Abstract
Lymphocyte Peyer patch adhesion molecule (LPAM) or α4β7 integrin is expressed on lymphocytes and is responsible for T-cell homing into gut-associated lymphoid tissues through its binding to mucosal addressin cell adhesion molecule (MAdCAM), which is present on high endothelial venules of mucosal lymphoid organs. We found in murine allogeneic bone marrow transplantation (BMT) models that recipients of α4β7– donor T cells had significantly less graft-versus-host disease (GVHD) morbidity and mortality compared with recipients of α4β7+ donor T cells. A kinetic posttransplantation analysis of lymphocytes in the intestines and mesenteric lymph nodes demonstrated a delayed invasion of lower numbers of α4β7+ T cells in recipients of α4β7– T cells compared with recipients of α4β7+ T cells. Histopathologic analysis of GVHD target organs revealed that recipients of α4β7– T cells developed less GVHD of the intestines and liver, whereas there was no difference in cutaneous and thymic GVHD between recipients of α4β7– or α4β7+ T cells. Finally, we found that in vivo GVT activity of α4β7– donor T cells was preserved. We conclude that the α4β7 integrin is important for the invasion of alloreactive donor T cells into the gut and the subsequent development of intestinal GVHD and overall GVHD morbidity and mortality.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an important therapeutic modality for malignancies of hematopoietic origin, some metastatic solid tumors, as well as for a variety of nonmalignant diseases. Donor T lymphocytes are primarily responsible for both the beneficial graft-versus-tumor (GVT) effect following HSCT, as well as graft-versus-host disease (GVHD), which remains an important cause of posttransplantation morbidity and mortality. Although potential alloantigens are expressed on all host tissues, acute GVHD develops only in certain organs (skin, intestine, liver, and thymus), whereas other organs remain unaffected (eg, heart and kidney).1,2 The reasons for this GVHD target organ specificity remain largely unknown but could be related to organ specific differences in (1) susceptibility to tissue damage by the conditioning regimen and GVHD-associated cytokines and effector cells; (2) the inflammatory cytokine response; (3) numbers, types, and activation of antigen-presenting cells, and (4) activation, proliferation, and infiltration of T cells (and other effector cells).
The expression of specific adhesion molecules and chemokine receptors on T cells in combination with a spatial and temporal expression pattern of the ligands for these receptors by cells in the tissues is responsible for the tissue tropism of T-cell migration.3-6 Recirculation begins with a tethering and rolling phase, during which T cells in the blood transiently and reversibly interact with vascular adhesion receptors (including selectins, selectin ligands, and integrins) and sample the endothelium for activating factors (often chemokines). On activation, a combination of additional adhesion molecules, chemokines, and other signals will lead to an arrest of the T cell, followed by transmigration across the endothelium and further localization directed by tissue-associated chemokine gradients.5
Naive T cells express receptors, such as CCR7 (a receptor for CCL19 and 21), which allow them to recirculate through secondary lymphoid organs (spleen, lymph nodes, and Peyer patches).7 On encountering antigen presented by antigen-presenting cells in the secondary lymphoid organs, T cells become activated, begin to proliferate and differentiate, and reprogram their homing receptors so that they can migrate to specific extra-lymphoid tissues.
The α4β7 or LPAM (lymphocyte Peyer patch adhesion molecule) integrin is expressed on T (and B) cells and acts as an intestinal homing receptor. α4β7 interacts with mucosal addressin cell adhesion molecule-1 (MAdCAM-1) on high endothelial venules in the Peyer patches and intestinal lamina propria.8,9 Lymphocytes in spleen and mesenteric lymph nodes (MLNs) have low levels of α4β7 expression but can up-regulate their α4β7 expression on activation.10,11
Previous studies have demonstrated that inhibition of L-selectin, MAdCAM-1, or α1 and α4 integrin can ameliorate the development of acute GVHD.12-14 Therefore, we hypothesized that α4β7 expression on donor T cells would be important for the development of intestinal GVHD. In this study, we analyzed the ability of α4β7+ and α4β7– selected donor T cells to infiltrate the intestinal mucosa of the recipient, cause systemic and organ-specific GVHD, and exert GVT activity.
Materials and methods
Cell line and antibodies
EL-4 is a murine T-cell leukemia/lymphoma derived originally from the C57BL/6 mouse and was obtained from ATCC (Manassas, VA). Cell culture medium consisted of Dulbecco modified essential medium (DMEM), supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 mg/mL streptomycin, and 2 mM l-glutamine.
Antimurine CD16/CD32 Fc block (2.4G2) and fluorochrome-labeled antimurine antibodies against CD3 (145-2C11), CD4 (RM4-5), CD8 (53-6.7), CD62L (MEL-14), Ly-9.1 (30C7), and α4β7 (DATK32) were all obtained from PharMingen (San Diego, CA).
Mice and BMT
Female CBA, B10.BR (H2k), C57BL/6 (B6, H-2b), C57BL/6 (Ly5.1+, H2b), and C3FeB6F1 (H2b/k) mice were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were used for the experiments between 8 and 12 weeks of age. BMT protocols were approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee. Briefly, BM cells were removed aseptically from the femurs and tibias and were T-cell depleted with anti–Thy-1.2 and low–TOX-M rabbit complement (Cedarlane Laboratories, Homby, ON, Canada). Purified splenic T cells were obtained in 2 different ways. The first method consisted of passage of splenocytes over a nylon wool column (to remove monocytes/macrophages and B cells), followed by RBC lysis and staining with anti-CD3–fluorescein isothiocyanate (FITC) and anti-α4β7–phycoerythrin (PE) antibodies. These cells were then sorted into α4β7+ and α4β7– CD3+ populations with the use of FACSVantage (BD Biosciences, San Jose, CA) or MoFlo (DakoCytomation, Fort Collins, CO) cell sorters in the Flow Cytometry Core Facility of the Memorial Sloan-Kettering Cancer Center. We opted for gating windows close to each other, so that we would capture sufficient α4β7+ and α4β7– cells for in vivo experiments. We considered it our highest priority to obtain sufficient numbers of α4β7– cells with as few as possible α4β7+ T cells. Our α4β7– fraction purity after sorting was 98% ± 1.6% and our α4β7+ fraction purity after sorting was 86.7% ± 4.9%. The second method of T-cell purification consisted of natural killer (NK) cell, B-cell, monocyte, granulocyte, and dendritic cell depletion of whole splenocytes by negative magnetic cell separation (Miltenyi Biotech, Auburn, CA), followed by anti-α4β7–PE antibody labeling and cell sorting into α4β7+ and α4β7– populations. The allograft consisted of 5 × 106 T-cell–depleted BM cells with or without 0.5 to 1 × 106 unselected or α4β7+/– selected T cells. These cells were resuspended in DMEM and infused into the tail vein of lethally irradiated recipients on day 0. Prior to transplantation, recipients received on day 0 total body irradiation (137Cs source) of 1300 cGy (CBA) or 1100 to 1200 cGy (C57BL/6, B10.BR) as a split dose with 3 hours between doses. Mice were housed in the specific pathogen-free facility of Memorial Sloan-Kettering Cancer Center in sterilized micro-isolater cages and received normal chow and auto-claved hyperchlorinated drinking water (pH 3).
Leukemia/lymphoma induction, assessment of GVHD, and determination of leukemic death
Animals received 1 × 105 EL-4 cells intravenously in a separate injection on day 0 of BMT after irradiation. Survival was monitored daily, and ear-punched mice were individually scored weekly for 5 clinical parameters (weight loss, hunched posture, activity level, fur ruffling, and skin lesions) on a scale from 0 to 2. A clinical GVHD score was generated by the summation of the 5 criteria scores as first described by Cooke et al,15 and mice scoring 5 or greater were killed. The cause of death (tumor versus GVHD) was determined by necropsy and histopathology. Briefly, all animals with macroscopic liver/spleen metastasis at autopsy or grossly visible abdominal masses were recorded as tumor deaths. Animals that did not exhibit any gross evidence of tumor had their liver and spleen histopathologically examined for microscopic evidence of leukemia/lymphoma by a veterinary pathologist (Dr Hai Nguyen, Cornell University Medical College, New York, NY), and cause of death was subsequently determined.
Histopathologic analysis
GVHD target organ pathology for small and large bowel, liver, and skin was assessed by experts (C.L., G.J.M.) in a blinded fashion. Formalin-preserved target organs were paraffin-embedded, sectioned, hematoxylin/eosin-stained, and scored with a semiquantitative scoring system as described before.16 In short, bowel and liver were scored for 19 to 22 different parameters associated with GVHD as previously described,17 and skin was evaluated for the number of apoptotic cells per millimeter of epidermis as previously described.18
IEL, LPL, and Peyer patch preparation
Mice were killed, and small intestine was dissected from the gastric-duodenal junction to the ileocecal junction. Intestines were washed/flushed by using a solution containing 10% HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 10% HBSS (Hanks balanced salt solution), and 80% ddH2O, cut into 1-cm–long pieces with luminal side exposed and placed in 50-mL conical tubes. Intestinal pieces were then incubated with DTE (dithioerythritol) for 20 minutes at 37°C, being continuously stirred. Following the incubation, the intestinal pieces were vortexed for 15 seconds, and the supernatant was collected. Incubation and collection of supernatant was repeated 2 more times, and then the supernatants were pooled and centrifuged at 1500 rpm for 5 minutes. The pellet was resuspended in 1 to 2 mL RPMI (without additives) and subsequently passed through a 0.3-g nylon wool column to enrich the T-cell population.
Flow cytometric analysis
Splenocytes and intraepithelial lymphocytes (IELs), lamina propria lymphocytes (LPLs), and Peyer patch lymphocytes were washed, incubated with anti-CD16/CD32 Fc block, and subsequently incubated with fluorochrome-labeled primary antibodies at saturating concentrations for 20 minutes at 4°C. Flow cytometric analysis of these cells was performed on a FACSCalibur (Becton Dickinson) with CellQuest software. Appropriate isotype controls were used with each experiment.
Intracellular staining
Cells were incubated for 5 hours with phorbol myristate acetate (PMA; 10 ng/mL) + ionomycin (2 μM). Brefeldin A (10 μg/mL) was added after 2 hours of incubation. The cells were then harvested, washed, and stained with fluorochrome-conjugated antibodies against cell surface antigens. Subsequently, cells were fixed and permeabilized with Cytofix/Cytoperm Kit reagents (PharMingen) and stained with PE-conjugated anti-interferon γ (IFNγ; XMG1.2) antibodies from PharMingen.
Carboxyfluorescein diacetate succinidyl ester (CFSE) labeling
Cells were labeled with CFSE as described previously.19 Briefly, cells (thymocytes or splenocytes) were incubated with CFSE at a final concentration of 2.5 μM in HBSS at 37°C for 10 minutes. Cells were then washed 3 times with HBSS before intravenous injection.
Statistics
Histopathologic scores and thymocyte counts were compared between groups using the Wilcoxon rank sum statistics. The log-rank statistic was applied for comparison of survival data between groups. The area under the curve (AUC) was used to summarize the GVHD trajectory of each mouse under study. The statistic used to test whether a differential GVHD change occurred between treatment groups was the pairwise difference in the AUC between groups, using all possible pairwise contrasts. Not all the mice were followed for the full length of the study. The primary reason for censoring was death or sacrifice. To account for informative dropouts, the AUCs were calculated up to the minimum follow-up time for each pairwise difference in the double sum above. A permutation distribution was used to compute the achieved significance level.
Results
Recipients of α4β7– donor T cells develop significantly less GVHD morbidity and mortality compared with recipients of LPAM+ donor T cells
To study the role of α4β7 expression on donor T cells in the development of intestinal GVHD, we used well-described clinically relevant major histocompatibility complex (MHC)–matched and -mismatched murine allogeneic BMT models. We chose as an MHC-matched model with a disparity in minor histocompatibility antigens B10.BR → CBA.20 As an MHC-mismatched model, we used the B10.BR → B6 strain combination.21 In both models, lethally irradiated recipients were infused with 5 × 106 T-cell–depleted donor bone marrow (TCD-BM) cells, and GVHD was induced by the addition of donor splenic T cells to the allograft.
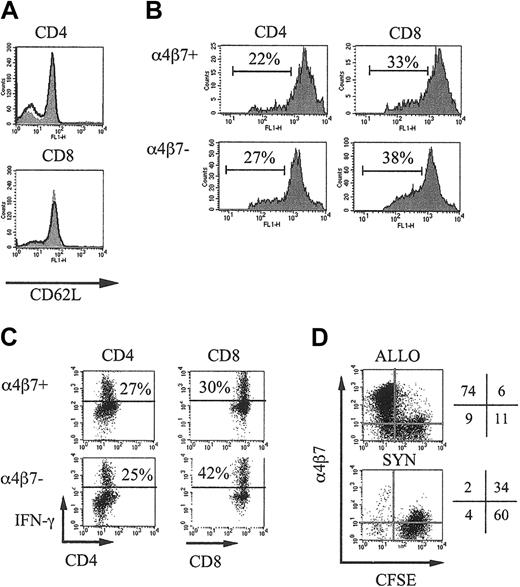
To determine the GVHD activity of α4β7+ and α4β7– donor T cells, we obtained purified populations of α4β7+ or α4β7– splenic donor T cells by flow cytometric cell sorting. We first analyzed if these α4β7+ or α4β7– selected cells differed in their expression of CD62L, which plays an important role in the initial interactions of naive T cells with the endothelium in secondary lymphoid organs, and we found no differences (α4β7+CD4+ CD62L+, 78%; α4β7– CD4+CD62L+, 66%; α4β7+CD8+ CD62L+, 89%; α4β7–CD8+ CD62L+, 86%; Figure 1A). We then assessed the alloreactive proliferation in vivo of the α4β7+ or α4β7– selected T cells by adoptive transfer after labeling with CFSE into irradiated allogeneic recipients and found no differences in the percentage of dividing cells and the number of divisions between the 2 selected populations (Figure 1B).
α4β7+ and α4β7– selected T cells do not differ in CD62L expression and alloreactive proliferation and cytokine secretion. (A) B10.BR splenic T cells were selected by magnetic separation, stained with anti-α4β7 antibodies, and separated in α4β7+ (filled curve) and α4β7– (bold line) populations by flow cytometric–assisted cell sorting. These populations were again analyzed for CD4, CD8, and CD62L expression after sorting. (B) α4β7+ and α4β7– sorted donor B10.BR T cells were labeled with CFSE and transferred into irradiated (750 cGy) CBA recipients, and donor splenic T cells from these recipients were analyzed after 40 hours. The number of dividing CD4+ or CD8+ CFSE-labeled donor T cells is indicated. (C) α4β7+ and α4β7– sorted B10.BR T cells and B10.BR TCD-BM were transferred into irradiated (750 cGy) CBA recipients, and 3 days later splenocytes from these recipients were incubated for 5 hours with PMA and Ionomycine. Brefeldin was added during the last 3 hours of this incubation. Donor (Ly9.1–) CD4+ or CD8+ T cells were analyzed for their intracellular IFN-γ expression. (D) B6 splenic T cells were labeled with CFSE and transferred into irradiated (750 cGy) syngeneic B6.Ly5.1 or allogeneic C3FeB6F1 recipients. After 3 days the expression of α4β7 on CFSE-labeled donor T cells in the spleen was determined.
α4β7+ and α4β7– selected T cells do not differ in CD62L expression and alloreactive proliferation and cytokine secretion. (A) B10.BR splenic T cells were selected by magnetic separation, stained with anti-α4β7 antibodies, and separated in α4β7+ (filled curve) and α4β7– (bold line) populations by flow cytometric–assisted cell sorting. These populations were again analyzed for CD4, CD8, and CD62L expression after sorting. (B) α4β7+ and α4β7– sorted donor B10.BR T cells were labeled with CFSE and transferred into irradiated (750 cGy) CBA recipients, and donor splenic T cells from these recipients were analyzed after 40 hours. The number of dividing CD4+ or CD8+ CFSE-labeled donor T cells is indicated. (C) α4β7+ and α4β7– sorted B10.BR T cells and B10.BR TCD-BM were transferred into irradiated (750 cGy) CBA recipients, and 3 days later splenocytes from these recipients were incubated for 5 hours with PMA and Ionomycine. Brefeldin was added during the last 3 hours of this incubation. Donor (Ly9.1–) CD4+ or CD8+ T cells were analyzed for their intracellular IFN-γ expression. (D) B6 splenic T cells were labeled with CFSE and transferred into irradiated (750 cGy) syngeneic B6.Ly5.1 or allogeneic C3FeB6F1 recipients. After 3 days the expression of α4β7 on CFSE-labeled donor T cells in the spleen was determined.
To detect potential differences in cytokine expression on alloactivation in vivo, we transferred α4β7+ and α4β7– sorted B10.BR T cells and B10.BR TCD-BM into irradiated (750 cGy) CBA recipients and 3 days later determined intracellular IFN-γ expression in the splenocytes from these recipients (Figure 1C). We could not detect any profound differences in IFN-γ expression of donor CD4+ or CD8+ T cells in these recipients.
Finally, we determined that alloreactive donor T cells up-regulate their α4β7 expression on alloactivation by adoptive transfer of unselected CFSE-labeled donor T cells into irradiated allogeneic recipients (Figure 1D).
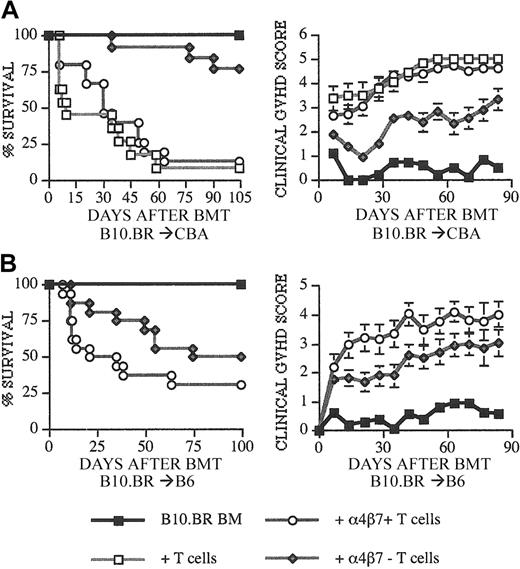
We then performed GVHD experiments by adding 0.5 × 106 (B10.BR → CBA) or 0.5 to 1 × 106 (B10.BR → B6) selected α4β7+ or α4β7– T cells to the TCD-BM containing allograft. Allografts with only TCD-BM or TCD-BM + unsorted T cells were injected as control groups. CBA recipients of α4β7– donor T cells had a significant delay and decrease in GVHD mortality and morbidity (Figure 2A) compared with recipients of α4β7+ or unsorted T cells. A similar delay in GVHD mortality and morbidity (Figure 2B) was observed when B6 recipients of α4β7– donor T cells were compared with recipients of α4β7+ T cells. From these results we conclude that α4β7– donor T cells have significantly less potential to induce GVHD than α4β7+ or unselected donor T cells.
Recipients of α4β7+ T cells have increased GVHD mortality and morbidity. Lethally irradiated (1300 cGy) CBA (A) and (1100-1200 cGy) C57BL/6 (B) recipients received transplants with B10.BR TCD-BM (5 × 106) and splenic T cells (0.5-1 × 106). T cells were included in the allograft as unsorted or α4β7+ CD3+ and α4β7– CD3+ sorted populations from B10.BR donors. (A-B) Kaplan-Meier and clinical GVHD score (± SEM) curves are shown that represent 4 (BM only), 8 to 11 (BM + T cells), and 13 to 16 (BM + α4β7+ or α4β7– T cells) recipients per group from 2 combined experiments. Statistical analysis is as follows: (A) left, ○ versus ♦ P = .007, □ versus ♦ P < .0001; right, ○ versus ♦ P < .01, □ versus ♦ P < .01. (B) Left, ○ versus ♦ P = .0179; right, ○ versus ♦ P < .02.
Recipients of α4β7+ T cells have increased GVHD mortality and morbidity. Lethally irradiated (1300 cGy) CBA (A) and (1100-1200 cGy) C57BL/6 (B) recipients received transplants with B10.BR TCD-BM (5 × 106) and splenic T cells (0.5-1 × 106). T cells were included in the allograft as unsorted or α4β7+ CD3+ and α4β7– CD3+ sorted populations from B10.BR donors. (A-B) Kaplan-Meier and clinical GVHD score (± SEM) curves are shown that represent 4 (BM only), 8 to 11 (BM + T cells), and 13 to 16 (BM + α4β7+ or α4β7– T cells) recipients per group from 2 combined experiments. Statistical analysis is as follows: (A) left, ○ versus ♦ P = .007, □ versus ♦ P < .0001; right, ○ versus ♦ P < .01, □ versus ♦ P < .01. (B) Left, ○ versus ♦ P = .0179; right, ○ versus ♦ P < .02.
Recipients of α4β7+ donor T cells have higher numbers of α4β7+ donor T cells in their intestinal mucosa
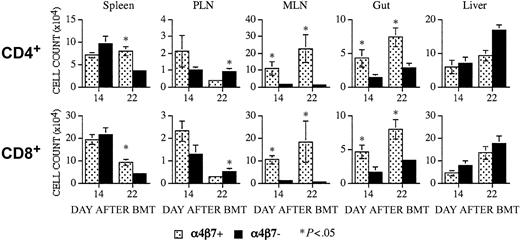
To analyze whether α4β7+ or α4β7– donor T cells differ in their capability to infiltrate into the intestinal mucosa of the recipient, we determined the numbers of B10.BR donor CD4+ or CD8+ α4β7+ T cells at 14 and 22 days after transplantation in the intestinal mucosa of the gut, spleen, peripheral lymph nodes, mesenteric lymph nodes, and liver in CBA recipients of α4β7+ or α4β7– donor T cells (Figure 3). As expected, we found significantly higher numbers of α4β7+ T cells (both CD4+ and CD8+) in the mesenteric lymph nodes and gut of recipients of α4β7+ T cells at both time points. Interestingly, we also observed more α4β7+ T cells in the spleens of recipients of α4β7+ T cells on day 22, whereas α4β7+ T cells were increased in the peripheral lymph nodes of recipients of α4β7– T cells on day 22.
Recipients of α4β7+ T cells have significantly higher numbers of α4β7+ donor T cells in their intestinal mucosa and mesenteric lymph nodes. CBA mice received transplants as described in Figure 2. Recipients of α4β7+ and α4β7– T cells were killed on days 14 and 22 after BMT. Infiltrating α4β7+ T cells of donor origin were determined by multicolor flow cytometry in multiple organs. Top panels represent averages of absolute cell numbers (± SE) of α4β7+ CD4+ donor cells in spleen (day +14, n = 8; day +22, n = 9), peripheral lymph nodes (PLNs; day +14, n = 4; day +22, n = 4), mesenteric lymph nodes (MLNs; day +14, n = 3-4; day +22, n = 3-5), small intestine (gut, day +14, n = 3-4; day +22, n = 4-5) and liver (day +14, n = 6-7; day +22, n = 8-9). Bottom panels represent averages of absolute cell numbers of α4β7+ CD8+ donor cells in the above mentioned organs. *Signifies statistically significant P values.
Recipients of α4β7+ T cells have significantly higher numbers of α4β7+ donor T cells in their intestinal mucosa and mesenteric lymph nodes. CBA mice received transplants as described in Figure 2. Recipients of α4β7+ and α4β7– T cells were killed on days 14 and 22 after BMT. Infiltrating α4β7+ T cells of donor origin were determined by multicolor flow cytometry in multiple organs. Top panels represent averages of absolute cell numbers (± SE) of α4β7+ CD4+ donor cells in spleen (day +14, n = 8; day +22, n = 9), peripheral lymph nodes (PLNs; day +14, n = 4; day +22, n = 4), mesenteric lymph nodes (MLNs; day +14, n = 3-4; day +22, n = 3-5), small intestine (gut, day +14, n = 3-4; day +22, n = 4-5) and liver (day +14, n = 6-7; day +22, n = 8-9). Bottom panels represent averages of absolute cell numbers of α4β7+ CD8+ donor cells in the above mentioned organs. *Signifies statistically significant P values.
Recipients of α4β7+ T cells develop intestinal and liver GVHD more rapidly than recipients of α4β7– T cells
Mortality in our mouse models is primarily determined by intestinal GVHD and mucositis-associated infections related to intestinal GVHD. To analyze whether recipients of α4β7+ T cells incurred more severe intestinal GVHD, we analyzed GVHD-associated organ damage in terminal ileum, colon, liver, skin (ear and tongue), and thymus. We performed semiquantitative histopathologic analysis in a blinded fashion on the terminal ileum, colon, and liver. Dyskeratotic indices were calculated to determine the number of apoptotic keratinocytes per millimeter of epidermis. Thymic GVHD was assessed by calculating thymic cellularity as we have described previously.22
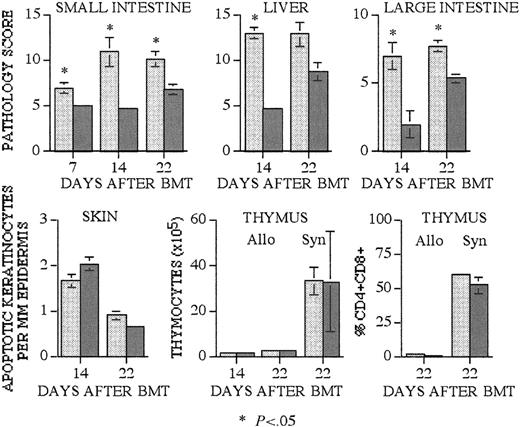
We found significantly higher histopathologic scores of terminal ileum, colon, and liver in recipients of α4β7+ T cells on days 14 and 22 after transplantation compared with recipients of α4β7– T cells (Figure 4A). The development of intestinal and liver GVHD in recipients of α4β7– cells seemed to be slower than in α4β7+ T-cell recipients, but histopathologic scores had increased to comparable levels by day 22 after transplantation.
Recipients of α4β7+ T cells have significantly increased GVHD organ pathology in intestines and liver. Lethally irradiated CBA (1300 cGy) mice received transplants as described in Figure 2. As a syngeneic BMT control for posttransplantation thymic cellularity, additional lethally irradiated (1100 cGy) B10.BR recipients received transplants. Recipients of B10.BR α4β7+ (light gray bars) and α4β7– (dark gray bars) T cells were killed on days 7, 14, and 22 after BMT, and tissues were obtained for histopathologic analysis. Small intestine, large intestine, and liver were scored for established organ-specific parameters in a blinded fashion (mean score ± SEM). Skin GVHD was determined by the number of apoptotic keratinocytes per millimeter of epidermis (±SEM), and thymic GVHD was assessed by the total number (±SEM) of thymocytes and the percentage (±SEM) of double-positive CD4+CD8+ thymocytes. Group sizes are as follows: small intestine, day 7 (3-4 animals), day 14 (4-6), and day 22 (6-7); liver, day 14 (3-6) and day 22 (7-9); large intestine, day 14 (3-5) and day 22 (7-8); skin, day 14 (14) and day 22 (12); thymus, day 14 (7-8) and day 22 (9); thymus syngeneic (5). Statistical analysis is as follows: small bowel, day 7 P = .03, day 14 P = .01, day 22 P = .006; liver, day 14 P = .02; large intestine, day 14 P = .037, day 22 P = .003.
Recipients of α4β7+ T cells have significantly increased GVHD organ pathology in intestines and liver. Lethally irradiated CBA (1300 cGy) mice received transplants as described in Figure 2. As a syngeneic BMT control for posttransplantation thymic cellularity, additional lethally irradiated (1100 cGy) B10.BR recipients received transplants. Recipients of B10.BR α4β7+ (light gray bars) and α4β7– (dark gray bars) T cells were killed on days 7, 14, and 22 after BMT, and tissues were obtained for histopathologic analysis. Small intestine, large intestine, and liver were scored for established organ-specific parameters in a blinded fashion (mean score ± SEM). Skin GVHD was determined by the number of apoptotic keratinocytes per millimeter of epidermis (±SEM), and thymic GVHD was assessed by the total number (±SEM) of thymocytes and the percentage (±SEM) of double-positive CD4+CD8+ thymocytes. Group sizes are as follows: small intestine, day 7 (3-4 animals), day 14 (4-6), and day 22 (6-7); liver, day 14 (3-6) and day 22 (7-9); large intestine, day 14 (3-5) and day 22 (7-8); skin, day 14 (14) and day 22 (12); thymus, day 14 (7-8) and day 22 (9); thymus syngeneic (5). Statistical analysis is as follows: small bowel, day 7 P = .03, day 14 P = .01, day 22 P = .006; liver, day 14 P = .02; large intestine, day 14 P = .037, day 22 P = .003.
In contrast, we observed no differences in skin and thymic GVHD in allogeneic or syngeneic recipients (Figure 4B) at 14 and 22 days after transplantation. These data suggest that intestinal and liver GVHD develops more rapidly in recipients of α4β7+ T cells, which is consistent with the selective homing tropism of alloreactive α4β7+ T cells. As expected, the development of skin and thymic GVHD is not affected by the expression of α4β7 by alloreactive T cells.
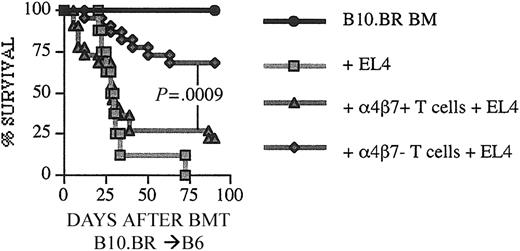
Recipients of α4β7– T cells have intact GVT activity
To assess if α4β7– expression of alloreactive T cells had any effect on GVT activity, we used the B10.BR → B6 strain combination and challenged recipients of an allograft containing α4β7+ or α4β7– T cells with EL-4 lymphoma cells on day 0. We performed autopsies on all animals that died after transplantation and determined the cause of death (GVHD or lymphoma). We observed again a significant higher GVHD mortality in recipients of α4β7+ T cells compared with recipients of α4β7– T cells, whereas GVT activity in recipients of α4β7–T cells was not diminished (Figure 5; Table 1). We conclude, therefore, that α4β7 expression of alloreactive T cells does not affect their GVT activity.
GVT activity is preserved in recipients of LPAM α4β7 T cells. C57BL/6 mice received transplants with B10.BR donor cells as described in Figure 1. Recipients were given 1 × 105 EL4 murine leukemia/lymphoma cells as a separate intravenous injection at the time of transplantation. Survival is depicted as a Kaplan-Meier curve representing mice that received TCD-BM only (n = 8), TCD-BM + EL4 (n = 8), TCD-BM + α4β7+ T cells + EL4 (n = 22), TCD-BM + α4β7– T cells + EL4 (n = 22). Statistical analysis: ▴ versus ♦ P = .0009.
GVT activity is preserved in recipients of LPAM α4β7 T cells. C57BL/6 mice received transplants with B10.BR donor cells as described in Figure 1. Recipients were given 1 × 105 EL4 murine leukemia/lymphoma cells as a separate intravenous injection at the time of transplantation. Survival is depicted as a Kaplan-Meier curve representing mice that received TCD-BM only (n = 8), TCD-BM + EL4 (n = 8), TCD-BM + α4β7+ T cells + EL4 (n = 22), TCD-BM + α4β7– T cells + EL4 (n = 22). Statistical analysis: ▴ versus ♦ P = .0009.
Cause of death (GVHD versus tumor) for all recipients that died during the course of the experiment
. | Tumor . | GVHD . | Not analyzed . |
|---|---|---|---|
| BM only | 0/8 | 0/8 | 0/8 |
| + EL4 | 8/8 | 0/8 | 0/8 |
| + α4β7+ T cells + EL4 | 8/22 | 8/22 | 1/22 |
| + α4β7- T cells + EL4 | 3/22 | 2/22 | 2/22 |
. | Tumor . | GVHD . | Not analyzed . |
|---|---|---|---|
| BM only | 0/8 | 0/8 | 0/8 |
| + EL4 | 8/8 | 0/8 | 0/8 |
| + α4β7+ T cells + EL4 | 8/22 | 8/22 | 1/22 |
| + α4β7- T cells + EL4 | 3/22 | 2/22 | 2/22 |
Discussion
Few studies have examined the role of integrin receptors in the development of GVHD. Tanaka et al12 found in a parent-into-F1 allogeneic HSCT model with a full MHC class I and II disparity that the treatment of lethally irradiated recipients with anti-α1, -α4, or -β7 antibodies resulted in a moderate amelioration of intestinal GVHD (as measured by histopathologic criteria: reduction of the villous-crypt ratio and mononuclear infiltrate), whereas the combination treatment with anti-α1 and -α4 antibodies completely prevented intestinal GVHD by histopathologic criteria. These results suggested that these 2 integrins, which both can associate with β7, are required for lymphocyte infiltration into the inflamed intestine during the development of GVHD. However, no data were provided regarding the effects of anti-α1 and -α4 antibody treatment on GVHD morbidity and mortality.
Li et al14 used an acute GVH reaction (a-GVHR) model and transferred B6 splenocytes into nonirradiated C.B-17 severe combined immunodeficient (SCID) recipients. Preincubation of donor splenocytes with anti-α4 and anti-CD62L antibodies resulted in a delay in a-GVHR mortality with 3 of 10 long-term survivors.
Murai et al13 recently demonstrated, using a-GVHR models, that the Peyer patches in the gut are required for the activation of donor antihost T cells. Their findings suggested that host dendritic cells in the subepithelial dome of Peyer patches express regulated on activation, normal T cell expressed and secreted (RANTES)/CCL5 during the development of intestinal a-GVHR, which results in the attraction of CCR5+CD8+ donor T cells. The infiltration of donor splenocytes into the Peyer patches of the recipient and the development of a-GVHR could be prevented by disruption of CCR5 in donor T cells, by inhibition of the interaction between α4β7 and MAdCAM-1 with neutralizing anti-MAdCAM-1 antibodies, or by using recipients, which lack Peyer patches.
These studies established the importance of α4β7 in the development of intestinal GVHD and demonstrated that the inhibition of the α4 integrin with neutralizing antibodies can ameliorate intestinal GVHD. Our experiments using true GVHD (not a-GVHR) models confirm that α4β7 is important for the development of intestinal GVHD and demonstrate that intestinal GVHD can be delayed by depleting α4β7+ T cells from the allograft. This delay in intestinal GVHD is associated with a significant decrease in GVHD morbidity and mortality. The association between a delay in the invasion of an epithelial target tissue by alloreactive T cells and a decrease in GVHD morbidity and mortality is reminiscent of the well-documented decrease in the GVHD alloresponse seen with delayed posttransplantation infusion of donor lymphocytes.23,24 This relationship between the timing of donor lymphocyte infusion and the severity of the GVHD alloresponse could be related to the degree of inflammation, tissue damage, and levels of inflammatory cytokines (“cytokine storm”) early after transplantation,25 as well as the turnover of host-derived antigen-presenting cells.26
Naive T cells have a uniform intermediate cell surface expression of α4β7, whereas the expression of α4β7 on CD44+ activated/memory T cells follows a bimodal pattern with a negative and a positive population (Williams and Butcher9 and A.P., M.R.M.v.d.B., our unpublished observations, February 27, 2003). For practical reasons, we selected in our experiments donor T cells only on their α4β7 expression. Therefore, both α4β7+ and α4β7– populations contained naive and activated/memory T cells, and we found equal percentages of CD62L expression in both populations. Several investigators have proposed that naive T cells express a variety of homing molecules, which allow them to recirculate through secondary lymphoid organs (spleen, lymph nodes, and Peyer patches).7,27 In support of this theory, Williams and Butcher found that naive CD44– splenic T cells, which had a uniform intermediate expression level of α4β7, homed equally well to spleen, Peyer patch, peripheral or mesenteric lymph nodes, when injected into congenic mice.9
On encountering antigen presented by antigen-presenting cells in the secondary lymphoid organs, T cells become activated, begin to proliferate and differentiate, and reprogram their homing receptors so that they can migrate to specific extra-lymphoid tissues. Therefore, we hypothesize that the naive T cells in our α4β7– selected population of donor T cells can become activated after encountering alloantigens in the mesenteric lymph nodes of the host resulting in a delayed (and decreased) infiltration of α4β7+ donor T cells in recipients of α4β7– selected T cells (Figure 3).
A similar delay between recipients of α4β7+ and α4β7– T cells was observed in the development of intestinal and liver GVHD. Recipients of α4β7+ T cells had developed extensive GVHD in liver and intestines at day +14 after transplantation, whereas recipients of α4β7– T cells developed maximal liver and intestinal GVHD by day +22 (Figure 4). Interestingly, the severity and kinetics of skin and thymic GVHD did not differ between recipients of α4β7+ and α4β7– T cells, which argues against a general decrease in alloreactivity of α4β7– T cells compared with α4β7+ T cells as an explanation for the differences in GVHD morbidity and mortality between recipients of α4β7+ and α4β7– T cells.
The higher numbers of α4β7+ T cells in the spleens of recipients of α4β7+ donor T cells at day +22 could be due to greater allostimulation at this later time point, when the recipients of α4β7+ donor T cells have developed more severe GVHD (see GVHD morbidity data in Figure 2A-B). This difference in overall GVHD morbidity could result in higher levels of immunostimulatory cytokines and chemokines, as well as more activated antigen-presenting cells. The higher numbers of α4β7+ T cells in the peripheral lymph nodes of recipients of α4β7– T cells reflect a more rapid decline in α4β7+ T cells in recipients of α4β7+ donor T cells. This could also be due to greater allostimulation in recipients of α4β7+ donor T cells, resulting in more rapid trafficking of alloactivated T cells from the peripheral lymph nodes into the target organs.
α4β7 has been demonstrated to play a role in homing of lymphoma cells to the liver sinusoids28 as well as migration of α4β7+ lymphocytes in primary sclerosing cholangitis in humans.29 Therefore, α4β7 could be involved in the homing of alloreactive T cells to the liver; however, we did not find significantly increased numbers of α4β7+ T cells in the livers of recipients of α4β7+ donor T cells. We did detect increased liver GVHD in recipients of α4β7+ T cells, which could be due to other mechanisms, such as non–T-effector cells or cytokines.
Finally, our finding that α4β7– alloreactive T cells have intact GVT activity is intriguing. The α4β7– alloreactive T cells are capable of inducing cutaneous and thymic GVHD comparable to α4β7+ alloreactive T cells, and this finding suggests that their alloreactivity is intact in all tissues except the intestines. Most leukemias and lymphomas are largely confined to the lymphohematopoietic niche in the early stages of their development, and we hypothesize, therefore, that the impaired migration of α4β7– alloreactive T cells has no effect on their GVT activity against leukemias and lymphomas. This hypothesis is supported by a recent study, which demonstrated reduced GVHD and intact GVT in recipients treated with a sphingosine-1-phosphate receptor agonist (FTY720).30 FTY720 binds to T-cell G protein–coupled receptors, which affects migration of lymphocytes and traps them in secondary lymphoid tissues.
In conclusion, this study demonstrates the importance of the expression of α4β7 by alloreactive donor T cells in the development of intestinal GVHD and overall GVHD morbidity and mortality. It supports the notion that strategies aimed at the inhibition of homing of alloreactive T cells to gut, including neutralizing antibodies against α4β7, could result in significant improvement in the prevention and/or treatment of intestinal GVHD and overall morbidity and mortality from GVHD.
Prepublished online as Blood First Edition Paper, October 16, 2003; DOI 10.1182/blood-2003-03-0957.
Supported by grants from the National Institutes of Health (RO1 grants HL69929 and HL72412) (M.R.M.v.d.B.), a translational research grant from the Leukemia & Lymphoma Society (M.R.M.v.d.B.), and a Leukemia Research Foundation Grant (A.P.). M.R.M.v.d.B. is the recipient of a Damon Runyon Scholar Award of the Cancer Research Fund and a research award from the V scholar program of the V Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal