Abstract
Adenovirus (Ad) infections are responsible for considerable morbidity and mortality, particularly in pediatric hematopoietic stem cell transplant (HSCT) recipients. To date there is no therapy. The present study was motivated by the potential for using adoptive immunotherapy as either prophylaxis or treatment for Ad infections and associated diseases. The authors have developed a protocol to reactivate Ad-specific memory T cells from peripheral blood mononuclear cells (PBMCs) using a clinical-grade adenoviral vector. Such lines contain a specific CD4 and CD8 T-cell component and are capable of recognizing and lysing target cells infected with wild-type Ad serotypes from different Ad groups. Furthermore, the frequency of Ad-specific precursors can be determined in PBMCs ex vivo and used as a means to assess changes in Ad-specific T-cell memory responses after infusion. This is the first report of a simple and reproducible method to activate and expand Ad-specific cytotoxic T lymphocytes (CTLs), which should be protective against the range of different Ad subtypes that affect transplant recipients. (Blood. 2004;103:1011-1019)
Introduction
Adenoviruses (Ads) are nonenveloped, lytic, DNA viruses capable of infecting most animal species. To date, 51 different serotypes of human Ads have been identified and grouped from A to F on the basis of genome size, composition, homology, and organization.1,2 Pathogenicity varies according to group and type. Infection ultimately results in cell destruction, but Ads do persist and can be detected months after primary exposure.3 In the immunocompromised human host, Ads are responsible for causing hepatitis, encephalitis, and hemorrhagic cystitis, associated with high mortality.4-6 For example, hematopoietic stem cell transplant (HSCT) recipients, particularly those receiving a T cell-depleted transplant, are prone to viral reactivations and/or primary infections from a variety of infectious agents, including Epstein-Barr virus (EBV),7 cytomegalovirus (CMV),8 and Ad.4 Antiviral drugs,9 monoclonal antibodies,10 or adoptively transferred virus-specific cytotoxic T lymphocytes (CTLs) have proved to be effective treatments for EBV11 and CMV,12 but there are no such therapies for Ad infections,13-18 which remain a major cause of morbidity and mortality, particularly in the pediatric population.5,6 Therefore, the treatment of Ad infections using an immunotherapeutic approach remains an attractive possibility.
To generate Ad-specific CTLs, T-cell precursors must be reactivated and expanded with Ad antigens. The use of intact virus as antigen is not feasible because of the risk of introducing infectious virus into the CTL cultures and thence into patients. It is also unclear which viral proteins induce protective immunity; therefore, rational antigen selection is not possible. The cellular immune response to Ad has been extensively studied in mouse models, where the immediate early proteins E1A and E1B have been identified as strong targets.19,20 However, analysis of Ad-specific T-cell immunity in the human host has indicated that a strong CD4+ T-cell response, detectable by both proliferation and enzyme-linked immunospot assay (ELISPOT) assay,21,22 can be induced by the virion proteins, hexon,23 penton, and fiber.24 On the basis of these data we therefore opted to characterize the immune response to replication-incompetent Ad vectors, which have a long history in clinical trials,25,26 and asked whether Ad-specific CTLs could be generated in response to these vectors.
For a safe clinical trial of Ad-specific CTLs, several requirements must be fulfilled. The methods used to generate the CTLs should be simple and reproducible and produce sufficient CTL numbers for an effective dose.27 Both CD4 T helper cells and CD8 CTLs should be generated to favor expansion and persistence in vivo.28 The CTL should be protective against the range of different adenovirus subtypes that affect transplant recipients, while having no reactivity against self-proteins. Finally, the reagents used for cell manufacture must be safe. To date, such methods have not been available. While we had previously reported reactivation of Ad-specific CTLs that recognized Ads from different serologic groups, these CTLs had unusual characteristics, being unable to kill target cells in a standard 4- to 6-hour assay (requiring 18 hours to kill), and could not be adequately expanded into CTL lines.29,30 This had precluded clinical application. We now present a system in which Ad-specific polyclonal T-cell lines, with both cytotoxic and helper function, can reproducibly be expanded from healthy donors in vitro using replication-defective Ad vectors. Further, these CTLs recognize and kill autologous cells infected with wild-type adenovirus isolates from multiple different serotypes and groups. We discuss the implications of these results for immunotherapy.
Materials and methods
Donors and cell lines
Peripheral blood mononuclear cells (PBMCs) and skin biopsies from Ad-seropositive healthy volunteers were obtained with informed consent. PBMCs were used to generate T-cell lines, dendritic cells (DCs), and lymphoblastoid cell lines (LCLs). LCLs were generated with concentrated supernatants of the B95-8 cell line.31 Both LCLs and fibroblasts were maintained in RPMI 1640 (HyClone, Logan, UT) supplemented with 5% and 10% fetal bovine serum (FBS) (HyClone), respectively, and 2 mM l-glutamine (GlutaMAX-I, Invitrogen, Carlsbad, CA).
Viruses and vectors
The Ad5GFP and the Ad5f35GFP 32 vectors were supplied by Dr Alan Davis (Baylor College of Medicine, Houston, TX). Ad5f35 has an Ad5 backbone with a chimeric Ad5/Ad35 fiber, selected for its ability to transduce hematopoietic cells.33 All multiplicities of infection (MOIs) used in this study are based on virus particles (vp); 1 infectious unit (iu) = 100 vp. The wild-type adenoviruses used in this study are prototype strains from the American Type Culture Collection (Rockville, MD).
Flow cytometry
For all flow cytometric analyses, a FACSCalibur instrument (Becton Dickinson [BD], Mountain View, CA) was used to acquire, and CellQuest software (BD) to analyze, data. Antibodies were purchased from BD or Immunotech (Marseille, France). Isotype controls for all antibody staining were immunoglobulin G1-phycoerythrin (IgG1-PE), IgG1-peridinin chlorophyll protein (IgG1-PerCP), and IgG1-fluorescein isothiocyanate (IgG1-FITC). Phosphate-buffered saline (PBS) (Sigma, St Louis, MO) with 2% FBS and 0.1% sodium azide (Sigma) was used as wash buffer. PBS with 0.5% paraformaldehyde (Sigma) was used as fixative solution. Cells were washed once with wash buffer, pelleted, and antibodies were added to the pellet in saturating amounts (5 μL). After 15-minute incubation at 4°C in the dark, cells were washed twice, fixed, and analyzed.
Surface staining. For DCs, the antibodies used were a lineage cocktail (CD3-PE, CD14-PE, CD16-PE, CD19-PE, CD56-PE), HLA-DR-PerCP, CD80-PE, CD86-PE, and CD83-PE. For responder T cells, the antibodies used were CD3-PerCP, CD4-PE, CD8-FITC, CD56-PE, T-cell receptor (TCR) αβ-PE, TCR γδ-PE, CD16-PE, and CD19-PE.
DC generation
DCs were prepared as previously described.34 Following maturation, DCs were harvested; assessed by flow cytometry for up-regulation of maturation markers CD80, CD83, and CD86; and used as stimulators following transduction with Ad vectors for 2 hours at the MOIs indicated in serum-free RPMI medium (HyClone).
CTL generation
For generation, 2 different protocols were used.
Cryopreserved nonadherent PBMCs were used as responders. Ad5f35GFP-transduced mature DCs or Ad5f35GFP-transduced autologous LCLs were used as stimulators. For the first stimulation, responders were plated as 2 × 106 per well in a 24-well plate in 2 mL CTL medium (RPMI 1640 supplemented by 45% Clicks medium, 2 mM GlutaMAX-I, and 10% FBS). The responder-to-stimulator (R/S; PBMC-to-DC) ratio was 10:1 for the first stimulation. On day 9 or 10, responders were harvested, plated as 2 × 106 per well, and stimulated at an R/S ratio of 10:1. Responders were fed every 2 to 3 days with a half-media change. Responders were harvested on day 17 or 18 and stimulated for a third time using an R/S ratio of 4:1 where stimulators were Ad5f35GFP-transduced LCLs.
Fresh PBMCs were transduced with Ad5f35GFP at an MOI of 200 and used as both stimulators and responders. For the first stimulation, cells were plated as 2 × 106 per well in a 24-well plate in 2 mL CTL medium; the precise R/S ratio was unknown, because the monocyte fraction varied from donor to donor. On day 9 or 10, responders were harvested, plated as 2 × 106 per well, and stimulated at an R/S ratio of 4:1 for the second time. Responders were fed every 2 to 3 days with a half-media change, harvested on day 17 or 18, and stimulated for a third time with Ad5f35-transduced LCLs at an R/S ratio of 4:1. No exogenous interleukin-2 (IL-2) was added until day 21.
Enzyme-linked immunospot assay
The ELISPOT assay, as previously described,34 was used to determine the precursor frequency of Ad- and EBV-specific interferon-γ (IFN-γ)-secreting T cells.22,35-37 As responder cells, peripheral blood CD4+ and CD8+ T cells were isolated using miniMACS (Miltenyi Biotec, Bergisch Gladbach, Germany) positive selection columns, according to the manufacturer's instructions, and stimulated either with autologous DCs alone or transduced with Ad5f35GFP at an MOI of 200, at 2 × 104 cells per well. Autologous LCLs were used at 1 × 105 per well. Each condition was run in triplicate.
For analysis of Ad-specific precursor frequency ex vivo using total PBMCs, cells were isolated by Ficoll (Lymphoprep, Nycomed, Oslo, Norway) gradient separation and used either unstimulated or incubated with Ad5f35GFP at an MOI of 200 and then serially diluted from 4 × 105 to 5 × 104 cells per well in triplicate. Monocytes in the mixed PBMC population become transduced by Ad5f35GFP and present adenoviral antigens to T cells (Figure 3).
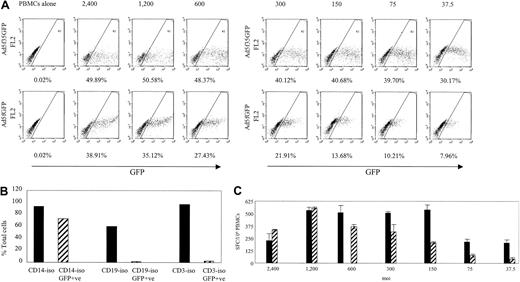
The Ad5f35GFP vector transduces PBMCs more efficiently than the Ad5GFP vector and is capable of inducing an Ad-specific T-cell immune response. (A) PBMCs were transduced using the Ad5f35GFP vector or the Ad5GFP vector at the MOIs indicated. The transduction efficiency was assessed based on percent GFP-positive cells. (B) Cells becoming transduced with the Ad5f35GFP vector were analyzed by isolating CD14+ cells, CD19+ cells, and CD3+ cells, transducing them with the Ad5f35GFP vector at MOI 200 and then assessing GFP expression after 24 hours. (C) PBMCs transduced with the Ad5f35GFP (▪) and Ad5GFP (▨) vectors were used to stimulate PBMCs in ELISPOT assay. Results are shown as SFCs per 106 PBMCs. Error bars indicate the standard error of 3 experiments.
The Ad5f35GFP vector transduces PBMCs more efficiently than the Ad5GFP vector and is capable of inducing an Ad-specific T-cell immune response. (A) PBMCs were transduced using the Ad5f35GFP vector or the Ad5GFP vector at the MOIs indicated. The transduction efficiency was assessed based on percent GFP-positive cells. (B) Cells becoming transduced with the Ad5f35GFP vector were analyzed by isolating CD14+ cells, CD19+ cells, and CD3+ cells, transducing them with the Ad5f35GFP vector at MOI 200 and then assessing GFP expression after 24 hours. (C) PBMCs transduced with the Ad5f35GFP (▪) and Ad5GFP (▨) vectors were used to stimulate PBMCs in ELISPOT assay. Results are shown as SFCs per 106 PBMCs. Error bars indicate the standard error of 3 experiments.
To determine the Ad-specific CTL precursor frequency in CTL lines, autologous LCLs were transduced with Ad5f35GFP at an MOI of 500 and used as stimulators after being irradiated at 40 Gy; negative controls used were nontranduced LCLs. Responder CTLs were serially diluted from 4 × 103 to 5 × 102 cells per well and plated with 1 × 105 stimulators per well in 200 μL ELISPOT medium. After 20 hours of incubation, plates were developed as previously described.34 After overnight drying at room temperature in the dark, plates were sent for evaluation to Zellnet Consulting, New York, NY. Spot-forming cells and input cell numbers were plotted, and a linear regression was calculated after excluding plateau data points. The adenovirus-specific precursor frequency was expressed as specific spot-forming cells (SFCs) after subtracting the background (ie, the frequency of unstimulated responding cells).
Cytotoxicity assay
A standard chromium 51 release assay was performed to assess the cytolytic activity of responders.31 Target LCL cells were either nontransduced or transduced with Ad5f35GFP at an MOI of 500 and used 24 hours after transduction. Target fibroblasts were infected with adenovirus at an MOI of 1 to 5 either 48 hours or as indicated before the assay and then treated with 100 U/mL IFN-γ (R&D Systems, Minneapolis, MN). Mock-infected and allogeneic targets were used as controls. The percent specific lysis was calculated as ([experimental release - spontaneous release]/[maximum release - spontaneous release]) × 100. Statistical analysis (Wilcoxon signed rank test) was performed using GB-STAT (Dynamic Microsystems, Silver Spring, MD).
Results
Adenovirus-specific T cells can be detected ex vivo in both the CD4 and CD8 T-cell compartments
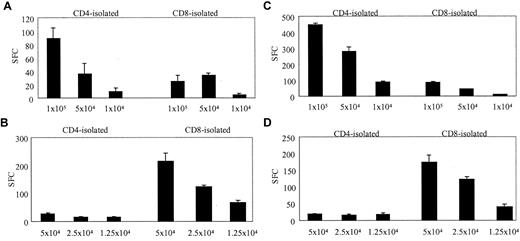
To determine the frequency and phenotype of T cells that would respond to a replication-defective Ad vector, we stimulated PBMCs with autologous DCs transduced with Ad5f35. DCs were incubated for 2 hours with Ad5f35GFP on day 7 or 8 of culture and then mixed immediately with PBMCs at a 10:1 PBMC/DC ratio in IFN-γ ELISPOT plates. The magnitude and phenotype of the Ad-specific response in 6 healthy donors were compared with their response to autologous EBV-transformed LCLs. Figure 1 illustrates that 2 representative donors, donor 4 (Figure 1A-B) and donor 5 (Figure 1C-D), showed significant reactivity against Ad and EBV. In each case the Ad-specific T-cell response was detected predominantly in the CD4+ T-cell compartment, while the EBV-specific response was detected primarily in the CD8+ fraction. In the 6 donors tested, the magnitude of Ad-specific responses ranged from 1120 to 5600 CD4 T cells per 106 isolated cells and 240 to 2880 CD8 T cells per 106 isolated cells, while the EBV-specific responses ranged from 200 to 3960 CD4 T cells per 106 isolated cells and 680 to 12 040 CD8 T cells per 106 isolated cells (Table 1), and in all but 1 donor the Ad-specific response was predominantly in the CD4 compartment. While the magnitude of the CD4 response to Ad5f35GFP was comparable to the CD4 response to EBV-LCL, the Ad-specific response was weaker than the EBV response in the CD8 compartment.
Identification of Ad-specific and EBV-specific T cells ex vivo using the ELISPOT assay of IFN-γ release. CD4-isolated and CD8-isolated T cells from 2 healthy seropositive donors, donor 4 and donor 5, were stimulated with autologous Ad5f35GFP-transduced DCs. T cells were used at different cell concentrations starting at 1 × 105 per well in the case of Ad-specific responses and 5 × 104 per well to detect EBV-specific responses. The assay was performed on triplicate samples, and the results shown, expressed as spot-forming cells (SFCs), are the averages of these triplicates, which had been corrected for background assessed using control wells containing T cells alone, APCs alone and, in the case of the Ad-specific response, nontransduced DCs plus T cells (mean, 142 SFCs per 105 cells for donor 4; and mean, 30.5 SFCs per 105 for donor 5). (A) The Ad-specific response detected ex vivo from donor 4. (B) The EBV-specific response detected from donor 4. (C-D) The Ad- and EBV-specific responses, respectively, detected from donor 5. Error bars indicate the standard error of 3 experiments.
Identification of Ad-specific and EBV-specific T cells ex vivo using the ELISPOT assay of IFN-γ release. CD4-isolated and CD8-isolated T cells from 2 healthy seropositive donors, donor 4 and donor 5, were stimulated with autologous Ad5f35GFP-transduced DCs. T cells were used at different cell concentrations starting at 1 × 105 per well in the case of Ad-specific responses and 5 × 104 per well to detect EBV-specific responses. The assay was performed on triplicate samples, and the results shown, expressed as spot-forming cells (SFCs), are the averages of these triplicates, which had been corrected for background assessed using control wells containing T cells alone, APCs alone and, in the case of the Ad-specific response, nontransduced DCs plus T cells (mean, 142 SFCs per 105 cells for donor 4; and mean, 30.5 SFCs per 105 for donor 5). (A) The Ad-specific response detected ex vivo from donor 4. (B) The EBV-specific response detected from donor 4. (C-D) The Ad- and EBV-specific responses, respectively, detected from donor 5. Error bars indicate the standard error of 3 experiments.
Ad- and EBV-specific precursor frequencies/106 T cells
| . | Adenovirus . | . | EBV . | . | ||
|---|---|---|---|---|---|---|
| Donor . | CD4 isolated . | CD8 isolated . | CD4 isolated . | CD8 isolated . | ||
| 1 | nt | nt | 3180 | 6 080 | ||
| 2 | 1320 | 500 | 780 | 6 380 | ||
| 3 | 1640 | 240 | 0 (seronegative) | 0 (seronegative) | ||
| 4 | 1860 | 680 | 540 | 4 300 | ||
| 5 | 5600 | 900 | 380 | 3 460 | ||
| 6 | nt | nt | 3060 | 12 040 | ||
| 7 | 2020 | 2880 | 380 | 4 100 | ||
| 8 | 1120 | 540 | nt | nt | ||
| 9 | nt | nt | 3200 | 6 500 | ||
| 10 | nt | nt | 3960 | 4 840 | ||
| 11 | nt | nt | 200 | 680 | ||
| 12 | nt | nt | 3420 | 9 200 | ||
| . | Adenovirus . | . | EBV . | . | ||
|---|---|---|---|---|---|---|
| Donor . | CD4 isolated . | CD8 isolated . | CD4 isolated . | CD8 isolated . | ||
| 1 | nt | nt | 3180 | 6 080 | ||
| 2 | 1320 | 500 | 780 | 6 380 | ||
| 3 | 1640 | 240 | 0 (seronegative) | 0 (seronegative) | ||
| 4 | 1860 | 680 | 540 | 4 300 | ||
| 5 | 5600 | 900 | 380 | 3 460 | ||
| 6 | nt | nt | 3060 | 12 040 | ||
| 7 | 2020 | 2880 | 380 | 4 100 | ||
| 8 | 1120 | 540 | nt | nt | ||
| 9 | nt | nt | 3200 | 6 500 | ||
| 10 | nt | nt | 3960 | 4 840 | ||
| 11 | nt | nt | 200 | 680 | ||
| 12 | nt | nt | 3420 | 9 200 | ||
nt indicates not tested.
Generation of Ad-specific CTLs for immunotherapy protocols
To determine if the Ad-specific, IFN-γ-secreting cells detected by ELISPOT could be restimulated and expanded in vitro and whether they had cytolytic activity, we cocultured PBMCs with autologous, Ad5f35GFP-transduced DCs at a responder-stimulator (R/S) ratio of 10:1 on day 0 and day 9 of culture. For the expansion of the Ad-specific T cells we switched to autologous Ad5f35GFP-transduced LCLs at an R/S ratio of 4:1 as stimulators, as these provided an unlimited supply of excellent antigen-presenting cells (APCs).34 Although the transduction efficiency of LCLs, using an MOI of 500, varied from 20% to 70% in a donor-dependent manner, they consistently produced at least 10-fold expansion of cells after 3 stimulations. The availability of the modified fiber of Ad5f35 was critical to this step, because the original Ad5 transduces LCLs poorly and with high toxicity.34 Isolated CD4 and CD8 T cells showed more than 95% purity by fluorescence-activated cell sorter (FACS) analysis and were used in ELISPOT and cytotoxicity assays to assess function. Figure 2 shows, in 2 representative lines from 10 donors studied, that both CD4 and CD8 T cells secreted IFN-γ in an Ad-specific manner (Figure 2Ai,Bi) and demonstrated statistically significant Ad-specific killing (Figure 2Aii, Aiii, Bii, Biii): P less than .01 for all samples; P equal to .017 for CD4 T-cell killing of autologous targets transduced with Ad in donor 12. Both lines were predominantly (more than 70%) CD4+, but Ad-specific CD8+ T cells were also expanded. Notably, no green fluorescent protein (GFP)-specific T-cell reactivity was detected in these CTL lines, and although Ad5f35GFP-transduced LCLs were used as APCs for the third stimulation, no EBV-specific T cells could be detected in either ELISPOT or cytotoxicity assays (Figure 2).
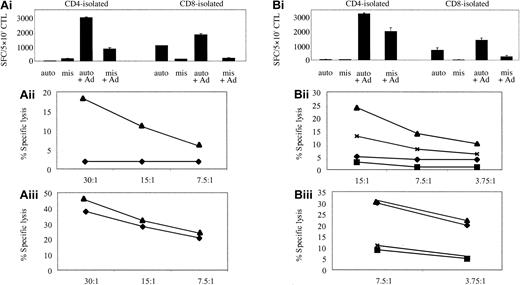
In vitro reactivation of Ad-specific CTL lines. The cell lines, from donors 1 (A) and 12 (B), were generated by PBMC stimulation with Ad5f35GFP-transduced DCs. In panels Ai and Bi, ELISPOT assays show the relative specificities of the CD4 and CD8 T cells. T cells were incubated with autologous LCL targets (auto) either alone or transduced with Ad5f35GFP (auto + Ad); negative controls were class I-mismatched LCLs alone (mis) or Ad5f35GFP transduced (mis + Ad). Results are expressed as SFCs per 5 × 103 cells. Panels Aii, Aiii, Bii, and Biii display the ability of these expanded T cells to kill autologous Ad-transduced targets in a standard chromium release assay using the same targets as described in the ELISPOT assay. Results are expressed as percent specific lysis of autologous LCLs alone (♦), allogeneic LCLs alone (▪), Ad5f35GFP-transduced autologous LCLs (▴), or Ad5f35GFP-transduced allogeneic LCLs (x). Panels Aii and Bii show isolated CD4+ T-cell cytotoxicity assay results, and panels Aiii and Biii show CD8-isolated T cells.
In vitro reactivation of Ad-specific CTL lines. The cell lines, from donors 1 (A) and 12 (B), were generated by PBMC stimulation with Ad5f35GFP-transduced DCs. In panels Ai and Bi, ELISPOT assays show the relative specificities of the CD4 and CD8 T cells. T cells were incubated with autologous LCL targets (auto) either alone or transduced with Ad5f35GFP (auto + Ad); negative controls were class I-mismatched LCLs alone (mis) or Ad5f35GFP transduced (mis + Ad). Results are expressed as SFCs per 5 × 103 cells. Panels Aii, Aiii, Bii, and Biii display the ability of these expanded T cells to kill autologous Ad-transduced targets in a standard chromium release assay using the same targets as described in the ELISPOT assay. Results are expressed as percent specific lysis of autologous LCLs alone (♦), allogeneic LCLs alone (▪), Ad5f35GFP-transduced autologous LCLs (▴), or Ad5f35GFP-transduced allogeneic LCLs (x). Panels Aii and Bii show isolated CD4+ T-cell cytotoxicity assay results, and panels Aiii and Biii show CD8-isolated T cells.
Ad-specific T-cell reactivation without DC generation
The generation of DCs to act as stimulator cells requires large blood volumes not always available from pediatric or National Marrow Donor Program (NMDP) donors. Therefore, we sought to determine if DCs were an absolute requirement for the activation of Ad-specific T cells. To determine if Ad5f35GFP-transduced PBMCs were able to stimulate Ad-specific T cells to produce IFN-γ ex vivo, we first asked whether cells contained in the PBMC fraction could be transduced by Ad vectors. We used 2 different vectors, Ad5GFP and Ad5f35GFP, gated on large cells and compared the transduction efficiency of both vectors at a variety of MOIs using GFP expression as a readout. Figure 3A shows GFP expression in PBMCs transduced with the Ad vectors in serial dilutions from 37.5 to 2400 vp per cell. At an MOI of 2400 both vectors caused cytopathic effects. Ad5f35GFP transduced PBMCs more efficiently than Ad5GFP; more than 30% of gated cells expressed GFP from Ad5f35GFP transduction at an MOI of 37.5, and a plateau of about 48% GFP-positive cells was reached at an MOI of 600 (Figure 3A, upper panel). By contrast, less that 8% of gated cells expressed GFP at an MOI of 37.5 when the Ad5GFP vector was used, and the maximum expression that could be achieved without toxicity was 35% at an MOI of 1200 (Figure 3A, lower panel).
To determine which cells were transduced by the Ad5f35 vector, CD14+ (monocytes), CD19+ (B cells), and CD3+ (T) cells were isolated from a PBMC fraction and transduced with Ad5f35GFP at an MOI of 200; 24 hours later these samples were assessed for GFP expression (Figure 3B). Only the CD14+ cells were transduced, and therefore we looked at their ability to stimulate Ad-specific memory T cells in an IFN-γ ELISPOT assay. Figure 3C shows that the transduced PBMC population provided both stimulators (monocytes) and responders (T cells). Ad5f35GFP was more efficient at stimulating T cells than Ad5GFP, being capable of inducing approximately 500 specific T cells per 106 PBMCs to secrete IFN-γ at an MOI of 150, while the Ad5 vector was able to stimulate a comparable response only at MOI of 1200. While transduced monocytes, the precursor of DCs, are likely responsible for stimulating specific T cells to secrete IFN-γ, we cannot exclude the possibility that other cells take up exogenous vector for processing and presentation to specific T cells. This observation will be of substantial pragmatic importance for the serial monitoring of CTL function after infusion, because only small blood volumes with low lymphocyte yields are available after hematopoietic stem cell transplantation.
Ad-specific CTLs can be reactivated and expanded without using DCs
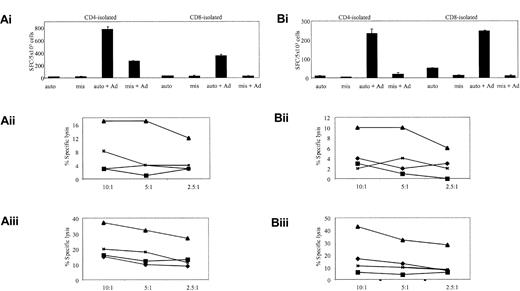
To determine if this method could be used for activating and expanding Ad-specific CTL lines, thus avoiding the requirement for DCs altogether, we used Ad5f35GFP-transduced autologous LCLs, at a R/S ratio of 4:1, for the second and subsequent stimulations of reactivated T cells. Then phenotype, specificity, and function were assessed. Again these lines were predominantly CD4 (more than 60%). Because Ad5f35GFP-transduced LCLs were introduced earlier, at the second stimulation, the CTL lines contained both an EBV- and Ad-specific component. Figure 4Ai shows that the EBV-specific T-cell response in donor 4 was predominantly CD8+, while the Ad-specific response was largely CD4+, with some Ad specificity also mapping to the isolated CD8 fraction. Similar results were obtained for donor 5 (Figure 4B). Both the frequency of IFN-γ-secreting cells and cytotoxic activity were in the range seen using DCs as APCs (Figure 2), validating this method for the detection and expansion of authentic virus-specific T cells. Notably, no Ad-specific cytotoxicity could be detected in the CD8-isolated T-cell populations from donors 4 and 5 (Figure 4Aiii,Biii) over the EBV-specific response. However, when fibroblast targets were available, Ad-specific, CD8 T-cell killing could be detected (data not shown). Thus, apart from the presence of an EBV-specific component, the characteristics of the CTL lines generated in this way did not differ dramatically from those observed earlier (Figure 2).
Ad5f35- and LCL-stimulated PBMCs activate both Ad- and EBV-specific T cells. (Ai) Donor 4 and (Bi) donor 5 represent ELISPOT assays to assess the relative specificities of the CD4 and CD8 T cells expanded by 1 stimulation with Ad5f35GFP-transduced PBMCs, followed by 2 stimulations with Ad5f35GFP-transduced autologous LCLs. T cells were incubated with autologous LCL targets (auto) either alone or transduced with Ad5f35GFP (auto + Ad); negative controls were class I-mismatched LCLs alone (mis) or Ad5f35GFP transduced (mis + Ad). Results are expressed as SFCs per 5 × 103 cells. Panels Aii, Aiii, Bii, and Biii display the ability of these expanded T cells to specifically kill autologous Ad-transduced targets in a standard chromium release assay using the same targets as described in the ELISPOT assay, and results are expressed as percent specific lysis. Results are expressed as percent specific lysis of autologous LCLs alone (♦), allogeneic LCLs alone (▪), Ad5f35GFP-transduced autologous LCLs (▴), or Ad5f35GFP-transduced allogeneic LCLs (×). Panels Aii and Bii show isolated CD4+ T-cell cytotoxicity assay results, and panels Aiii and Biii show CD8-isolated T cells.
Ad5f35- and LCL-stimulated PBMCs activate both Ad- and EBV-specific T cells. (Ai) Donor 4 and (Bi) donor 5 represent ELISPOT assays to assess the relative specificities of the CD4 and CD8 T cells expanded by 1 stimulation with Ad5f35GFP-transduced PBMCs, followed by 2 stimulations with Ad5f35GFP-transduced autologous LCLs. T cells were incubated with autologous LCL targets (auto) either alone or transduced with Ad5f35GFP (auto + Ad); negative controls were class I-mismatched LCLs alone (mis) or Ad5f35GFP transduced (mis + Ad). Results are expressed as SFCs per 5 × 103 cells. Panels Aii, Aiii, Bii, and Biii display the ability of these expanded T cells to specifically kill autologous Ad-transduced targets in a standard chromium release assay using the same targets as described in the ELISPOT assay, and results are expressed as percent specific lysis. Results are expressed as percent specific lysis of autologous LCLs alone (♦), allogeneic LCLs alone (▪), Ad5f35GFP-transduced autologous LCLs (▴), or Ad5f35GFP-transduced allogeneic LCLs (×). Panels Aii and Bii show isolated CD4+ T-cell cytotoxicity assay results, and panels Aiii and Biii show CD8-isolated T cells.
Monitoring infused Ad-specific CTL lines following infusion
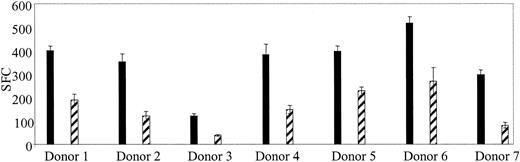
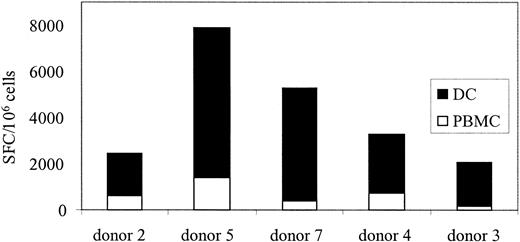
If CTLs are used in a clinical trial to treat Ad infections, a means to monitor their function, persistence, and expansion in vivo will be required. After stem cell transplantation, lymphocyte counts are low, and because children are at highest risk for Ad infections a test using small blood volumes is required. We showed, in Figure 3C, that Ad-specific T cells could be reactivated and stimulated to produce IFN-γ ex vivo using Ad5f35GFP-transduced PBMCs. So, to determine if Ad-specific T-cell immunity could be quantitated in PBMCs without DCs, 7 additional healthy seropositive donors were screened by transducing their PBMCs with Ad5f35GFP and then measuring IFN-γ production by ELISPOT. Figure 5 shows that responses ranged from 190 to 1345 Ad-specific T cells per 106 PBMCs. By comparison, the Ad-specific T cell precursor frequency in response to Ad5f35GFP-transduced DCs at the optimal R/S ratio was 1360 to 8480 specific T cells per 106 isolated cells (Table 1). Therefore, this method underestimates the true Ad-specific T-cell frequency by about 1 log, as demonstrated in Figure 6 where 5 donors screened for Ad-specific precursors ex vivo using both methods are compared. However, using this modified method, we will be able to measure relative changes in frequency of Ad-specific CTLs resulting from CTL infusion, even from small blood volumes.
Ad-specific T-cell frequency can be detected ex vivo from healthy seropositive donors. PBMCs were isolated from 7 seropositive donors and screened for Ad-specific T cells without prior stimulation. Responses for each donor are shown at 4 × 105 PBMCs (▪) and 2 × 105 (▨). Error bars indicate the standard error of 3 experiments.
Ad-specific T-cell frequency can be detected ex vivo from healthy seropositive donors. PBMCs were isolated from 7 seropositive donors and screened for Ad-specific T cells without prior stimulation. Responses for each donor are shown at 4 × 105 PBMCs (▪) and 2 × 105 (▨). Error bars indicate the standard error of 3 experiments.
Ad-specific T-cell frequency detected from PBMCs underestimates the true Ad frequency by a log. PBMCs from 5 seropositive donors were screened for Ad-specific T cells without prior stimulation, using either Ad5f35GFP-transduced DCs (▪) or PBMCs (□).
Ad-specific T-cell frequency detected from PBMCs underestimates the true Ad frequency by a log. PBMCs from 5 seropositive donors were screened for Ad-specific T cells without prior stimulation, using either Ad5f35GFP-transduced DCs (▪) or PBMCs (□).
Cross-reactivity of adenovirus-specific CTL lines
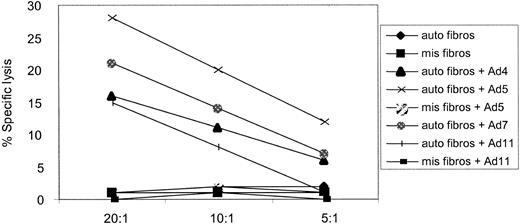
In total, there are 51 different adenovirus serotypes, in 6 distinct groups. A variety of these serotypes have been isolated from patients suffering from Ad infections after HSCT. We therefore wanted to determine whether a CTL line generated using DCs transduced with an Ad5f35 (Ad type 5 vector backbone) would also kill target cells infected with serotypes from different subgroups. We used a CTL line, from donor 2, in a cytotoxicity assay against autologous fibroblasts infected with Ad2 (subgroup C), Ad4 (subgroup E), Ad5f35 (chimeric subgroups C and B), Ad7, and Ad11 (subgroup B). Allogeneic, HLA-mismatched fibroblasts, either alone or infected with Ad5f35GFP and Ad11, and autologous uninfected fibroblasts were not killed. However, the autologous targets infected with all wild-type Ad serotypes tested were recognized and killed (Figure 7). Results from 3 of 3 CTL lines tested so far have confirmed this result (data not shown) and validate the use of one adenovirus serotype to generate subgroup and serotype cross-reactive CTL lines for patient infusion.
Ad-specific CTL lines are capable of subgroup and serotype cross-reactive recognition. An in vitro-reactivated CTL line from donor 2, generated using the Ad5f35 vector, is capable of recognizing and killing autologous fibroblast targets (▪) transduced with Ad2 (subgroup C), Ad4 (subgroup E), Ad5 (chimeric subgroups C and B), Ad7, and Ad11 (subgroup B) but not autologous fibroblasts alone or allogeneic targets infected with Ad5 and Ad11 at 3 effector-target (E/T) ratios: 20:1, 10:1, and 5:1.
Ad-specific CTL lines are capable of subgroup and serotype cross-reactive recognition. An in vitro-reactivated CTL line from donor 2, generated using the Ad5f35 vector, is capable of recognizing and killing autologous fibroblast targets (▪) transduced with Ad2 (subgroup C), Ad4 (subgroup E), Ad5 (chimeric subgroups C and B), Ad7, and Ad11 (subgroup B) but not autologous fibroblasts alone or allogeneic targets infected with Ad5 and Ad11 at 3 effector-target (E/T) ratios: 20:1, 10:1, and 5:1.
Discussion
Adenovirus infections, particularly in pediatric HSCT recipients, continue to be responsible for significant levels of morbidity and mortality.4 In a recent report of 132 consecutive pediatric patients, 27% tested positive for Ad by polymerase chain reaction (PCR), and 73% of patients with detectable Ad in peripheral blood developed fatal disseminated Ad disease.6 Because no effective antiviral drugs are available, the present study was motivated by the potential for using adoptive immunotherapy, either as prophylaxis for patients at risk for developing adenoviral infections after transplantation or as treatment for patients suffering from adenovirus disease.4,6 Any effective treatment will first require a substantial increase in our understanding of virus immune control in healthy individuals. Here we demonstrate that T cells specific for Ad capsid antigens are detectable ex vivo and are composed of both a CD4 and CD8 component, although the response is predominantly CD4. Ad-specific CTL lines can be reactivated in vitro without a requirement for DCs as APCs and expanded to levels necessary for patient infusion. Importantly, resultant CTL lines are capable of recognizing Ads of other serotypes and from different subgroup families, thus validating this method of CTL generation for general clinical application.
We first addressed the question of whether Ad-specific T cells could be detected in healthy seropositive donors using precise assays for the measurement of T-cell responses ex vivo. As a control, we measured the response to EBV in the same individuals to use as a reference for comparing the magnitude and compartmentalization of Ad-specific T-cell immunity. We utilized an Ad vector, Ad5f35, to detect specific T-cell immunity because this vector had previously been shown to have a broad cell tropism.32,33 Ad-specific T cells were found mainly in the CD4+ T-cell fraction, in accordance with other reports,21,22 but specific CD8+ T cells could also be detected. In contrast, when the cellular immune response directed against EBV latent antigens was assessed in the same individuals, EBV-specific T-cell memory was detected mainly in the CD8 T-cell compartment.
Compartmentalization of Ad- and EBV-specific T cells in the CD4 and CD8 fractions, respectively, may reflect the virus tropism in vivo; EBV antigens are expressed endogenously in infected B cells, processed into peptides, and presented preferentially on HLA class I molecules for recognition by specific CD8+ T cells,38 whereas Ads preferentially infect non-APCs,1,2 so the main stimulation may come from cross-priming. However, we cannot rule out a bias in the experimental set-up based on the different APCs and exogenous versus endogenous sources of antigen used to stimulate the Ad- and EBV-specific T cells, respectively. Furthermore, this study is limited to analysis of T-cell immunity against the Ad capsid proteins. However, because adenoviruses encode multiple early gene products dedicated to evading the immune system,39 we suggest that only the immediate early proteins, which were not analyzed in the present study,2 are alternative T-cell targets. Ad vectors, widely available as a clinical-grade product, have a number of features that make them useful tools for this type of analysis. They are internalized into the endosomes by receptor-mediated endocytosis. From here the vector escapes from the endosomal/lysosomal pathway where virion proteins have the potential to access the HLA class II pathway, into the cytosol where antigen becomes available for proteasomal processing and entry into the HLA class I pathway.2 Thus we have the potential to reactivate circulating specific CD4 and CD8 T cells. Ultimately the question of whether virion-specific T cells can provide protective immunity in humans can be tested only in a clinical trial.
The magnitude of the EBV-specific T-cell response detected by ELISPOT assay (Figure 1) was consistently greater than the magnitude of the Ad-specific response detected in the same individuals. In 6 seropositive donors screened for Ad-specific precursors, the magnitude of the response was found to be 1360 to 8480 Ad-specific T cells per 106 isolated cells, while the EBV precursor frequency ranged from 880 to 16 000 specific CD8 T cells per 106 (Table 1). These differences are likely attributable to the fact that EBV is a persistent virus that provides chronic stimulation to circulating memory T cells. In contrast, Ad infections are cleared by the immune response. However, repeated exposure over time is perhaps responsible for retaining the circulating memory T-cell response at this relatively high level. Interestingly, a recent study of the magnitude of CD8+ T-cell responses directed against influenza viruses, RNA viruses that cause annual epidemics reminiscent of Ad, reported a mean frequency of 2800 influenza-specific CD8 T cells per 106 cells,40 which is within the range that we can detect with Ad, adding further weight to this hypothesis. In addition, there have been reports of a latency associated with Ad, which may be a contributing factor, but as yet the reservoir for this latent state has not been described.1
Having detected Ad-specific memory T cells ex vivo, we wanted to address whether we could reactivate and expand such cells in vitro to prepare therapeutic lines for infusion. To this end, we stimulated PBMCs with Ad5f35GFP-transduced DCs and expanded virus-specific cells using Ad5f35GFP-transduced autologous LCLs. These lines displayed recognition of the Ad5f35 vector in an autologous but not in an allogeneic setting (Figure 2) and were shown to contain Ad-specific CD4 and CD8 T-cell components. Interestingly, the lines were always predominantly CD4+, reflecting the ex vivo components of Ad-specific immunity (Figure 1). The CD4-restricted Ad-specific IFN-γ secretion in response to allogeneic Ad5f35GFP-transduced targets (Figure 2Ai) may be due to identities in the HLA antigens not analyzed in our HLA typing panel, such as HLA-DP. Importantly, the cytolytic activity detected in both Ad-specific CD4 and CD8 T cells suggests that CD4+ T cells may function not just as helper cells but also in the clearance of infection.41 In Figure 1 we established that Ad-specific T cells could be detected ex vivo using DCs as APCs and isolated CD4 and CD8 T cells as responders in an ELISPOT assay. However, it may not always be feasible to use this method for generating Ad-specific CTL lines for patients, because blood volumes are a limiting factor and DC production requires large blood volumes and is time consuming and expensive. Therefore, in an effort to simplify the CTL generation protocol, we attempted to reactivate Ad-specific CTL lines in vitro without the DCs. We confirmed that PBMCs provide both stimulators and responders to the Ad5GFP and Ad5f35GFP vectors (Figure 3A) and identified monocytes as the main targets for Ad vectors (Figure 3B). As expected, the Ad5f35GFP vector was more efficient at transducing hematopoietic cells than the Ad5GFP vector because the Ad5 virus has an absolute requirement for the expression of the coxsackie-adenovirus receptor (CAR) on target cells. In contrast, the Ad5f35 receptor is currently unknown but appears to be more broadly expressed on a variety of different cell types.33 Importantly, Ad5f35- and Ad5-transduced monocytes were able to induce Ad-specific memory T cells to secrete IFN-γ, but the Ad5f35 vector could induce optimal T-cell reactivity at a lower MOI than the Ad5 vector, again most likely due to the differential infectivity of the vectors.
Using Ad5f35GFP-transduced PBMCs as the initial stimulus, followed by 2 stimulations with autologous, Ad5f35GFP-transduced LCLs, specific reactivity against both EBV and Ad was expanded.24 This was not surprising since (1) more than 90% of individuals are EBV seropositive and will therefore have preexisting immunity, (2) the ex vivo precursor frequency of LCL-reactive T cells is higher than the Ad-specific T cell precursor frequency, and (3) these CTL lines were exposed to EBV antigens at day 9 when viable EBV-specific memory T cells must remain in the culture. Importantly, the Ad-specific response in these bispecific CTL lines was detectable in both CD4- and CD8-isolated T-cell fractions, while the EBV-specific T-cell response was mainly detected in the CD8+ T-cell compartment. Because EBV-specific CD4+ T-cell epitopes have been previously identified,35,36 the lack of EBV-specific CD4 response may reflect the manner of antigen presentation or competition for class II molecules from Ad proteins, which may be overrepresented in the endosomal compartment. However, the infusion of bispecific CTL lines into HSCT patients should, in fact, be advantageous to the patient because EBV as well as Ad reactivations are common problems in this patient cohort.
Following CTL infusion an effective monitoring system to catalog the ability of infused T cells to reconstitute immunity to Ads and to evaluate persistence is essential for evaluation purposes. We used Ad5f35GFP-transduced PBMCs to screen 7 seropositive donors for detectable Ad-specific T-cell reactivity ex vivo and found that the responses detected ranged from 190 to 1345 Ad-specific T cells per 106 PBMCs. In contrast, the magnitude of Ad-specific responses detected using DCs as stimulators at an optimal R/S ratio detected 1360 to 8480 Ad-specific T cells per 106 T cells. There are 2 possible explanations for this: (1) Monocytes are not as efficient as DCs at antigen uptake, processing, and presentation, and/or (2) the monocyte fraction in PBMCs, generally 3% to 5%, does not offer the optimal R/S for T-cell stimulation. However, using Ad5f35GFP-transduced PBMCs we can monitor the relative frequencies of Ad-specific T cells and look for changes in the magnitude of the response before and over time after CTL infusion.
In total, there are 6 different human adenovirus subgroups containing 51 different serotypes. In retrospective studies of transplant recipients a spectrum of serotypes including 2, 5, 7, 9, 11, 34, and 35 have been found to be responsible for disease.5,6,42 Any immunotherapeutic approach to patient treatment must control Ad infections caused by a broad range of serotypes. We therefore analyzed whether CTL lines, generated using an Ad5f35 vector, could recognize and lyse target cells infected with a cross-section of different wild-type virus serotypes from various subgroups. Using autologous and allogeneic fibroblast targets we demonstrated that CTL lines generated using the Ad5f35 vector can lyse target cells infected with Ad2 (subgroup C), Ad4 (subgroup E), Ad5f35 (chimeric subgroups B and C), Ad7 (subgroup B), and Ad11 (subgroup B) in an HLA-restricted manner (Figure 7). We had previously demonstrated similar cross-reactivity; however, the CTLs reported at that time killed only in an 18-hour cytotoxicity assay,30 and significant recognition of Ad-infected allogeneic targets was also detected. In our hands cross-reactive recognition of Ad can be detected in a standard chromium release assay and only in an autologous setting.
We conclude from our present work that Ad is a good target for immunotherapy. Specific CTL lines can be simply and reproducibly generated in vitro using small blood volumes and a clinical-grade Ad5f35 vector to reactivate memory T cells. We can demonstrate that Ad-specific CD4 and CD8 T cells are expanded, both of which are capable of specific IFN-γ secretion and lytic activity. Most important, these CTL lines demonstrate serotype and subgroup cross-reactive recognition, implying that the virion proteins are responsible for generating at least some of our in vivo T-cell memory response to Ad and that T-cell epitopes that are recognized are conserved between different serotypes. Thus, Ad immunotherapy is a practical objective that will determine whether virion proteins provide protective CTL epitopes.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-07-2449.
Supported by grants from the National Institutes of Health (NIH1RO1 CA61384) and the Dana Foundation to C.M.R. and the Center for Cell and Gene Therapy. B.S. is supported by the Elizabeth Glaser Pediatric Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Helen Huls for excellent technical assistance, Stephen Gottschalk for critical reading of the manuscript, and Tatiana Goltsova for flow cytometric analysis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal