrombin-TM complex plays a central role in the regulation of coagulation by converting protein C (PC) to its activated form (APC), a process that is facilitated by the endothelial protein C receptor (EPCR).1 APC has antithrombotic properties, together with its cofactor protein S (PS), by inactivating factor Va and VIIIa, and has profibrinolytic properties by forming complexes with plasminogen activator inhibitor type 1 (PAI-1), the main inhibitor of plasminogen activation.1,2 In addition to these anticoagulant properties, APC also attenuates several inflammatory responses. The anti-inflammatory activities of APC are mainly due to inhibition of leukocyte activation, tumor necrosis factor (TNF) production, and E-selectin–mediated cell adhesion to endothelial cells.3-7 Evidence indicates that the expression and function of TM and EPCR are diminished in sepsis in humans; this together with decreased PC levels may cause reduced generation of APC.8,9 Such a reduction in endogenous APC activity had dramatic consequences in experimental sepsis in baboons. Indeed, either infusion of anti-APC or anti-EPCR antibody and the administration of C4b binding protein, reducing the bioavailability of PS, exacerbated the response to an intravenous Escherichia coli challenge, converting a sublethal model into a severe shock response associated with lethality.10-12 In accordance, administration of exogenous APC prevented tissue injury and death induced by infusion of a lethal dose of E coli.10 Moreover, recently it has been reported that treatment with APC reduced mortality in patients with severe sepsis.13
Also in the pulmonary compartment, APC exerts strong antiinflammatory effects. Intravenous infusion of APC protected endotoxemic rats against lung injury by inhibition of leukocyte activation14 and intratracheal administration of APC attenuated bleomycin-induced lung inflammation in mice.15 Interestingly, TM expression varies in different organs. In lungs TM mRNA and TM antigen are both expressed at high levels in comparison with other organs.16-21 Together these findings led us to postulate a possible relationship between TM and pulmonary inflammation in vivo. In the present study, we sought to examine this relationship by making use of mice with a mutation in the TM gene that results in a minimal capacity for APC generation.22 We compared procoagulant and inflammatory responses in the lungs of these mice with responses of wild-type (Wt) mice after pulmonary instillation of Streptococcus pneumoniae, the predominant cause of Gram-positive pneumonia; Klebsiella pneumoniae, a frequently encountered Gram-negative respiratory pathogen; or lipopolysaccharide (LPS) to induce a sterile inflammation.
Materials and methods
Animals
Mice with a single amino acid substitution (Glu404Pro) in the gene for TM, kindly provided by Dr R. D. Rosenberg (Massachusetts Institute of Technology, Cambridge), were generated on a C57BL/6 (and B6D2F1) background, as previously described.22 Homozygous mutant TMpro/pro mice exhibit a decrease of approximately 1000-fold with respect to PC activation and approximately 100-fold with respect to binding of thrombin at physiological levels of the enzyme.22 Yet in contrast to TM gene–deficient mice, which die in the embryonic stage,23 TMpro/pro mice develop to term and possess normal reproductive performance. All mice were on a C57BL/6 background. The wild types of the TMpro/pro mice were derived from original littermates. All experiments were approved by the Committee on Use and Care of Animals of the Academic Medical Center, Amsterdam, The Netherlands.
APC measurement in the alveolar compartment
Mice were anesthetized by inhalation of isoflurane (Upjohn, Ede, The Netherlands). The trachea was exposed through a midline incision. A total of 16 wild-type and 16 TMpro/pro mice received one of the following treatments (dissolved in 50 μL sterile normal saline) via direct intratracheal injection (4 mice per strain for each treatment): (1) saline only (control), (2) thrombin (1 μg), (3) human protein C (50 μg), or (4) thrombin and human protein C. Directly thereafter the wound was sutured. After 30 minutes, mice were anesthetized again using an intraperitoneal injection with Hypnorm (Janssen Pharmaceutica, Beerse, Belgium) and midazolam (Roche, Mijdrecht, The Netherlands). The trachea was exposed and cannulated with a sterile 22-gauge Abbocath-T catheter (Abbott, Sligo, Ireland). A bronchoalveolar lavage (BAL) was performed by instilling 2 aliquots of 0.5 mL sterile saline containing 10 mM citrate and 20 mM benzamidine (pH 7.4) to reversibly inhibit APC. Thrombin, protein C, and activated protein C (APC) were generous gifts from Dr W. Kisiel (University of New Mexico, Albuquerque). APC levels were determined in BAL fluid (BALF) as described by Liaw et al.24 After recalcification of the BAL samples, APC was captured by a specific calcium-dependent monoclonal antibody (HAPC1555), generously provided by C.T.E. The activity of the bound APC could be determined toward the chromogenic substrate S2366 (Chromogenix, Mölndal, Sweden) after removal of benzamidine by a washing step. A dilution curve of APC in BALF was taken as a standard.
Induction of lung inflammation
Pneumococcal pneumonia was induced as described previously.25,26 S pneumoniae (ATCC 6303; American Type Culture Collection, Rockville, MD) were suspended in sterile isotonic saline at approximately 0.5 × 107 to 1 × 107 colony-forming units (CFUs) per milliliter, as determined by plating serial 10-fold dilutions on sheep-blood agar plates. For induction of Gram-negative pneumonia, K pneumoniae serotype 2 (ATCC 43816) was used. Bacteria were diluted in sterile isotonic saline at approximately 3 × 104 CFUs per milliliter. LPS (10 μg; from E coli 0111:B4) was obtained from Sigma (St Louis, MO) and dissolved in 50 μL sterile saline. Mice were lightly anesthetized by inhalation of isoflurane (Abbott, Queensborough, United Kingdom), and 50 μL bacterial suspension or LPS was inoculated intranasally. Control mice were instilled intranasally with 50 μL sterile saline. The time points at which mice were killed were chosen because they were found to be representative for a particular model (ie, S pneumoniae pneumonia, 48 hours; K pneumoniae pneumonia, 24 hours; LPS inflammation, 6 hours)25,26 (data not shown).
Preparation of lung homogenates
At 6, 24, or 48 hours after inoculation, mice were anesthetized by intraperitoneal injection with Hypnorm (Janssen Pharmaceutica) and midazolam (Roche), and blood was collected from the inferior caval vene. Whole lungs were harvested and homogenized at 4°C in 5 volumes of sterile isotonic saline with a tissue homogenizer (Biospect Products, Bartlesville, OK) that was carefully cleaned and desinfected with 70% ethanol after each homogenization. Serial 10-fold dilutions in sterile saline were made from these homogenates (and blood), and 50 μL volumes were plated onto sheep-blood agar plates and incubated at 37°C and 5% CO2. CFUs were counted after 16 hours. For cytokine measurements, lung homogenates were lysed in lysis buffer (300 mM NaCl, 15 mM Tris [tris(hydroxymethyl)aminomethane], 2 mM MgCl, 2 mM Triton X-100), pepstatin A, leupeptin, and aprotinin (20 ng/mL) (pH 7.4) and spun at 1500g at 4°C for 15 minutes; the supernatant was frozen at –20°C for cytokine measurement.
Cell counts in BALF
BAL was performed as described above; 0.9 to 1 mL lavage fluid was retrieved per mouse, and total cell numbers were counted from each sample in a hemacytometer. BALF differential cell counts were determined on cytospin preparations stained with modified Giemsa stain (Diff-Quick; Baxter, McGraw Park, IL).
Histologic examination
After 24-hour fixation of lungs in 10% formalin and embedding in paraffin, 4-μm-thick sections were stained with hematoxylin and eosin. All slides were coded and scored by a pathologist without knowledge of the genotype of mice and treatment. For fibrin(ogen) staining, slides were deparaffinized and endogenous peroxidase activity was quenched with a solution of methanol/0.03% H2O2 (Merck, Darmstadt, Germany). After digestion with a solution of pepsin 0.25% (Sigma) in 0.01M HCl, the sections were incubated in 10% normal goat serum (Dako, Glostrup, Denmark) and then exposed to biotin-labeled goat antimouse fibrin(ogen) antibody (Ixell; Accurate Chemical & Scientific, Westbury, NY). After washes, slides were incubated in a streptavidin-ABC solution (Dako) and developed using 1% H2O2 and 3.3′-diaminobenzidine-tetra-hydrochloride (Sigma) in Tris-HCl. The sections were mounted in glycerin gelatin without counterstaining and analyzed.
For TM staining, endogenous peroxidase activity was quenched by a solution of 1.5% H2O2 (Merck) in phosphate-buffered saline (PBS) and nonspecific binding blocked by incubation with Teng-T, pH 8.0. Slides were then incubated with a rat antimouse TM monoclonal antibody (mAb) (kindly provided by Dr S. J. Kennel, Oak Ridge National Laboratory, Oak Ridge, TN). Slides were then exposed to a biotinylated rabbit antirat polyclonal Ab (Dako), further incubated in a streptavidin-ABC solution (Dako), and developed using 1% H2O2 and 3.3′-diaminobenzidine-tetrahydrochloride (Sigma) in Tris-HCl. The sections were mounted in glycerin gelatin after a slight methyl green counterstaining and analyzed.
Assays
Cytokine and chemokine levels were measured by using commercially available enzyme-linked immunosorbent assays (ELISAs), in accordance with the manufacturers' recommendations: TNF, interleukin-6 (IL-6) (Pharmingen, San Diego, CA), interleukin-1β (IL-1β), macrophage inflammatory protein-2 (MIP-2), and KC (R&D Systems, Abingdon, United Kingdom). Detection limits were 150 pg/mL (TNF and IL-1β), 75 pg/mL (IL-6), 47 pg/mL (MIP-2), and 12 pg/mL (KC). Myeloperoxidase (MPO) activity was measured as described previously.27 Briefly, lungs were homogenized in 10% (wt/vol) homogenization buffer (50 mM phosphate buffer [pH 6.0]) containing 0.5% hexadecyltrimethylammonium bromide). After centrifugation (4500g for 20 minutes at 4°C), 0.1 mL supernatant was added to 0.55 mL of 0.1 M phosphate buffer (pH 6.0) containing 1.25 mg/mL o-dianisidine and 0.05% hydrogen peroxide. After 5 minutes, the change in absorbance at 460 nm was measured with a spectrophotometer. Purified MPO was used as a standard. Results were expressed as units of MPO activity per gram of tissue. Thrombin-antithrombin complexes (TATc's) were measured in heparin-anticoagulated plasma and in bronchoalveolar lavage fluid with an ELISA-based method. Briefly, rabbits were immunized with mouse thrombin or rat antithrombin. Antithrombin antibodies were used as capture antibody, digoxigenin-conjugated anti-antithrombin antibodies were used as detection antibodies, horseradish peroxidase–labeled sheep antidigoxigenin (anti-DIG) F(ab′) fragments (Boehringer Mannheim, Mannheim, Germany) were used as staining enzyme, and o-phenylene-diamine dihydrochloride (OPD, Sigma) was used as substrate. Dilutions of mouse serum (Sigma) were used for the standard curve, yielding a lower detection limit of 0.3 ng/mL.28
Statistical analysis
Data are expressed as means ± SEM unless indicated otherwise. Comparisons between groups were conducted using the Mann-Whitney U test. Survival curves were compared by log-rank test. A P value of less than .05 was considered to represent a statistically significant difference.
Results
Protein C activation in the alveolar space
To evaluate whether PC activation takes place in the alveolar space and whether TM regulates this activation, we administered thrombin, protein C, or both intratracheally in Wt and TMpro/pro mice and measured APC in BALF 30 minutes later (Table 1). Administration of saline or thrombin alone did not result in APC generation in Wt or TMpro/pro mice. Administration of PC only resulted in minimal APC generation in Wt mice, suggesting that the normal alveolar space contains little thrombin. Indeed, the combined administration of PC and thrombin induced profound generation of APC in Wt mice, whereas TMpro/pro mice produced less than 4% of the APC that was generated in Wt mice. These data indicate that PC activation occurs in the alveolar space and at least in part is regulated by TM.
Reduced protein C activation in BALF of TMpro/pro mice
Administration . | Wt mice, ng/mL . | TMpro/pro mice, ng/mL . |
|---|---|---|
| Saline | 0.3 ± 0.1 | 0.3 ± 0.3 |
| Thrombin | 0.8 ± 0.4 | 0* |
| Protein C | 4.7 ± 0.6 | 1.5 ± 0.4 |
| Thrombin and PC | 838.5 ± 66.9 | 32.9 ± 7.3* |
Administration . | Wt mice, ng/mL . | TMpro/pro mice, ng/mL . |
|---|---|---|
| Saline | 0.3 ± 0.1 | 0.3 ± 0.3 |
| Thrombin | 0.8 ± 0.4 | 0* |
| Protein C | 4.7 ± 0.6 | 1.5 ± 0.4 |
| Thrombin and PC | 838.5 ± 66.9 | 32.9 ± 7.3* |
Thrombin and/or protein C (PC) were administered intratracheally in Wt and TMpro/pro mice. A BAL was performed after 30 minutes, and human APC (ng/mL) was measured herein. Data are means ± SE of 4 mice per group for each treatment.
P < .05 versus Wt mice.
Gram-positive pneumonia
We used S pneumoniae as a Gram-positive respiratory pathogen. This bacterium is the most frequently encountered pathogen in community-acquired pneumonia29 and is able to induce a procoagulant state on endothelial cells in vitro.30,31 To determine whether pneumococcal pneumonia activates coagulation in the systemic compartment, TATc's were measured in plasma. Plasma concentrations of TATc's did not differ from uninfected control mice at 48 hours after administration of S pneumoniae (data not shown).
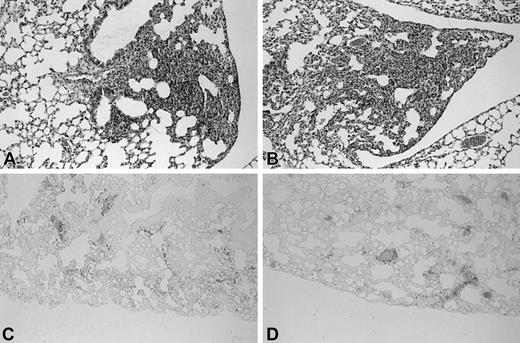
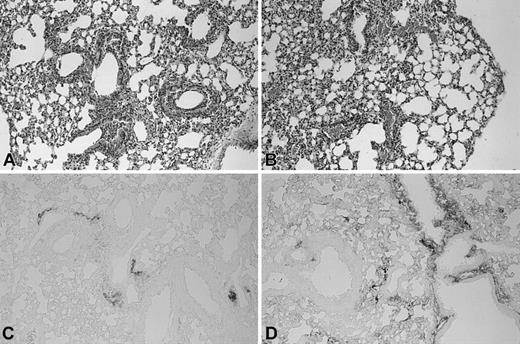
Furthermore, no difference was found between Wt and TMpro/pro mice after induction of pneumonia. We next determined whether coagulation was activated at a local level in the alveolar compartment. However, neither group had detectable levels of TATc's in BALF 48 hours after induction of pneumococcal pneumonia. Besides TATc measurements to evaluate coagulation activation, we also examined lung tissue deposition of fibrin(ogen). Antifibrin(ogen) immunostaining showed fibrin(ogen) deposition that colocalized with the inflammatory infiltrates. No fibrin clots were seen in pulmonary vessels. TMpro/pro mice showed more extensive fibrin(ogen) deposition compared with Wt mice and a strong staining of the pleura (Figure 1C-D).
Enhanced fibrin(ogen) deposition in lungs of TMpro/pro mice during pneumococcal pneumonia. Histologic sections of lungs of Wt (A) and TMpro/pro (B) mice 48 hours after inoculation with S pneumoniae (hematoxylin and eosin [HE] staining). Fibrin(ogen) immunostaining of lung 48 hours after inoculation with S pneumoniae in Wt (C) and TMpro/pro (D) mice. Original magnification, × 50. Representative slides are shown from a total of 5 mice per group.
Enhanced fibrin(ogen) deposition in lungs of TMpro/pro mice during pneumococcal pneumonia. Histologic sections of lungs of Wt (A) and TMpro/pro (B) mice 48 hours after inoculation with S pneumoniae (hematoxylin and eosin [HE] staining). Fibrin(ogen) immunostaining of lung 48 hours after inoculation with S pneumoniae in Wt (C) and TMpro/pro (D) mice. Original magnification, × 50. Representative slides are shown from a total of 5 mice per group.
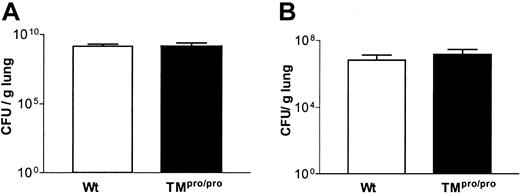
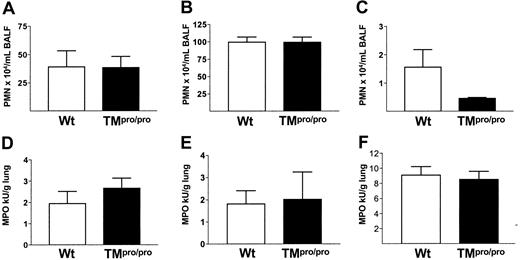
Having established that TM is important for the inhibition of coagulation at tissue level during pneumococcal pneumonia, we next determined the role of APC generation in host defense 48 hours after inoculation. The numbers of CFUs recovered from the lungs (Figure 2A) and blood (data not shown) were similar in TMpro/pro and Wt mice. Because APC has been shown to influence pulmonary cell recruitment after intravenously administered LPS,14 we assessed neutrophil influx into the alveolar compartment. No difference was seen in the number of neutrophils in BALF between the 2 groups at 48 hours after inoculation with S pneumoniae (Figure 3A). In addition, MPO was measured in lung homogenates, as a neutrophil marker enzyme, and found to be similar in Wt and TMpro/pro mice (Figure 3D). Histologically, the inflammation was comparable in both groups, although more perivascular edema was present in TMpro/pro mice (Figure 1A-B). During infection local cytokine responses are necessary to mount an adequate inflammatory reaction.32 We measured TNF, IL-1β, IL-6, KC, and MIP-2 in lung homogenates and found no difference between Wt and TMpro/pro mice (data not shown). Finally, mortality did not differ between Wt and TMpro/pro mice during a 10-day follow-up (11 of 11 versus 9 of 11 mice, respectively; nonsignificant).
TM does not influence bacterial outgrowth during pneumococcal or Klebsiella pneumonia. (A) S pneumoniae CFUs in lungs of Wt (□) and TMpro/pro (▪) mice 48 hours after intranasal inoculation. (B) K pneumoniae CFUs in lungs of Wt and TMpro/pro mice 24 hours after intranasal inoculation. Data are mean ± SEM; n = 8 per group per time point.
TM does not influence bacterial outgrowth during pneumococcal or Klebsiella pneumonia. (A) S pneumoniae CFUs in lungs of Wt (□) and TMpro/pro (▪) mice 48 hours after intranasal inoculation. (B) K pneumoniae CFUs in lungs of Wt and TMpro/pro mice 24 hours after intranasal inoculation. Data are mean ± SEM; n = 8 per group per time point.
TM does not influence neutrophil recruitment to the lungs during pneumonia or LPS-induced inflammation. Neutrophil influx in BALF 48 hours, 24 hours, and 6 hours, respectively, after intranasal inoculation with S pneumoniae (A), K pneumoniae (B), and LPS (C) in Wt (□) and TMpro/pro (▪) mice. Mean ± SEM; n = 6 per group. Myeloperoxidase content in lung homogenates of Wt (□) and TMpro/pro (▪) mice 48 hours, 24 hours, and 6 hours, respectively, after intranasal inoculation with S pneumoniae (D), K pneumoniae (E), and LPS (F). Mean ± SEM; n = 8 per group.
TM does not influence neutrophil recruitment to the lungs during pneumonia or LPS-induced inflammation. Neutrophil influx in BALF 48 hours, 24 hours, and 6 hours, respectively, after intranasal inoculation with S pneumoniae (A), K pneumoniae (B), and LPS (C) in Wt (□) and TMpro/pro (▪) mice. Mean ± SEM; n = 6 per group. Myeloperoxidase content in lung homogenates of Wt (□) and TMpro/pro (▪) mice 48 hours, 24 hours, and 6 hours, respectively, after intranasal inoculation with S pneumoniae (D), K pneumoniae (E), and LPS (F). Mean ± SEM; n = 8 per group.
Gram-negative pneumonia
Next, we evaluated whether TM is involved in the pulmonary response to Klebsiella pneumoniae, a common pathogen in Gram-negative bacterial pneumonia. First we examined whether this Gram-negative bacterium was able to induce coagulation either systemically or locally. TATc's in plasma or BALF did not differ in infected Wt and TMpro/pro mice compared with uninfected control mice (data not shown). At tissue level activation of coagulation could be detected in mice with Gram-negative pneumonia. Fibrin(ogen) was mainly observed in the pulmonary interstitium and around vessels in both mouse strains to the same extent (Figure 4C-D).
Similar pathological findings in lungs of Wt and TMpro/pro mice during Klebsiella pneumonia. Histologic sections of lungs of Wt (A) and TMpro/pro (B) mice 24 hours after inoculation with K pneumoniae (HE staining). Fibrin(ogen) immunostaining of lung 24 hours after inoculation with K pneumoniae in Wt (C) and TMpro/pro (D) mice. Original magnification, × 50. Representative slides are shown from a total of 5 mice per group.
Similar pathological findings in lungs of Wt and TMpro/pro mice during Klebsiella pneumonia. Histologic sections of lungs of Wt (A) and TMpro/pro (B) mice 24 hours after inoculation with K pneumoniae (HE staining). Fibrin(ogen) immunostaining of lung 24 hours after inoculation with K pneumoniae in Wt (C) and TMpro/pro (D) mice. Original magnification, × 50. Representative slides are shown from a total of 5 mice per group.
With respect to host defense, no difference in the number of CFUs was found in lungs and blood of TMpro/pro and Wt mice 24 hours after inoculation (Figure 2B). To determine whether the inflammatory response to K pneumoniae was influenced by TM, we assessed the number of neutrophils recruited to the alveoli. No difference in neutrophil counts was found in BALF of Wt and TMpro/pro mice 24 hours after inoculation (Figure 3B). Also, lung MPO activity did not differ between the 2 groups (Figure 3E). Histopathology showed no difference in composition and distribution of the inflammatory infiltrates (Figure 4A-B). Cytokine and chemokine levels in lung homogenates were similar in both groups (data not shown).
LPS-induced lung inflammation
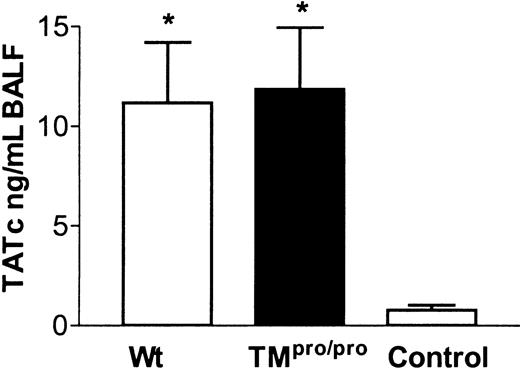
LPS is a major pathogenetic factor in Gram-negative sepsis. Systemic LPS administration caused hemodynamic and inflammatory changes but also lung damage. Recombinant soluble TM and APC were able to prevent these effects.14,33-35 To investigate whether TM has a similar role after local exposure to LPS in vivo, we inoculated Wt and TMpro/pro mice intranasally with LPS. No significant increase in plasma TATc's was seen in Wt mice and TMpro/pro mice compared with control mice (data not shown). However, at the local level, there was a brisk increase of TATc's in BALF of Wt and TMpro/pro mice compared with control mice (Figure 5). No difference in BALF TATc levels was found between TMpro/pro and Wt mice. Fibrin(ogen) deposition was mainly seen around large vessels and was slightly more pronounced in TMpro/pro mice. Thrombi were not observed (Figure 6C-D).
TM deficiency does not influence generation of TATc's in the alveolar compartment after local LPS administration. TATc's in BALF 6 hours after intranasal administration of 10 μg LPS in Wt, TMpro/pro, and control mice (inoculated with sterile saline). *P < .05 versus control. Error bars indicate SEM.
TM deficiency does not influence generation of TATc's in the alveolar compartment after local LPS administration. TATc's in BALF 6 hours after intranasal administration of 10 μg LPS in Wt, TMpro/pro, and control mice (inoculated with sterile saline). *P < .05 versus control. Error bars indicate SEM.
Similar pathological findings in LPS-induced lung inflammation in lungs of Wt and TMpro/pro mice. Histologic sections of lungs of Wt (A) and TMpro/pro (B) mice 6 hours after inoculation with LPS (HE staining). Fibrin(ogen) immunostaining of lung 6 hours after inoculation with LPS in Wt (C) and TMpro/pro (D) mice. Representative slides are shown from a total of 5 mice per group. Original magnification, × 50.
Similar pathological findings in LPS-induced lung inflammation in lungs of Wt and TMpro/pro mice. Histologic sections of lungs of Wt (A) and TMpro/pro (B) mice 6 hours after inoculation with LPS (HE staining). Fibrin(ogen) immunostaining of lung 6 hours after inoculation with LPS in Wt (C) and TMpro/pro (D) mice. Representative slides are shown from a total of 5 mice per group. Original magnification, × 50.
We evaluated the inflammatory response in the lung by determining the influx of neutrophils. No difference could be found in the recruitment of neutrophils into the BALF of Wt and TMpro/pro mice (Figure 3C). MPO content in lung homogenates did not significantly differ between the 2 groups (Figure 3). On histopathological examination the lungs showed endothelialitis, perivascular inflammation, and interstitial inflammation to the same extent in Wt and TMpro/pro mice (Figure 6A-B). Cytokine and chemokine levels in lung homogenates were similar in both groups (data not shown).
TM expression
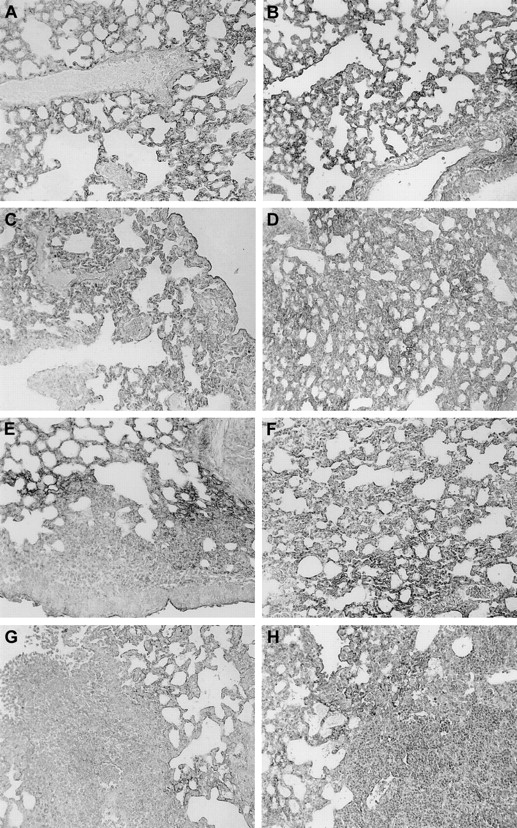
In patients with severe sepsis, TM expression is down-regulated in the dermal microvasulature.8 To evaluate the possibility that this also occurs in the lung during pulmonary infection and inflammation, we compared the expression of TM in normal and inflamed lungs using anti-TM immunostaining in Wt and TMpro/pro mice (Figure 7). Normal lungs showed a delicate network of TM expression along the interalveolar capillaries (Figure 7A-B). Decreased TM staining was observed after LPS administration (Figure 7C-D). After K pneumoniae and S pneumoniae infection, TM staining was strongly diminished in the inflamed areas (Figure 7E-H). The pattern of immunoreactivity for TM was similar in TMpro/pro mice (Figure 7B,D,F,H).
Reduced TM expression in areas of lung inflammation in Wt and TMpro/pro mice. Immunostaining for TM in the lungs of Wt (A) and TMpro/pro (B) mice, in lungs 6 hours after LPS administration in Wt (C) and TMpro/pro (D) mice, 24 hours after K pneumoniae in Wt (E) and TMpro/pro (F) mice, and 48 hours after S pneumoniae in Wt (G) and TMpro/pro (H) mice. Slides are representative for n = 5 for each group; magnification, × 20.
Reduced TM expression in areas of lung inflammation in Wt and TMpro/pro mice. Immunostaining for TM in the lungs of Wt (A) and TMpro/pro (B) mice, in lungs 6 hours after LPS administration in Wt (C) and TMpro/pro (D) mice, 24 hours after K pneumoniae in Wt (E) and TMpro/pro (F) mice, and 48 hours after S pneumoniae in Wt (G) and TMpro/pro (H) mice. Slides are representative for n = 5 for each group; magnification, × 20.
Discussion
APC, both endogenously produced and exogenously administered, plays an important role in the regulation of coagulation and inflammation. The production of endogenous APC requires an interaction between thrombin and TM. The function of TM in disease cannot be studied using TM gene–deficient mice, because these die in the embryonic stage.23 Therefore, in the present study we made use of mice with a point mutation in the TM gene, resulting in viable mice with a severely reduced capacity to generate APC.22 We were interested in TM function during lung inflammation, because TM is preferentially expressed in the pulmonary compartment16-21 and APC has been reported to exert potent anti-inflammatory effects in lungs.14,15 Moreover, the beneficial effect of recombinant human APC in a recent clinical sepsis trial was obtained in a study population that predominantly consisted of patients with pneumonia.13 We here report that experimental Gram-positive (S pneumoniae) and Gram-negative (K pneumoniae) pneumonia is associated with fibrin(ogen) depositions in lung tissue, which were modestly enhanced in TMpro/pro mice in pneumococcal pneumonia only. Local LPS administration resulted in generation of TATc's in the alveolar compartment, which was not altered by functional TM deficiency. Furthermore, we showed that TM is neither important for antibacterial defense in the lung nor for lung inflammation in response to bacteria or LPS.
Previous studies have established that TMpro/pro mice exhibit a 100-fold reduction with respect to binding of thrombin and a 1000-fold reduction with respect to PC activation in the circulation when compared with Wt mice; the incapacity of TMpro/pro mice to activate PC was demonstrated using an indirect approach (ie, mice were intravenously injected with human PC, after which human APC levels were determined using an immunocapture assay).22 In the present investigation we wished to exclude the possibility that even in Wt mice TM is not expressed to a biologically significant extent in the alveolar space; if this were the case, TM would not be expected to play a role in the regulation of coagulation in this compartment. We therefore intratracheally administered human PC with or without thrombin and measured APC in BALF 30 minutes later. PC alone induced little if any APC generation, suggesting that thrombin is not present in the normal alveolar space. Administration of PC with thrombin, however, resulted in a strong rise in APC levels in BALF of Wt mice. TMpro/pro mice, however, displayed a strongly reduced capacity to generate APC in their alveolar space (a 25-fold reduction when compared with Wt mice). Although these data indicate that indeed APC can be generated in the alveolar space and that TM plays a major role herein, it should be noted that the extent of reduction in APC generation in TMpro/pro mice is less than that previously reported for APC generation capacity in the circulation.22 It therefore remains to be established whether other factors can contribute to APC generation in the alveolar compartment.
In all 3 models studied, TM expression was reduced in areas of lung inflammation in both TMpro/pro and Wt mice. This finding is in line with a recent investigation showing diminished TM expression in the dermal microvasculature of children with severe meningococcemia8 and raises the possibility that the almost complete absence of functional TM in TMpro/pro mice does not have a major effect on the pulmonary host response because already in normal Wt mice TM expression and APC generation are reduced to a significant extent.
In Gram-negative sepsis the importance of the coagulation system has been well documented. Treatment with DEGR-FXa (a selective inhibitor of thrombin generation) or heparin prevented the coagulopathy related to sepsis but did not increase survival.36 In contrast, treatment with recombinant human TM (rh-TM) or APC decreased tissue injury as well as mortality in animal models of disseminated intravascular coagulation (DIC).10,37,38 Moreover, recombinant APC significantly reduced mortality in septic patients, which was associated with reduced circulating d-dimer and IL-6 levels.13 Interventions inhibiting the endogenous TM-PC-PS pathway strongly enhanced mortality after intravenous infusion of E coli.10-12 In accordance, TMpro/pro mice displayed a significantly reduced survival compared with Wt mice after intraperitoneal administration of LPS.39 Thus, TM does play a very important role with respect to hemostasis and inflammatory responses during sepsis and endotoxemia, both providing severe systemic challenges.
Interestingly, while our studies were in progress Conway et al reported several anti-inflammatory properties of endogenous TM that were unrelated to its anticoagulant properties.40 These authors generated transgenic mice that lack the NH2-terminal (lectin) domain of TM; these TMLeD/LeD mice were shown to have normal TM antigen levels and to retain the capacity to generate APC. TMLeD/LeD mice were found to have a reduced survival after intraperitoneal injection of LPS together with elevated circulating cytokine levels. In an experiment that resembled one of the studies described in the present paper, TMLeD/LeD mice were exposed to LPS via a nebulizer and neutrophil influx in BALF was measured 3 hours later. At this time point absolute neutrophil counts were 2-fold higher in TMLeD/LeD mice. In addition, TMLeD/LeD mice not challenged with LPS were found to have relatively more neutrophils in their lung parenchyma than Wt mice. In a series of in vitro experiments, these authors further showed that the lectin domain of TM interferes with neutrophil adhesion to endothelial cells by intracellular adhesion molecule-1–dependent and –independent pathways through the suppression of extracellular signal-regulated kinase (ERK)1/2 activation.40 These elegant studies clearly indicate that TM, and in particular its lectin domain, has anti-inflammatory properties that can be dissected from the anticoagulant properties of TM. In this respect it is important to realize that the TMpro/pro mice used here have an intact TM lectin domain. It is therefore possible that intact TM does play a role in the pulmonary infection and inflammation models described here. Nonetheless, our studies clearly reveal that the capacity of TM to interact with thrombin to generate APC (ie, the anticoagulant properties of TM) are unlikely to be involved in the regulation of lung inflammatory responses.
Notably, several studies have revealed that exogenously administered (recombinant human) TM and APC are both able to attenuate lung injury related to DIC by inhibiting leukocyte activation14,33-35,41 and that intratracheal administration of APC exerts anti-inflammatory effects in bleomycin-induced lung fibrosis.15 Furthermore, leukocyte activation was also inhibited by APC after renal and spinal cord injury in rats.42-44 On the other hand, APC did not influence pulmonary leukocyte accumulation in an intestinal ischemia/reperfusion model or in the skin in immune complex–mediated skin inflammation.45 This demonstrates that the effects of APC on leukocyte activation and recruitment differ between models.
TMpro/pro mice display a prethrombotic state and an increased susceptibility to thrombosis.22,39 During pneumonia in humans the coagulation system has been shown to become activated in the alveolar space.46-48 The results of the present study suggest that coagulation is activated after exposure of the lung to S pneumoniae, K pneumoniae, and LPS, as indicated by fibrin(ogen) depositions in the parenchyma of the lung. However, only after LPS instillation was there activation of the coagulation system in the alveolar space as well. Accordingly, adult respiratory distress syndrome (ARDS) patients showed more profound alterations in the alveolar hemostatic balance than patients with bronchopneumonia.46 In addition, it is possible that in the LPS challenge model the brisk and strong proinflammatory stimulus that remains within the alveolar space is sufficient to cause a significant rise in BALF TATc levels, whereas this is not the case in the lung infection models in which the bacterial load gradually increases primarily in lung tissue. We found a remarkable variation in fibrin(ogen) deposition in lung tissue in the 3 different models used. These fibrin(ogen) deposits colocalized with the inflammatory infiltrates. When TMpro/pro mice were exposed to S pneumoniae, K pneumoniae, and LPS, only during pneumococcal pneumonia, enhanced fibrin deposition was observed in the lung and in the pleura in TMpro/pro mice compared with Wt mice. This suggests that only during pneumococcal pneumonia is TM necessary for adequate anticoagulant activity. Other natural inhibitory pathways, such as tissue factor pathway inhibitor (TFPI), antithrombin, nitric oxide (NO) synthetase, and prostacyclin synthetase, may counteract this defect in K pneumoniae and LPS-induced lung injury.
TM is a widely expressed receptor that can mediate both anticoagulant and anti-inflammatory effects. Recent evidence indicates that these 2 properties at least in part can be separated—that is, the NH2-terminal lectin domain of TM displays several anti-inflammatory properties that are unrelated to the anticoagulant properties of TM.40 Using TMpro/pro mice, which have a strongly reduced capacity to interact with thrombin and to generate APC in the circulation22 and in the alveolar space (the present paper), we provide evidence that the anticoagulant properties of TM do not importantly contribute to the host pulmonary response to locally administered bacteria or LPS, at least not in the experimental settings used here. Further investigations are warranted to determine the role of the lectin domain of TM in the host response to bacterial pneumonia and LPS-induced lung inflammation.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2002-05-1380.
Supported by grants from the Dutch Association for Scientific Research (NWO) to A.W.R. and S.W. H.T.C. is a clinical established investigator of the Netherlands Heart Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank I. Kopp and J. B. Daalhuijsen for expert technical assistance and N. Claessen for immunostaining.

![Figure 1. Enhanced fibrin(ogen) deposition in lungs of TMpro/pro mice during pneumococcal pneumonia. Histologic sections of lungs of Wt (A) and TMpro/pro (B) mice 48 hours after inoculation with S pneumoniae (hematoxylin and eosin [HE] staining). Fibrin(ogen) immunostaining of lung 48 hours after inoculation with S pneumoniae in Wt (C) and TMpro/pro (D) mice. Original magnification, × 50. Representative slides are shown from a total of 5 mice per group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/5/10.1182_blood-2002-05-1380/6/m_zh80050457820001.jpeg?Expires=1765913256&Signature=AjuthAX2RdewV9cgysAv8kdKougcwAzUM~CDDbXRzZaSETXtGEwd4hh86ni5p1uv92j8gCvUo0B8qzTcfoTKUCDmucq08pEZkD2uZsdbQWSQZbFTr~flQll1id2ze4PEWpzTPfzeEoyLoK6spyIkOm4Yt5pHq~jsM1EYLAyOVCLofWcP~HIlAOCQZPx-5kCY4tB09AC9A7b-~dUymWElmBnr1Wy2bgPDeXQTXpdcp7fLtQMXTzuYJ9KSGpsoAFySDm~oLNcQuVTW0CZ3ZCAc8wJxdFzcH5-NQxF4i7fFcq0IH3kgwaADn8wX2fjq3bHPY46m1WiUArqZs8Flr6B-yA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal