Abstract
Chronic myelogenous leukemia (CML) is a malignant myeloproliferative disease arising from the clonal expansion of a stem cell expressing the bcr/abl oncogene. CML patients frequently respond to treatment with interferon-α (IFN-α), even though the mechanisms of the response remain unclear. In the present study, we evaluated the role of IFN-α in differentiation and activity of monocyte-derived dendritic cells (DCs) from CML patients as well as in modulation of the cell response to lipopolysaccharide (LPS). Treatment of CML monocytes with IFN-α and granulocyte-macrophage colony-stimulating factor (GM-CSF) resulted in the rapid generation of activated DCs (CML-IFN-DCs) expressing interleukin-15 (IL-15) and the antiapoptotic bcl-2 gene. These cells were fully competent to induce IFN-γ production by cocultured autologous T lymphocytes and expansion of CD8+ T cells. LPS treatment of CML-IFN-DCs, but not of immature DCs generated in the presence of IL-4/GM-CSF, induced the generation of CD8+ T cells reactive against autologous leukemic CD34+ cells. Altogether, these results suggest that (1) the generation of highly active monocyte-derived DCs could be important for the induction of an antitumor response in IFN-treated CML patients and (2) IFN-α can represent a valuable cytokine for the rapid generation of active monocyte-derived DCs to be utilized for vaccination strategies of CML patients. (Blood. 2004;103:980-987)
Introduction
Chronic myelogenous leukemia (CML) is a malignant myeloproliferative disease arising from the clonal expansion of a stem cell characterized by the typical Philadelphia (Ph) chromosome cytogenetic abnormality.1 Ph-positive tumor cells express the bcr/abl oncogene, which encodes a protein with enhanced tyrosine kinase activity involved in maintaining the malignant phenotype.2 CML is characterized by an initial chronic phase that invariably evolves to an accelerated phase, and then to a fatal blast crisis, as the malignant cells lose their ability to differentiate.3
A historic advancement in the management of patients with CML has been achieved by the clinical use of interferon-α (IFN-α), which has represented the first-line therapy in patients who are not candidates for allogeneic stem cell transplantation. IFN-α induces complete hematologic (60% to 80%) and major cytogenetic (10% to 20%) responses in patients with chronic-phase CML leading to a significant improvement of survival rates.4,5 Although the search for more effective treatment modalities has recently led to the clinical utilization of imatinib mesylate (formerly STI571), a potent abl tyrosine kinase inhibitor, as a new anti-CML agent,6 until now IFN-α has still an important role in the management of CML patients.5,7
IFN-α is a group of cytokines belonging to the type I IFN family, which are endowed with potent antiviral, antitumor, and immunoregulatory activities.8,9 In spite of many years of current use of IFN-α in patients with CML and other malignancies, the mechanisms of the antitumor action of these cytokines are still a matter of debate. For a long time, it was thought that the direct inhibitory effects on tumor cell growth/functions were the major mechanisms important in the antitumor response in patients. In fact, IFN-α can directly inhibit the proliferation of tumor cells in vitro and can exert other direct effects on tumor cells, including the down-regulation of oncogene expression and induction of tumor suppressor genes.10 In addition to the direct effects on tumor cells, IFN-α exerts several effects on host immune cells that may play a more central role in the overall antitumor response.11-13 Some reports have recently underscored the potential importance of effects of IFN-α on the host immune system for the generation of a long-lasting antitumor response in CML patients. In particular, the recent findings of a strong correlation between the clinical response to IFN-α therapy and the presence of T cells specific for an epitope targeted by CML-specific T cells,14,15 as well as of antibodies against CML-associated antigens,16 suggest that IFN-α might induce clinical responses by promoting the expansion of autologous leukemia-specific effector T cells and/or B cells. Recent studies have shown that IFN-α can exert a variety of effects on dendritic cells (DCs), which may play an important role in the induction of an antitumor immunity (reviewed by Belardelli et al17 and Belardelli and Ferrantini18 ).
DCs are professional antigen-presenting cells (APCs), derived from hematopoietic progenitor cells, acting as sentinels of the immune system and playing a pivotal role in the induction of the immune response.19 Results from pilot clinical trials have suggested that DCs can represent powerful cellular adjuvants for vaccination strategies in cancer patients.20,21 The most commonly used methods for preparing human DCs for clinical studies are based on the generation of immature DCs from monocytes after several days of treatment with interleukin-4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF) followed by an incubation step with specific maturation factors.22,23 Recent studies have shown that type I IFNs are a group of cytokines endowed with a marked capability to promote the differentiation/activation of both mouse24 and human25-29 DCs. In particular, we have reported that exposure of freshly isolated human monocytes from healthy donors to IFN-α and GM-CSF results in the rapid generation of highly active partially mature DCs.28,29
Recently, the use of autologous DCs has been considered as an attractive approach to anticancer therapy in CML patients.30-32 One particular feature of CML is that DC-based vaccines may not require antigen loading, because DCs themselves express putative tumor-associated antigens, including the bcr/abl oncogene. In fact, monocyte-derived DCs from CML patients have proven to be capable of inducing a primary CML-directed cytotoxic immune response in vitro without requiring additional exogenous antigens.33 There are a few published studies describing the effects of type I IFN on DCs from CML patients.34-36 However, a detailed characterization of the phenotype and function of DCs from CML patients generated after few days of treatment of monocytes with IFN-α, in association or not with a subsequent cell exposure to typical maturation factors, is still missing.
In the present study, we have characterized the phenotype and functional properties of DCs generated from monocytes of CML patients, harvested at the time of the diagnosis, after 3 days of in vitro treatment with either IFN-α and GM-CSF (CML-IFN-DCs) or IL-4 and GM-CSF (CML-IL-4-DCs); moreover, we have evaluated the response of these 2 types of DCs to lipopolysaccharide (LPS), a typical DC maturation signal. We report that CML-IFN-DCs exhibit the phenotype of partially mature DCs, similar to the DCs generated in the presence of IFN-α and GM-CSF from monocytes of healthy donors.28,29 Notably, CML-IFN-DCs exhibited an up-regulation of the expression of factors associated with DC activation, including IL-15, interferon-inducible protein-10 (IP-10), and bcl-2, and showed an intrinsic attitude to undergo full maturation. Of interest, LPS treatment of these cells resulted in induction of apoptosis, associated with down-regulation of bcl-2, along with the acquisition of a full capability to promote the expansion of autologous CD8+ T cells. These results further suggest the possible importance of IFN-α-induced effects on DC differentiation/activation for the generation of antitumor response in patients and underline the potential advantage of using IFN-α for obtaining, within a short time of in vitro culture, highly active monocyte-derived DCs to be used for DC-based active immunotherapy in CML patients.
Patients, materials, and methods
Samples and culture of DCs
Peripheral blood DCs were obtained from 20 CML patients at diagnosis, 7 women and 13 men, aged 25 to 69 years. All patients were in the chronic stage of the disease. In 9 samples, an extensive phenotypic and functional characterization of the DCs was carried out. Peripheral blood mononuclear cells (PBMCs) were obtained from 20 mL heparinized peripheral blood (PBL) from patients with CML by Ficoll density gradient centrifugation (Cedarlane, Hornby, ON, Canada). CD34 progenitor cells were enriched using positive immunoselection by microbeads (MACS Cell Isolation Kit, Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the selected cells was confirmed using monoclonal antibody anti-CD34 (BD Pharmingen, San Jose, CA) and flow cytometry. Monocytes were obtained by standard Percoll density gradient centrifugation of the negative fraction obtained from the CD34 enrichment. Monocytes were further enriched by adherence seeding 1.5 × 106 cells per milliliter in 6-well plates at 3 mL per well. The plates were incubated at 37°C and 5% CO2 for 2 hours. In parallel, fractions of monocytes were cryopreserved in medium consisting of 90% fetal calf serum (FCS; EuroClone, West York, United Kingdom) and 10% dimethyl sulfoxide (DMSO; Sigma Aldrich, St Louis, MO) for use in further assays. After the adherence, the resulting cell population was analyzed for CD14 antigen expression as means by flow cytometry to assess the purity, which was 90% to 95%. Blood-derived monocytes were plated at the concentration of 1 × 106 to 1.5 × 106 cells per milliliter in RPMI 1640 (BioWhittaker, Verviers, Belgium) supplemented with 10% FCS (EuroClone). The following cytokines were added alone or in combination: GM-CSF (500 U/mL) (Peprotech, London, United Kingdom), IL-4 (1000 U/mL) (R&D Systems, Minneapolis, MN), and natural human IFN-α (104 IU/mL) (Alfa Wassermann, Pescara, Italy). After 3 days of culture, nonadherent and loosely adherent cells were collected and used for subsequent analysis. At this time point, the morphology of CML-IFN-DCs and CML-IL-4-DCs strongly resembled that of the corresponding type of DCs generated from monocytes of healthy subjects.29 In most of the experiments, some DC cultures were further incubated for 20 hours with LPS (100 ng/mL) (Sigma Aldrich) or with medium alone.
Immunophenotypic analysis
DCs were washed and resuspended in phosphate-buffered saline (PBS) containing 10% human serum AB (HS; EuroClone) and incubated with a series of monoclonal antibodies (mAbs), conjugated with either fluorescein isothiocyanate (FITC) or phycoerythrin (PE) for 30 minutes at 4°C. The following mAbs were used: anti-CD14, -CD80, -CD86, -CD83, -CD40, -HLA-DR, and -CCR5 (all from BD Pharmingen). The samples were analyzed using a FACSort flow cytometer and CellQuest software (Becton Dickinson, Mountain View, CA). Cells were electronically gated according to light scatter properties to exclude cell debris and contaminating lymphocytes.
Phenotypic analysis of proliferating T cells in autologous cocultures with DCs was assessed by flow cytometry using mAbs against CD4 and CD8 (both from BD Pharmingen). The samples were analyzed as previously described for DCs.
Mixed lymphocyte reaction and in vitro proliferative assays of autologous lymphocytes
T lymphocytes from healthy donors and CML patients were obtained by Ficoll and subsequently Percoll density gradient centrifugations. PBLs from healthy donors were seeded into 96-well plates at 2 × 105 cells per well in RPMI 1640 supplemented with 5% HS (EuroClone), 2 mM l-glutamine, 100 IU/mL penicillin/streptomycin, 1 mM sodium pyruvate, 1 mM nonessential amino acids, and 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (all from BioWhittaker) (named complete RPMI 1640 medium). DCs from CML patients, generated as previously described, were added to each well in triplicate at different ratios. After 5 days, 100 μCi (3.7 MBq) [methyl-3H]thymidine (Amersham Pharmacia Biotech, Piscataway, NJ) was added to each well and incubation was continued for an additional 18 hours. Cells were then collected by a Mach II Mcell (Tomtec, Hamden, CT) harvester, and thymidine uptake was quantified by liquid scintillation counting on a 1205 Betaplate (Amersham Pharmacia Biotech). CD4+ and CD8+ T cells from CML patients were further enriched by microbead-positive immunoselection (Miltenyi Biotec), and T-cell enrichment was checked by immunofluorescence. T cells were then seeded into 96-well plates in triplicate at 105 cells per well in complete RPMI 1640 medium. Autologous DCs (5 × 104 cells per well) generated as previously described were added to each well. After 5 days, 100 μCi (3.7 MBq) [methyl-3H]thymidine was added to each well for further 18 hours and then the assay was performed. In parallel, T cells were seeded into 24-well plates at the concentration of 1 × 106 cells per milliliter in complete RPMI 1640 medium with or without autologous DCs at the concentration of 0.5 × 106 cells per milliliter. On day 6, culture supernatants were harvested and stored at -80°C for performing cytokine enzyme-linked immunosorbent assays (ELISAs).
RT and PCR
The total RNA from DCs was extracted by Trizol (Gibco, Carlsbad, CA) and processed as described previously.29 Reverse transcription was performed by incubation of 1 μg total RNA at 37°C for 60 minutes in 20 μL of final volume with 200 units of Moloney murine leukemia virus reverse transcriptase (RT) (Gibco), 10 mM dithiothreitol (DTT), 1:5 (vol/vol) 5 × RT buffer (Gibco), 5 μM pd(N)6 random hexamer, (Amersham Pharmacia Biotech), and 0.5 mM of each deoxyribonucleoside triphosphate (dNTP) (Pharmacia). The polymerase chain reaction (PCR) was performed by mixing an aliquot of RT product, which corresponded to 1 μg total RNA, to a final volume of 50 μL with 1.25 units of Taq DNA polymerase (Applied Biosystems, Roche, NJ), 0.25 mM dNTP, 1 μM 5′ sense and 3′ antisense primer, 1 mM MgCl2, and 1:10 (vol/vol) 10 × PCR buffer (Applied Biosystems). The mixture was amplified for a specific number of cycles, depending on the considered gene, by the GeneAmp PCR thermal cycler (PE Applied Biosystems, Norwalk, CT). The durations and temperatures used for annealing were as follows: IP-10 (35 cycles, 62°C), Bcl-2 (32 cycles, 62°C), IL-15 (30 cycles, 60°C), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (21 cycles, 62°C). The specific primer pairs used for the analyzed genes were as follows: IP-10 (5′-TGATTTGCTGCCTTATCTTTCTGA, 3′-CAGCCTCTGTGTGGTCCATCCTTG), IL-15 (5′-CTCGTCTAGAGCCAACTGGGTGAATGTAATAAG, 3′-TACTTACTCGAGGAATCAATTGCAATCAAGAAGTG), bcl-2 (5′-GGAAAGGCTCGAAATACAAGC, 3′-ATTGTTCCTCCCTCCCACCC), and GAPDH (5′-CCATGGAGAAGGCTGGGG, 3′-CAAAGTTGTCATGGATGACC).
Ten microliters of the PCR product were loaded onto 1% agarose gel containing 0.5 μg/mL ethidium bromide and electrophoresed at 100 V for 30 minutes.
Terminal dUTP nick-end labeling (TUNEL) assay
The presence of DNA nicking in apoptotic cells was assayed, with minor modifications, according to Gorczyca et al.37 In brief, cell samples were collected and fixed in 1% formaldehyde in PBS on ice, washed once with PBS, resuspended in cold 70% ethanol in PBS, and stored at -20°C. After rehydration, cells were resuspended in cacodylate buffer, 25 mM CoCl2, and 0.5 nM biotin-deoxyuridine triphosphate (biotin-dUTP) in the presence or absence of 10 units of terminal deoxynucleotidyl transferase (TdT) enzyme (Boehringer Ingelheim, Mannheim, Germany) and incubated at 37°C. After washing in PBS, cells were resuspended in saline citrate buffer containing 4 × saline-sodium citrate (SSC), 2.5 μg/mL FITC-avidin (Boehringer Ingelheim), 0.1% Triton X-100 (Sigma Aldrich), and 5% nonfat dry milk. After a 30-minute incubation in the dark, cells were washed in PBS with 0.1% Triton X-100 and 0.5% BSA and resuspended in Sorter medium (Quality Biological, Gaithersburg, MD). dUTP incorporation by individual cells was measured by green fluorescence on a FACScan flow cytometer using CellQuest data software (Becton Dickinson).
In vitro expansion of CD8+ T cells and evaluation of CD8+ T-cell reactivity against autologous leukemic cells by ELISPOT assay
T lymphocytes from CML patients and enrichment of CD4+/CD8+ T cells were obtained as described above. T-cell enrichment was more than 95% as evaluated by flow cytometry. CD4+/CD8+ T cells were seeded into 24-well plates at the concentration of 1 × 106/mL in complete RPMI 1640 medium with autologous DCs at the concentration of 0.5 × 106/mL (generated with either GM-CSF/IFN-α or with GM-CSF/IL-4 as described). On days 7 and 14, the cells were harvested, cultured at 1 × 106 cells/mL, and restimulated with 0.5 × 106 DCs per milliliter previously thawed and generated as described above. Recombinant human IL-2 (Collaborative Biomedical Products, Bedford, MA) was added to the cultures on day 5 (10 U/mL) after the first stimulation and on day 3 (100 U/mL) after each restimulation. On day 21, lymphocytes were cultured with autologous CD34+ cells for an additional 24 hours. Then, CD8+ T cells were positively selected with MACS microbeads (Miltenyi Biotec) and tested in an enzyme-linked immunospot assay (ELISPOT) assay for IFN-γ production (EuroClone). Briefly, 100 μL cell suspension (3 × 104 cells in RPMI 1640 supplemented with 2 mM l-glutamine and 10% FCS) was dispensed in a 96-well anti-γ-IFN antibody-coated plate; after overnight incubation and cell lysis, trapped cytokine molecules were revealed by a secondary biotinylated detection antibody and developed by incubating with streptavidin-alkaline phosphatase followed by incubating with 5-bromo-4-chloro-3-indolyl-phosphate-4-toluidine (BCIP) substrate in a gel overlay. Colored spots were enumerated on an inverted microscope at a magnification of 40 ×.
Statistical analysis
For statistical analysis, the analysis of variance (ANOVA) test paired with Fisher comparison was used, and a P value of less than .05 was considered as significant.
Results
Addition of IFN-α and GM-CSF to monocytes from CML patients results in the generation of activated DCs expressing IL-15 and IP-10 and primed for LPS-induced full maturation
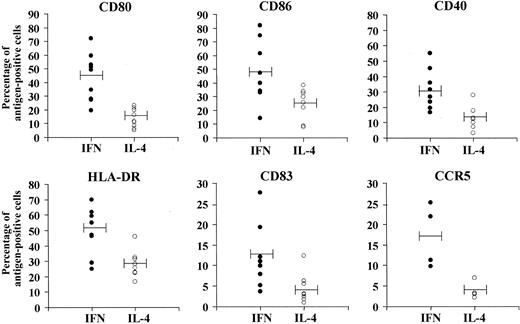
In a first set of experiments with monocytes from different CML patients, we evaluated whether the cell culture in the presence of GM-CSF and IFN-α could result in a time-dependent up-regulation of typical markers of DCs, in comparison with the corresponding cultures treated with GM-CSF and IL-4. As shown in Figure 1, cultures generated after 3 days of treatment of monocytes with IFN-α/GM-CSF (CML-IFN-DCs) showed a higher percentage of cells expressing costimulatory molecules (CD80, CD86, CD40) and HLR-DR antigens with respect to cells treated with IL-4/GM-CSF (CML-IL-4-DCs). Of interest, CML-IFN-DCs exhibited a significant increase in the percentage of cells expressing the DC maturation marker CD83 as compared with CML-IL-4-DCs. Moreover, while the expression of the chemokine CCR5 receptor was barely detected in CML-IL-4-DCs, a considerable expression of this antigen was observed in CML-IFN-DCs.
Up-regulation of differentiation and activation surface markers in monocyte-derived DCs from CML patients cultivated in the presence of IFN-α/GM-CSF. Comparison with the effects induced by IL-4/GM-CSF. Freshly isolated monocytes were prepared from CML patients at diagnosis as described in “Materials and methods.” Cells were treated with cytokines as described in “Materials and methods” and, after 3 days of culture, stained with immunofluorescent antibodies to the indicated antigens and analyzed by flow cytometry. Each solid or empty dot represents the percentage of antigen-positive cells in DC cultures derived from a single patient, generated from monocytes after treatment with GM-CSF and either IFN-α or IL-4. Horizontal bars represent the mean values (P values were the following: CD80, P < .001; CD86, P < .02; CD40, P < .001; HLA-DR, P < .005; CD83, P < .02; CCR5, P < .01).
Up-regulation of differentiation and activation surface markers in monocyte-derived DCs from CML patients cultivated in the presence of IFN-α/GM-CSF. Comparison with the effects induced by IL-4/GM-CSF. Freshly isolated monocytes were prepared from CML patients at diagnosis as described in “Materials and methods.” Cells were treated with cytokines as described in “Materials and methods” and, after 3 days of culture, stained with immunofluorescent antibodies to the indicated antigens and analyzed by flow cytometry. Each solid or empty dot represents the percentage of antigen-positive cells in DC cultures derived from a single patient, generated from monocytes after treatment with GM-CSF and either IFN-α or IL-4. Horizontal bars represent the mean values (P values were the following: CD80, P < .001; CD86, P < .02; CD40, P < .001; HLA-DR, P < .005; CD83, P < .02; CCR5, P < .01).
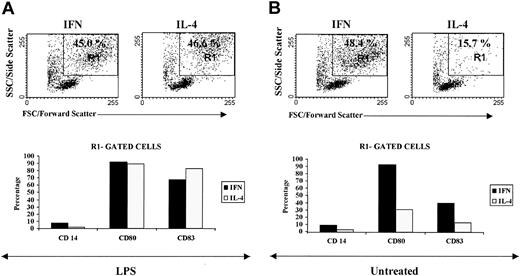
Notably, the fluorescence-activated cell sorter (FACS) analysis of CML-IFN-DCs suggested that a large portion of these cells exhibited a highly differentiated/activated DC phenotype, as revealed by the dot plot in the R1-gated region, which was poorly detected in CML-IL-4-DCs (Figure 2A). Virtually all the R1-gated CML-IFN-DCs were positive for the CD80 antigen, and approximately 40% of this selected cell population expressed the CD83 DC maturation marker. Notably, the few CML-IL-4-DCs expressing CD80 and CD83 were found in the R1-gated region (Figure 2A and data not shown).
IFN-α promotes the generation of DCs with a highly differentiated/activated phenotype. Monocyte-derived DCs were generated after 3 days of treatment with either IFN-α/GM-CSF or IL-4/GM-CSF as described in “Materials and methods.” Cells were then further treated with LPS for 20 hours (A) or left untreated (B). Flow cytometric analysis was performed on day 4. Top panels show the morphologic parameters (side versus forward scatter) of cells generated in the presence of the different cytokine combinations containing either IFN-α or IL-4 and subsequently exposed to LPS or left untreated. Staining with antibodies to the differentiation markers CD83 and CD14 or to the activation molecule CD80 is shown for the R1-gated cell cultures (bottom panels). Similar results were obtained using monocyte-derived DCs from 3 other patients.
IFN-α promotes the generation of DCs with a highly differentiated/activated phenotype. Monocyte-derived DCs were generated after 3 days of treatment with either IFN-α/GM-CSF or IL-4/GM-CSF as described in “Materials and methods.” Cells were then further treated with LPS for 20 hours (A) or left untreated (B). Flow cytometric analysis was performed on day 4. Top panels show the morphologic parameters (side versus forward scatter) of cells generated in the presence of the different cytokine combinations containing either IFN-α or IL-4 and subsequently exposed to LPS or left untreated. Staining with antibodies to the differentiation markers CD83 and CD14 or to the activation molecule CD80 is shown for the R1-gated cell cultures (bottom panels). Similar results were obtained using monocyte-derived DCs from 3 other patients.
Addition of LPS during the last 20 hours of culture to CML-IL-4-DCs resulted in the appearance of a greater number of differentiated/activated DCs (Figure 2B), which was comparable to that observed in cultures treated only with IFN-α and GM-CSF (Figure 2A). As expected, the expression of CD80 and CD83 was higher in LPS-treated CML-IL-4-DCs with respect to the untreated counterparts, and such expression was preferentially detected in the R1-gated region (Figure 2B and data not shown). Although the LPS treatment of IFN-DCs did not increase further the percentage of differentiated/activated DCs (see the R1-gated region in Figure 2B), an enhancement in the percentage of CD83+ cells was detected in the R1-gated population (Figure 2B).
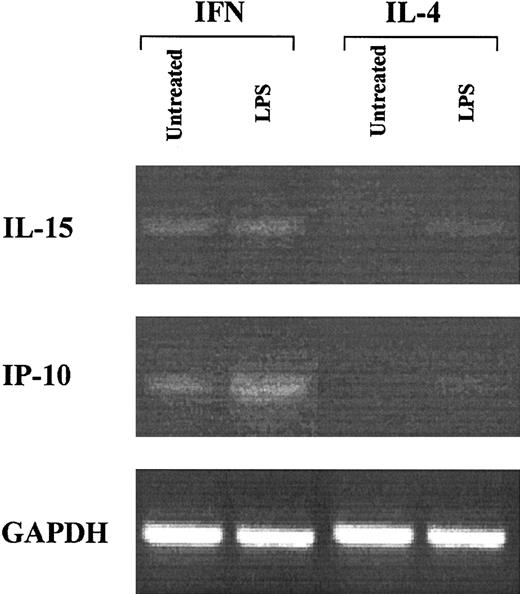
One of the important parameters characterizing the activation stage of DCs is represented by the expression profile of certain cytokines/chemokines. Thus, it was of interest to evaluate whether CML-IFN-DCs expressed factors, such as IL-15 and IP-10, which are generally produced by activated DCs and have been detected in DCs generated from monocytes of healthy donors after few days of in vitro treatment with IFN-α and GM-CSF.29,38,39 As shown in Figure 3, CML-IFN-DCs expressed considerable levels of mRNA for IL-15 and IP-10, which were undetectable in CML-IL-4-DCs. However, LPS treatment of CML-IL-4-DCs induced some expression of both IL-15 and IP-10 mRNA. Notably, LPS treatment of CML-IFN-DCs resulted in an enhanced expression of IP-10 mRNA (Figure 3) as well as in some release of IL-15 in the culture supernatants (data not shown).
Expression of IL-15 and IP-10 in IFN-DCs from CML patients. DC cultures were generated by treating freshly isolated monocytes from CML patients with either IFN-α/GM-CSF or IL-4/GM-CSF for 3 days, followed by a further 20-hour incubation in the presence or absence of LPS. At that time point, total RNA was extracted from the different cell samples and RT-PCR was performed. PCR for IL-15, IP-10, and GAPDH was carried out as described in “Materials and methods.” The results obtained with cells from 1 representative patient out of 3 are shown.
Expression of IL-15 and IP-10 in IFN-DCs from CML patients. DC cultures were generated by treating freshly isolated monocytes from CML patients with either IFN-α/GM-CSF or IL-4/GM-CSF for 3 days, followed by a further 20-hour incubation in the presence or absence of LPS. At that time point, total RNA was extracted from the different cell samples and RT-PCR was performed. PCR for IL-15, IP-10, and GAPDH was carried out as described in “Materials and methods.” The results obtained with cells from 1 representative patient out of 3 are shown.
Overall, these results indicate that IFN-α treatment of monocytes from CML patients results in the rapid generation of activated DCs expressing IL-15 and IP-10, tuned to undergo full maturation after LPS stimulation.
CML-IFN-DCs express high levels of bcl-2 and undergo apoptosis after LPS-induced stimulation
In a set of 5 experiments with cells from different patients, the percentages of viable cells recovered after DC differentiation/maturation under the different culture conditions were found to be comparable: The average percentages (mean ± SE) were 59% ± 6% and 57% ± 8% for CML-IL-4-DCs and CML-IFN-DCs, respectively. However, when the 2 different DC types were further treated with LPS, a reduction in the percentage of recovered viable cells was observed in CML-IFN-DCs as compared with LPS-treated CML-IL-4-DCs (45% ± 5% versus 55% ± 10%).
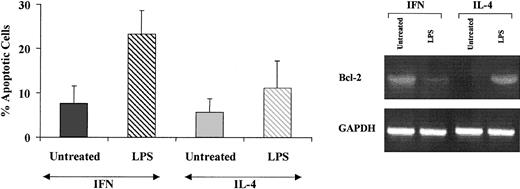
Because the expression of bcl-2 is known to control the activation stage of DCs and play a role in the regulation of the life span of mature DCs in vivo,40 it was of interest to evaluate whether the expression of this gene was up-regulated in CML-IFN-DCs as compared with CML-IL-4-DCs. As shown in Figure 4, bcl-2 mRNA was expressed at high levels in CML-IFN-DCs, while it was undetectable in CML-IL-4-DCs. Treatment of CML-IFN-DCs with LPS resulted in a marked decrease of bcl-2 mRNA levels. Conversely, LPS induced an up-regulation of bcl-2 expression in CML-IL-4-DCs.
Monocyte-derived DCs generated in the presence of IFN-α express high levels of bcl-2 and undergo apoptosis after LPS-induced stimulation. Three-day CML-DCs were generated as described in the legend to Figure 2 and either left untreated or treated with LPS for 20 hours. Total RNA was then extracted from the different cell samples and assayed for the expression of bcl-2 by RT-PCR. At the same time point, apoptosis was assayed by terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL). Values are the mean ± SE of the results of 5 experiments performed with cells from different patients. The differences between CML-LPS/IFN-DCs and CML-LPS/IL-4-DCs were statistically significant (P < .05).
Monocyte-derived DCs generated in the presence of IFN-α express high levels of bcl-2 and undergo apoptosis after LPS-induced stimulation. Three-day CML-DCs were generated as described in the legend to Figure 2 and either left untreated or treated with LPS for 20 hours. Total RNA was then extracted from the different cell samples and assayed for the expression of bcl-2 by RT-PCR. At the same time point, apoptosis was assayed by terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL). Values are the mean ± SE of the results of 5 experiments performed with cells from different patients. The differences between CML-LPS/IFN-DCs and CML-LPS/IL-4-DCs were statistically significant (P < .05).
In addition, whereas low levels of spontaneous apoptosis were observed in both CML-IL-4-DCs and CML-IFN-DCs, LPS treatment induced a significant enhancement in the percentage of apoptotic cells only in CML-IFN-DCs, which paralleled the marked bcl-2 down-modulation (Figure 4).
Taken together, these results suggest that the up-regulation of bcl-2 expression in CML-IFN-DCs may reflect a well-defined activation stage of DCs; a similar bcl-2-positive activation stage can be achieved in CML-IL-4-DCs after LPS stimulation. On the contrary, the down-regulation of bcl-2 expression observed in LPS-treated CML-IFN-DCs is consistent with induction of apoptosis occurring in these cultures at a final stage of maturation, which may represent a homeostatic mechanism regulating survival/death of activated DCs.
CML-IFN-DCs exhibit an intrinsic attitude to induce IFN-γ production by cocultured autologous lymphocytes and expansion of CD8+ cells: effects of LPS
Consistent with data from other authors,41,42 most (more than 80%) CML-IFN-DCs and CML-IL-4-DCs carried the oncogenic bcr/abl fusion protein, as revealed by the detection of the Ph chromosome by fluorescence in situ hybridization (FISH) of interphase nuclei (data not shown). Because CML-IFN-DCs showed a DC activation stage not detectable in CML-IL-4-DCs, it was of interest to evaluate whether the 2 types of DCs obtained from the same patients could exhibit different alloreactivity and capability to stimulate an autologous T-lymphocyte response, which could eventually correlate with a putative response to CML-specific antigens, including the oncogenic bcr/abl fusion protein. Figure 5 shows results of allogeneic and autologous T-cell proliferation assays performed by using either CML-IFN-DCs or CML-IL-4-DCs from 1 representative patient out of 4. CML-IFN-DCs and CML-IL-4-DCs exhibited a similar capacity to stimulate the proliferation of unrelated donor T lymphocytes. However, upon LPS treatment CML-IFN-DCs acquired a higher capacity to induce allogeneic T-cell proliferation than the corresponding LPS-treated CML-IL-4-DCs (Figure 5A). Untreated CML-IFN-DCs did not induce the proliferation of autologous T lymphocytes, even though they showed an increased ability, with respect to CML-IL-4-DCs, to induce lymphocyte-derived IFN-γ production in cocultures. Interestingly, LPS treatment of CML-IFN-DCs rendered these cells capable of inducing an autologous T-cell proliferation, which was associated with marked IFN-γ production, while the corresponding CML-IL-4-DCs were poorly effective (Figure 5B-C). Notably, the percentage of CD83+ cells in CML-IFN-DCs were approximately 18%, while CML-IL-4-DCs did not show any significant expression of this DC maturation marker. In both types of DCs from this patient, LPS strongly enhanced the percentage of cells expressing the CD83 antigen (Figure 5D).
Mixed lymphocyte reaction (MLR) and proliferation and IFN-γ production by autologous CML T lymphocytes after coculture with DCs generated in the presence of IFN-α: effect of LPS. Monocytes from CML patients were treated with either IFN-α/GM-CSF or IL-4/GM-CSF for 3 days. Cell cultures were further incubated for 20 hours in the presence or absence of LPS. (A) The proliferation of allogeneic T cells cocultured with the different DC populations at the indicated ratios. After 5 days of coculture, [3H]thymidine was added for 18 hours, and the cells were analyzed for thymidine uptake (*P < .01, **P < .05). (B) The proliferation of autologous T cells stimulated by the different DC populations as determined by a [3H]thymidine assay (*P < .01). The results in panels A and B represent the mean ± SE of data obtained from 3 single cultures. (C) The IFN-γ production by DC-stimulated autologous T cells. Supernatants from cocultures of DCs, generated as previously indicated, and autologous T cells were harvested after 6 days and tested for IFN-γ production by ELISA. (B-C). The single values are subtracted from the relative controls represented by basal [3H]thymidine incorporation and spontaneous IFN-γ production of DCs cultured alone. The values of spontaneous proliferation of T cells were under 300 cpm, and no IFN-γ production was observed in control cultures. (D) CD83 expression of the DC populations used in the coculture experiments illustrated above. Representative data obtained with cells from 1 patient out of 4 are shown.
Mixed lymphocyte reaction (MLR) and proliferation and IFN-γ production by autologous CML T lymphocytes after coculture with DCs generated in the presence of IFN-α: effect of LPS. Monocytes from CML patients were treated with either IFN-α/GM-CSF or IL-4/GM-CSF for 3 days. Cell cultures were further incubated for 20 hours in the presence or absence of LPS. (A) The proliferation of allogeneic T cells cocultured with the different DC populations at the indicated ratios. After 5 days of coculture, [3H]thymidine was added for 18 hours, and the cells were analyzed for thymidine uptake (*P < .01, **P < .05). (B) The proliferation of autologous T cells stimulated by the different DC populations as determined by a [3H]thymidine assay (*P < .01). The results in panels A and B represent the mean ± SE of data obtained from 3 single cultures. (C) The IFN-γ production by DC-stimulated autologous T cells. Supernatants from cocultures of DCs, generated as previously indicated, and autologous T cells were harvested after 6 days and tested for IFN-γ production by ELISA. (B-C). The single values are subtracted from the relative controls represented by basal [3H]thymidine incorporation and spontaneous IFN-γ production of DCs cultured alone. The values of spontaneous proliferation of T cells were under 300 cpm, and no IFN-γ production was observed in control cultures. (D) CD83 expression of the DC populations used in the coculture experiments illustrated above. Representative data obtained with cells from 1 patient out of 4 are shown.
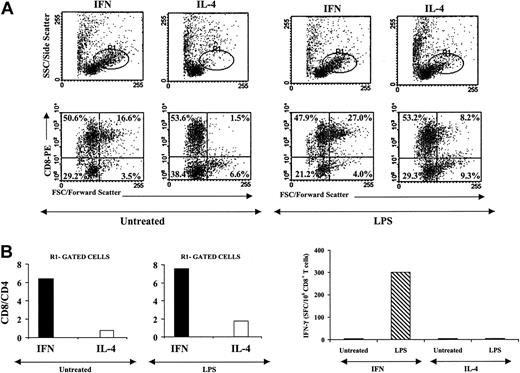
We then evaluated the features of the autologous T lymphocytes generated after coculture with the 2 types of CML-DCs. After 6 days of coculture with either untreated or LPS-treated CML-DCs, T cells were analyzed by flow cytometry. Figure 6A shows the forward scatter versus side scatter dot plot profiles of the recovered cells, which reveal a highly activated phenotype of the T lymphocytes cocultured with CML-IFN-DCs, as evidenced by the higher percentage of cells in the R1-gated region compared with CML-IL-4-DC-stimulated T lymphocytes. Of interest, the dot plot profiles showing CD8 staining versus forward scatter parameters revealed a remarkable percentage of activated CD8+ T cells in T lymphocytes cocultured with CML-IFN-DCs, which were barely detected in CML-IL-4-DC-stimulated T lymphocytes. In addition, the analysis of CD8+ and CD4+ T cells showed that the former population was significantly higher in T lymphocytes stimulated by CML-IFN-DCs than in those induced by CML-IL-4-DCs, as indicated by the CD8/CD4 ratios (data not shown). Interestingly, the CD8/CD4 ratio of R1-gated cells was strongly enhanced, suggesting the prevalence of CD8+ T cells in this region (Figure 6B). The further analysis of the T lymphocytes cocultured with CML-IFN-DCs treated with LPS revealed that, although there was only a detectable increment in the CD8/CD4 ratio, the percentage of CD8+ T cells showing an activated phenotype was significantly increased (Figure 6A). In contrast, T lymphocytes cocultured in the presence of CML-IL-4-DCs treated with LPS exhibited a mild increase in the percentage of activated CD8+ T cells and a moderate increment in the CD8/CD4 ratio, as evaluated in ungated and R1-gated cells (Figure 6B and data not shown). In a further experiment, purified CD8+ and CD4+ T cells from the same patient were stimulated twice with autologous CML-IFN-DCs or CML-IL-4-DCs, treated with LPS or left untreated. The frequencies of CD8+ T cells expanded after cocultivation with autologous leukemic DCs were analyzed by IFN-γ ELISPOT assays. As shown in Figure 6C, LPS-treated CML-IFN-DCs proved capable of inducing the expansion of CD8+ T cells, as revealed by the IFN-γ ELISPOT results, while no induction of CD8+ T cells could be detected in cocultures stimulated with the other types of CML-DCs.
CML-IFN-DCs treated with LPS are capable of inducing expansion of CML-specific CD8+ T cells. (A-B) Autologous T cells were cocultured with DCs generated from monocytes from CML patients as previously indicated. After 6 days of culture, phenotypic analysis of the proliferating T lymphocytes was performed by means of flow cytometry using fluorochrome-labeled antibodies to CD8 and CD4 antigens. (A) The morphologic parameters and the staining for CD8+ T lymphocytes (the differentiated/activated lymphocyte population is gated as R1). (B) The CD8/CD4 ratio of lymphocytes as generated in the different culture conditions. (C) CD8+ and CD4+ T cells from CML patients were magnetically sorted and cocultured with autologous DCs (generated with either GM/IFN-α or with GM/IL-4 in the presence or absence of LPS as described). On days 7 and 14, the cells were harvested, cultured, and restimulated with DCs previously thawed and generated as described above. Recombinant human IL-2 (hIL-2) was added to the cultures on day 5 after the first stimulation and on day 3 after each restimulation. On day 21, the lymphocytes were stimulated with autologous CD34+ cells for an additional 24 hours, and then CD8+ T cells were magnetically sorted by positive immunoselection and tested in an ELISPOT assay for the production of IFN-γ. LPS-treated IFN-DCs did not induce any generation of IFN-γ production by the autologous T cells when CD34+ cells from HLA-matched healthy individuals were used for the final stimulation. Representative data obtained with cells from 1 patient out of 3 are shown.
CML-IFN-DCs treated with LPS are capable of inducing expansion of CML-specific CD8+ T cells. (A-B) Autologous T cells were cocultured with DCs generated from monocytes from CML patients as previously indicated. After 6 days of culture, phenotypic analysis of the proliferating T lymphocytes was performed by means of flow cytometry using fluorochrome-labeled antibodies to CD8 and CD4 antigens. (A) The morphologic parameters and the staining for CD8+ T lymphocytes (the differentiated/activated lymphocyte population is gated as R1). (B) The CD8/CD4 ratio of lymphocytes as generated in the different culture conditions. (C) CD8+ and CD4+ T cells from CML patients were magnetically sorted and cocultured with autologous DCs (generated with either GM/IFN-α or with GM/IL-4 in the presence or absence of LPS as described). On days 7 and 14, the cells were harvested, cultured, and restimulated with DCs previously thawed and generated as described above. Recombinant human IL-2 (hIL-2) was added to the cultures on day 5 after the first stimulation and on day 3 after each restimulation. On day 21, the lymphocytes were stimulated with autologous CD34+ cells for an additional 24 hours, and then CD8+ T cells were magnetically sorted by positive immunoselection and tested in an ELISPOT assay for the production of IFN-γ. LPS-treated IFN-DCs did not induce any generation of IFN-γ production by the autologous T cells when CD34+ cells from HLA-matched healthy individuals were used for the final stimulation. Representative data obtained with cells from 1 patient out of 3 are shown.
Discussion
Few studies are available on the effects of IFN on DCs from CML patients. One study focused on the comparative characterization of DCs derived from bone marrow progenitors of healthy donors or CML patients after cultivation in the presence of GM-CSF, tumor necrosis factor-α (TNF-α), and IL-4, with or without the addition of IFN-α.36 The results of this study indicated that DCs derived from patients with CML exhibited a decreased stimulatory activity toward allogeneic T cells in MLRs as compared with DCs obtained from healthy donors. Addition of IFN-α to DCs generated from CML patients significantly enhanced their stimulatory capacity in allogeneic MLRs to near normal levels and decreased the proportion of DCs carrying the Philadelphia chromosome. In another study performed using monocytes from CML patients,35 it has been shown that treatment with GM-CSF and IFN-α for 7 days can induce DC differentiation. However, the specific features of DCs generated in the presence of IFN were poorly defined in comparison with the typical DCs generated with IL-4. A promoting effect of IFN-α on the differentiation of DCs from CML patients has also recently been shown by Paquette et al,34 whose study suggests that the therapeutic activity of IFN-α in CML may be due to its ability to stimulate the generation of DCs capable of presenting CML-specific antigens.
In the present study, we have characterized the DCs generated from monocytes of untreated CML patients after 3 days of in vitro treatment with GM-CSF and IFN-α following culture conditions previously used for the generation of DCs from healthy subjects.28 We have shown that a single-step treatment of monocytes from CML patients at diagnosis with IFN-α and GM-CSF results in the generation of activated DCs (CML-IFN-DCs) exhibiting some characteristics (pattern of expression of IL-15, IP-10, and bcl-2) similar to the mature DCs obtained when the same monocytes were cultured in medium containing IL-4 and GM-CSF and were subsequently stimulated with LPS, a typical DC maturation signal. CML-IFN-DCs expressed higher levels of costimulatory molecules (CD80, CD86, and CD40) and HLA-DR antigens than the corresponding cultures generated after treatment with IL-4 and GM-CSF (CML-IL-4-DCs) (Figure 1). Of note, a considerable portion of the CML-IFN-DCs proved to be CD83+ and CCR5+. FACS analyses revealed that a large percentage of the CML-IFN-DCs exhibited the typical characteristics of highly activated CD80+ DCs, partially positive for CD83, whose generation was observed in CML-IL-4-DCs only after their exposure to LPS (Figure 2). Notably, CML-IFN-DCs resembled the DCs generated under similar conditions using monocytes from healthy donors,28,29 not only for their phenotype but also for their capability to express IL-15 and IP-10, which are generally associated with the DC competence of promoting a T helper-1 (Th-1) type of immune response.43,44
A typical functional feature of the CML-IFN-DCs is their capacity to stimulate IFN-γ production by autologous lymphocytes in coculture experiments (Figure 5) and to induce a clear-cut expansion of activated CD8+ T cells, a characteristic not observed in CML-IL-4-DCs unless these cultures were also treated with LPS to induce DC maturation (Figure 6A-B). Notably, an expansion of CD8+ lymphocytes reactive against autologous leukemic CD34+ cells was only observed using LPS-treated CML-IFN-DCs as stimulators (Figure 6C). These results suggest that IFN-DCs can lead to a bystander proliferation of autologous CD8+ T cells, while a further maturation stimulus (ie, LPS) is required to induce CD8+ T cells reactive against tumor cells. Previous studies in mice have shown that type I IFN can induce the in vivo expansion of CD8+ lymphocytes45 and that cytokines such as IL-15 can play a role in this response.46 Thus, we may envisage that the production of IL-15 by CML-IFN-DCs can mediate, at least in part, the expansion of activated CD8+ lymphocytes and that an additional activation/maturation step of these IFN-DCs can render these cells fully capable of presenting CML-specific antigens and induce tumor-specific CD8+ effector cells, which represents an important requisite for achieving an immune-mediated control of tumor progression in CML patients.15,30 Intriguingly, CML-IFN-DCs expressed high levels of the antiapoptotic gene bcl-2, which was undetectable in IL-4-DCs, unless these cultures were also treated with LPS (Figure 4). Signaling via bcl-2 is one of several mechanisms dedicated to DC survival and strictly correlated to DC maturation. It has been suggested that the very rapid down-regulation of bcl-2 in mature DCs could represent a mechanism by which the immune system controls the presence of powerful APCs in T-cell areas of lymphoid organs.40 Indeed, the down-regulation of bcl-2, as induced by an inflammatory stimulus, controls DC longevity.40,47 In this regard, we report that CML-IFN-DCs showed a clear-cut down-regulation of bcl-2 along with a significant increase in apoptosis following LPS treatment (Figure 4). We assume that LPS-treated CML-IFN-DCs might represent competent fully mature DCs partially committed to cell death as a mechanism to restrict their longevity. Notably, in addition to the levels of bcl-2 expression, other important features (including expression of IL-15, IP-10, and capability to induce expansion of CD8 T cells) are shared by both CML-IFN-DCs and LPS-treated CML-IL-4-DCs, suggesting that the treatment of CML-derived monocytes with IFN only is sufficient to achieve the maturation stage induced by LPS treatment of CML-IL-4-DCs. Because CD8+ T reactive against autologous leukemic CD34+ cells were only observed using LPS-treated CML-IFN-DCs as stimulators, we may conclude that a single-step treatment of monocytes can result not only in the generation of DCs with characteristics regarding their activation stage similar to those of classic mature DCs but can also prime these APCs to acquire a marked capability to induce T-cell proliferation and expansion of CD8+ T cells specific against endogenous tumor-associated antigens. These results support the concept, recently suggested by others,34,35 that IFN-induced effects on DC differentiation/activation can be important for the clinical response observed in IFN-treated CML patients, often associated with the presence of a CML-specific T-cell response15 as well as with the detection of antibodies against CML-associated antigens.16
The use of DC-based immunotherapy in CML patients is aimed at achieving the immune-mediated control of the disease especially when tumor burden is markedly reduced following the current therapeutic strategies. This has been a long-sought goal over many years in the clinical setting of a therapy-induced status of minimal residual disease and holds even greater expectations today, when a high proportion of cytogenetic remissions can be obtained following imatinib mesylate treatment.48 In addition, the encouraging results of phase 1 and phase 2 clinical studies with the tyrosine kinase inhibitor imatinib mesylate,49 together with in vitro studies,50 suggest that combination regimens with DC-based immunotherapy could represent a potentially important new therapeutic approach for CML patients.51 DC-based immunotherapy may be considered as a new approach for the treatment of CML patients,30,52 which may result in an antitumor response without the considerable side effects often observed in cytokine-treated patients.53 The observation that highly active DCs, expressing Th-1-promoting factors and capable of efficiently inducing the expansion of autologous T cells, can be generated after a few days of culture in the presence of IFN-α of monocytes from CML patients opens new perspectives for DC-based therapy of this important human malignancy.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-03-0981.
Supported by grants from the Istituto Superiore di Sanità, Rome, Italy; the Associazione Italiana per la Ricerca sul Cancro (F.B. and R.F.), Milan, Italy; and the Italian Ministry of Health (Project on Cytokines no. 98/JB/T).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Cinzia Gasparrini and Anna Ferrigno for secretarial assistance.

![Figure 5. Mixed lymphocyte reaction (MLR) and proliferation and IFN-γ production by autologous CML T lymphocytes after coculture with DCs generated in the presence of IFN-α: effect of LPS. Monocytes from CML patients were treated with either IFN-α/GM-CSF or IL-4/GM-CSF for 3 days. Cell cultures were further incubated for 20 hours in the presence or absence of LPS. (A) The proliferation of allogeneic T cells cocultured with the different DC populations at the indicated ratios. After 5 days of coculture, [3H]thymidine was added for 18 hours, and the cells were analyzed for thymidine uptake (*P < .01, **P < .05). (B) The proliferation of autologous T cells stimulated by the different DC populations as determined by a [3H]thymidine assay (*P < .01). The results in panels A and B represent the mean ± SE of data obtained from 3 single cultures. (C) The IFN-γ production by DC-stimulated autologous T cells. Supernatants from cocultures of DCs, generated as previously indicated, and autologous T cells were harvested after 6 days and tested for IFN-γ production by ELISA. (B-C). The single values are subtracted from the relative controls represented by basal [3H]thymidine incorporation and spontaneous IFN-γ production of DCs cultured alone. The values of spontaneous proliferation of T cells were under 300 cpm, and no IFN-γ production was observed in control cultures. (D) CD83 expression of the DC populations used in the coculture experiments illustrated above. Representative data obtained with cells from 1 patient out of 4 are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/3/10.1182_blood-2003-03-0981/6/m_zh80030455730005.jpeg?Expires=1765977031&Signature=re9J3wINhuFI-fHetPxo6ludY03lInjnvFetQweZzer4MIxbVwyc8Td71NXaB~qbGmBf1i3sVddafZg8f-WYrDAVRiqDHD-r7eBoVK6mUkeqbS4cZdXmiuT8EjbLfqnv8~k18wi8DquTPcsBoen8aRtD16StMeV6OM~GfdlY2tbvIzhvrpo1~~WzSrcrAlnh0kLkG9EmxpicGHrABhhiOLIBoUR5-1h6-UvI-z4L5wgrCblRotj2lk3xispWlbOiRpTI-wdukCOqqgV8myQ07-6PL5Rljn~VB8GBfZ4TSkCKpYoKxH84DzUd08Itt3Kbu6KbMyqD0xNjL3NSKlt9tg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal