Abstract
Patients with chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) typically exhibit thymic hypoplasia, conotruncal cardiac defects, and hypoparathyroidism. The immunodeficiency that results from the thymic hypoplasia has been extensively described and consists primarily of T-cell lymphopenia. A curious feature of the T-cell lymphopenia is that the age-related rate of decline of T-cell numbers is slower in patients than controls. This leads to T-cell numbers in adulthood that are minimally decreased compared with controls. This suggests that homeostatic mechanisms might be acting to preserve the peripheral blood T-cell numbers in patients. We characterized changes in CD4/CD45RA and CD4/CD45RO T-cell populations in patients and controls of various ages and determined T-cell recombination excision circles and telomere length within the CD4/CD45RA population. Patients had evidence of accelerated conversion of naive to memory cells and had evidence of more extensive replicative history within the CD4/CD45RA compartment compared with controls. Oligoclonal T-cell receptor (TCR) Vβ families and missing Vβ families were seen more often in patients than controls. These data are consistent with homeostatic proliferation of T cells in patients with limited T-cell production due to thymic hypoplasia. (Blood. 2004;103:1020-1025)
Introduction
Chromosome 22q11.2 deletion syndrome is a disorder that is classically associated with conotruncal cardiac anomalies, thymic hypoplasia, and hypoparathyriodism.1 DiGeorge syndrome, velocardiofacial syndrome, Opitz/GBBB syndrome, Coloboma, heart defects, atresa of choanae, retardation of growth and development, genital anomalies, ear anomalies (CHARGE) syndrome, and conotruncal anomaly face syndrome have been associated with the deletion. The thymic hypoplasia is seen in more than 80% of the patients with the deletion regardless of the other clinical manifestations of the syndrome. The immunodeficiency arising as a consequence of the thymic hypoplasia in patients with the deletion syndrome has been studied for nearly 50 years, and it is now known to be extremely variable.2-5 The spectrum of immunodeficiency ranges from absent T cells due to thymic aplasia to normal T-cell numbers.2,6 T-cell function is typically preserved, although standard mitogen proliferation assays are usually diminished when the T-cell count is very low, corresponding to the diminished numbers of cells competent to respond to the stimulus.4 Immunoglobulin A (IgA) deficiency, hypogammaglobulinemia, and defects in functional antibody production have been occasionally described and are thought to be secondary to the T-cell defect.7-9
The clinical picture for patients with chromosome 22q11.2 deletion syndrome is similarly diverse. Early descriptions of the syndrome emphasized life-threatening infections; however, most of these patients were ascertained due to absent or nearly absent T cells.3,10 Recent descriptions of populations with the deletion have noted that the very severe immunodeficiency is seen in less than 1% of patients.6,11 In 2 large surveys, none of the deaths were primarily ascribed to the immunodeficiency.6,11 Most patients have a mild to moderate decrement in circulating T-cell numbers and are not predisposed to opportunistic infections. In a survey of infections and T-cell counts in patients with chromosome 22q11.2 deletion syndrome, the majority of young children had diminished T-cell numbers and frequent infections.12 Adults with the deletion were found to have nearly normal numbers of T cells, and approximately one third continued to have recurrent infections.2 Furthermore the rate of age-related decline of T-cell numbers in patients was less than what was seen in healthy controls. Decline in peripheral blood T-cell counts is thought to be due to thymic involution. The slower decline in patients with the deletion was unexpected and suggested that there may be compensatory mechanisms serving to sustain T-cell counts. This study examined one potential mechanism that could sustain T-cell counts in patients with T-cell lymphopenia due to thymic hypoplasia.
Understanding mechanisms underlying T-cell preservation has significance for the patients themselves and for understanding regulation of peripheral T-cell maintenance in settings where reconstitution is required. Chromosome 22q11.2 deletion syndrome is extremely common with a frequency of approximately 1:4000 births.13 While much work has been done to examine the immunodeficiency in childhood, little is known about the long-term natural history of this disorder. There are comparatively few adults available for study because patients with significant cardiac lesions did not survive until the advent of wide-scale cardiac bypass surgery in the 1980s. Patients without cardiac lesions were often not recognized. This is the first study to specifically examine the immune system in older patients with chromosome 22q11.2 deletion syndrome. Our data are suggestive of a homeostatic mechanism that preserves the peripheral blood T-cell count in the face of limited thymic output.
Patients, materials, and methods
Patients
The patients reported in the study were all confirmed as having the chromosome 22q11.2 deletion by fluorescence in situ hybridization with the N25 probe (Vysis, Downers Grove, IL). The controls were recruited over the same time frame and were examined using the same procedures and instrumentation. Controls were excluded for chronic or acute illness. They were generally healthy siblings of patients or unrelated controls recruited from well-child clinics. Patients were excluded if acutely ill. This study was approved by the institutional review board of the Children's Hospital of Philadelphia, and all patients and controls provided informed consent for this study. A subset of 195 patients was reported previously as age quartile T-cell numbers.2 This current study includes 409 patients and 131 age-matched controls.
Flow cytometry
Laboratory analyses were performed in the Clinical Immunology Laboratory or the Immunology Flow Cytometry Core Facility at the Children's Hospital of Philadelphia. Flow cytometry (2- or 3-color) was performed on a Coulter EPICS XL (Birmingham, United Kingdom) (or FACSCalibur [Becton Dickinson, Franklin Lakes, NJ] for recent samples) to define the following lymphocyte populations: CD3+, CD3+/CD4+, CD3+/CD8+, CD4/CD45RA, and CD4/CD45RO. CD14 and CD45 were used to confirm the purity of the lymphocyte population. Absolute counts were obtained by multiplying the lymphocyte subset fraction by the absolute lymphocyte count obtained simultaneously.
Proliferative assays
Proliferative responses to phytohemagglutinin (PHA) were measured by incorporation of [3H] thymidine in triplicate cultures of 3 dilutions of stimulus harvested 72 hours after stimulation. Proliferative responses to candida, tetanus, and diphtheria antigens were measured in triplicate cultures of 3 dilutions of stimulus harvested at 5 days. Both raw counts per minute and stimulation index were recorded for each patient and control.
Cell purification
Peripheral blood mononuclear cells (PBMCs) were obtained from patients and controls using Ficoll-Paque Plus (Amersham Pharmacia, Uppsala, Sweden). PBMCs were washed twice in phosphate-buffered saline (PBS) with 2% fetal bovine serum (FBS) and resuspended at 0.5 × 107 cells/mL. CD4+ T cells were isolated using the CD4 Positive Isolation Kit (Dynal, Oslo, Norway), following the manufacturer's instructions. The supernatant was removed and placed in a separate tube to use for the B-cell-negative isolation. CD4+ T cells were washed and detached from beads using DETACHaBEAD (Dynal). CD45RA/RO cells were isolated from the CD4+ cells using CELLection Pan Mouse IgG Kit (Dynal), following the manufacturer's instructions. Briefly, CELLection beads were prepared by adding 1.5 μg CD45RA antibody to washed beads. CD4+ T cells were added to the prepared beads and incubated for 20 minutes at 2°C to 8°C. The supernatant containing the CD4/CD45RO cells was collected via magnetic separation. CD4/CD45RA cells were recovered by resuspending the beads in DNase Releasing Buffer (Dynal, Lake Success, NY). B cells were isolated using B Cell Negative Isolation Kit (Dynal), following the manufacturer's instructions. Briefly, CD4- cells were counted and resuspended at 1 × 107 cells in 200 μL PBS/0.1% BSA. Antibody mix was added to the cells and incubated for 10 minutes at 4°C. Cells were washed with 1 mL PBS/0.1% BSA and centrifuged for 8 minutes at 500g. The cells were resuspended in 0.9 mL PBS/0.1% BSA with 100 μL washed Depletion Dynabeads (Dynal). The supernatant containing the B cells was removed and beads were washed 3 times with PBS/0.1% BSA. Supernatants were added together and B cells were counted. Each population was approximately 95% pure by flow cytometry.
Replicative history analyses
T-cell recombination excision circles (TRECs) were measured on purified cell populations using real-time polymerase chain reaction (PCR) on an ABI 7700 (Applied Biosystems, Foster City, CA). Our standards were validated repeatedly by comparison with published standards.14,15 The primers and probes were synthesized and used according to previously published methods.16 Purified DNA (100 ng, PureGene; Gentra, Minneapolis, MN) was used per well for analysis. Data are expressed as TRECs per microgram of DNA. Telomere length was determined by digestion of genomic DNA from purified cell populations with HinfI and RsaI overnight. Digested DNA was run on a 0.8% agarose gel along with size standards and transferred to nitrocellulose. The resulting Southern blot was probed with an end-labeled oligo consisting of multiple telomeric repeats ((C3TA2)3). The peak signal was determined by Peak Finder software (Molecular Dynamics, Sunnyvale, CA) on the STORM 840 phosphorimager (Amersham Biosciences, San Francisco, CA). The size corresponding to the peak was defined by calculation from the size standards.
T-cell receptor (TCR) Vβ analysis
CDR3 size was defined by multiplex PCR as described.17,18 Patients were selected based on age (>18 years) and the status of all from having nearly normal T-cell numbers. Controls were matched for age. Data were collected using ABI 310 collection software and were analyzed using Genescan and Genotyper software (Applied Biosystems). Oligoclonal peaks were defined as those having a height more than 2-fold the mean of the surrounding 2 peaks. Vβ family dropout was defined as an undetectable signal for the amplification or less than 10% of the mean of the signals for that Vβ family. Each sample was run with a known normal control to facilitate interpretations of Vβ family dropout.
Results
Peripheral blood T-cell populations
We have previously reported that the slope of age-related decline of T-cell counts in patients with chromosome 22q11.2 deletion syndrome is slower than in age-matched controls.2 The rate of decline in our control group is comparable with that seen in other studies.19 Table 1 demonstrates that the vast majority of infants have T-cell counts below the lower limit of normal for age, while only about half of the adults have T-cell counts that are below the lower limit of normal. Additionally, the mean CD3 and CD4 T-cell counts are approximately two thirds of the control means in infancy, while they are nearly normal in the adult population. Figure 1 illustrates the differences in the decline of the T-cell numbers with age.
Profile of T-cell subsets in patients with chromosome 22q 11.2 deletion syndrome
. | 0 to 12 months of age . | 18 to 50 years of age . |
|---|---|---|
| Patients* | ||
| Mean CD3 count, cells/mm3 | 1996 | 1288 |
| Range of CD3 counts, cells/mm3 | 98-5730 | 645-2709 |
| Patients below lower limit of normal in CD3 count, % | 85 | 47 |
| Mean CD4 count, cells/mm3 | 1380 | 765 |
| Range of CD4 counts, cells/mm3 | 39-3704 | 433-1591 |
| Patients below lower limit of normal in CD4 count, % | 88 | 47 |
| Mean CD8 count, cells/mm3 | 550 | 467 |
| Range of CD8 counts, cells/mm3 | 39-2013 | 136-1161 |
| Patients below lower limit of normal in CD8 count, % | 91 | 50 |
| Controls† | ||
| Mean CD3 count, cells/mm3 | 3889 | 1497 |
| Mean CD4 count, cells/mm3 | 2781 | 945 |
| Mean CD8 count, cells/mm3 | 993 | 512 |
. | 0 to 12 months of age . | 18 to 50 years of age . |
|---|---|---|
| Patients* | ||
| Mean CD3 count, cells/mm3 | 1996 | 1288 |
| Range of CD3 counts, cells/mm3 | 98-5730 | 645-2709 |
| Patients below lower limit of normal in CD3 count, % | 85 | 47 |
| Mean CD4 count, cells/mm3 | 1380 | 765 |
| Range of CD4 counts, cells/mm3 | 39-3704 | 433-1591 |
| Patients below lower limit of normal in CD4 count, % | 88 | 47 |
| Mean CD8 count, cells/mm3 | 550 | 467 |
| Range of CD8 counts, cells/mm3 | 39-2013 | 136-1161 |
| Patients below lower limit of normal in CD8 count, % | 91 | 50 |
| Controls† | ||
| Mean CD3 count, cells/mm3 | 3889 | 1497 |
| Mean CD4 count, cells/mm3 | 2781 | 945 |
| Mean CD8 count, cells/mm3 | 993 | 512 |
For patients 0 to 12 months of age, n = 138; for 18 to 50 years of age, n = 36.
For controls 0 to 12 months of age, n = 31; for 18 to 50 years of age, n = 30.
The decline of CD3 T cells with age in patients and controls. CD3 absolute counts from patients (○) and controls (▪) are displayed according to age in years. CD3 T cells are lower in patients at all ages than in controls but have a slower rate of decline than controls.
The decline of CD3 T cells with age in patients and controls. CD3 absolute counts from patients (○) and controls (▪) are displayed according to age in years. CD3 T cells are lower in patients at all ages than in controls but have a slower rate of decline than controls.
To further examine the kinetics of change of T-cell populations with age, we determined the fraction of CD45RA- and CD45RO-positive cells within the CD4 compartment (Figure 2). Patients clearly have an accelerated decline of CD45RA and an accelerated increase in CD45RO. This could be due to impaired thymic production of naive cells, accelerated conversion of CD4/CD45RA cells to the CD4/CD45RO cell phenotype (due to infectious exposures or homeostatic proliferation), or selective loss or redistribution of the cells with the naive phenotype. Each of these possibilities has distinct implications for the patients.
CD45RA and CD45RO expression within CD4 T cells in patients and controls. The accumulation of CD45RO cells within the CD4 population (expressed as a percent of the CD4 population) is greatly accelerated in patients (A) compared with controls (B).
CD45RA and CD45RO expression within CD4 T cells in patients and controls. The accumulation of CD45RO cells within the CD4 population (expressed as a percent of the CD4 population) is greatly accelerated in patients (A) compared with controls (B).
TRECs are diminished in patients compared with controls
CD4/CD45RA and CD4/CD45RO cells were purified using magnetic bead separation from adolescent and adult patients and controls. Purity was approximately 95%. Approximately half of the patients had TREC analysis performed on both CD4/CD45RA and CD4/CD45RO DNA. In no case did we did detect TRECs in the CD4/CD45RO population. Figure 3 demonstrates that the TRECs in CD4/CD45RA cells are diminished at all ages in patients compared with controls. Most of the patients were young adults, and the mean TREC counts for patients aged 18 to 30 years was 7491 TRECs per microgram of DNA from CD4/CD45RA purified cells compared with 19 594 TRECs per microgram of DNA from CD4/CD45RA purified cells from controls aged 18 to 30 years (Wilcoxon P = .0017).
TRECs within the CD4/CD45RA purified population. TREC content within the CD4/CD45RA purified population is expressed per microgram of DNA. Patients have diminished TRECS compared with controls.
TRECs within the CD4/CD45RA purified population. TREC content within the CD4/CD45RA purified population is expressed per microgram of DNA. Patients have diminished TRECS compared with controls.
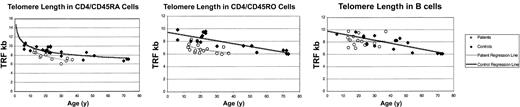
Telomere analysis of replicative history
The slow decline in T-cell numbers, the accelerated conversion of CD4/CD45RA to CD4/CD45RO, and the diminished TREC numbers are all suggestive of homeostatic proliferation. We measured TRECs within a purified CD4/CD45RA T-cell population; however, it is still difficult to distinguish between the impaired thymic output that is known to occur in this syndrome and increased proliferation in the periphery. To confirm that patient cells experience more rounds of replication than controls, we also examined telomere length in purified CD4/CD45RA T cells and CD4/CD45RO T cells (Figure 4). The mean and median telomere length within the CD4/CD45RA population from patients aged 20 to 30 years (n = 7) was 7.8 kb and 8.1 kb, respectively, whereas in controls (n = 10) it was 9.0 kb and 9.0 kb, respectively (Wilcoxon P = .05). The mean and median telomere length within the CD4/CD45RO population from patients aged 20 to 30 years was 6.5 kb and 6.5 kb, respectively, while in controls it was 8.2 kb and 8.3 kb, respectively (Wilcoxon P = .01). The expected difference in the telomere length between the CD4/CD45A and CD4/CD45RO populations was seen. CD45RO cells are typically about 1.4 kb shorter because they have proliferated on exposure to antigen during the conversion from naive to memory phenotype.20 The difference in patient and control telomere length in the CD4/CD45RA population corresponds to roughly 5 cell divisions.21-24 To ensure the phenomenon was T-cell specific, as expected, telomeres were measured in purified B cells (Figure 4). The mean and median telomere length within the B-cell population from patients aged 20 to 30 years was 8.3 kb and 8.1 kb, respectively, whereas in controls it was 8.3 kb and 8.3 kb, respectively (Wilcoxon P = .95).
Terminal restriction fragment (TRF) length was defined by Southern blot. TRFs were shorter in patients than controls in both CD4/CD45RA and CD4/CD45RO populations. TRFs were equivalent in B cells.
Terminal restriction fragment (TRF) length was defined by Southern blot. TRFs were shorter in patients than controls in both CD4/CD45RA and CD4/CD45RO populations. TRFs were equivalent in B cells.
TCR Vβ family analysis
One prediction arises from the data presented. The young adult patients had nearly normal T-cell numbers on average. This could be due to delayed involution of the thymus and would thus represent normal T-cell development via a “catch-up” mechanism. The other possibility is that the thymic output was poor and peripheral T cells proliferated via a homeostatic mechanism. If homeostatic expansion occurred to compensate for poor thymic output, then the T-cell repertoire should reflect the consequence of peripheral proliferation as opposed to a repertoire that arose within the thymus. Multiplex PCR was used to evaluate CDR3 size variability in patients and controls. Although patients were selected with normal or nearly normal T-cell numbers to eliminate a dilution effect, patients had twice the number of oligoclonal bands as controls (Wilcoxon P = .179) and 10 times the number of missing Vβ families (Wilcoxon P = .0008) (Table 2). Within the patient group, there was no correlation between number of oligoclonal Vβ families and CD3, CD4, or CD4/CD45RA T-cell counts, nor was there any correlation with TRECs.
T-cell repertoire analysis
. | Age, y . | Mean CD3 count . | Mean CD4 count . | Mean no. of oligoclonal families . | Mean no. of missing Vβ families . |
|---|---|---|---|---|---|
| Patients; n = 7 | 18-37 | 1232 ± 726 | 718 ± 452 | 5.3 | 2.7 |
| Controls; n = 11 | 20-32 | 1490 ± 540 | 851 ± 417 | 2.4 | 0.3 |
| P | .195 | .391 | .179 | .0008 |
. | Age, y . | Mean CD3 count . | Mean CD4 count . | Mean no. of oligoclonal families . | Mean no. of missing Vβ families . |
|---|---|---|---|---|---|
| Patients; n = 7 | 18-37 | 1232 ± 726 | 718 ± 452 | 5.3 | 2.7 |
| Controls; n = 11 | 20-32 | 1490 ± 540 | 851 ± 417 | 2.4 | 0.3 |
| P | .195 | .391 | .179 | .0008 |
The telomere analyses suggested that the CD4/CD45RO population had telomeres near the critical minimal length to support proliferation. We therefore compared proliferative responses in adult patients and controls. Patient (n = 35) mean counts per minute (cpm) responses ± standard deviation to PHA were 59 760 ± 30 185 compared with controls (n = 11) with 66 288 ± 34 180 (P = .55). However, when responses to specific antigens were measured, patients had diminished responses. Patient (n = 31) responses to candida were 4382 ± 4350 cpm, whereas control (n = 10) responses were 8862 ± 6466 cpm (Wilcoxon P = .02). Similarly, patient responses to tetanus were 11 863 ± 11 322 cpm, while control responses were 16 792 ± 7910 cpm (Wilcoxon P = .03). Responses to diphtheria were 10 983 ± 11 478 cpm for patients, while control responses were 13 065 ± 6837 (P = .59). Control cultures in the absence of stimulation were run with each type of stimulation and were comparable between patients and controls.
Discussion
It has previously been difficult to study adults with chromosome 22q11.2 deletion syndrome because there are so few known. Our study suggests that while the T-cell numbers may appear relatively robust in adults with the deletion, there are important differences between the T cells of the patients and those of age-matched controls. Our data support a model in which reduced thymic production leads to compensation via peripheral proliferation such as has been described in mice.25
Homeostatic proliferation has been difficult to study in human disorders, but clearly has relevance for older patients recovering from stem cell transplantation or with advanced HIV where peripheral proliferation could contribute significantly to the total T-cell population. The peripheral blood T-cell compartment is regulated in principle by the combined effects of thymic output and peripheral proliferation counterbalanced by apoptosis and loss. Grafting extra thymic lobes into mice increases the peripheral blood T-cell counts, and thymectomizing mice leads to a reduction in T-cell counts.26-31 Thus, thymic output is an important regulator of the peripheral T-cell pool. Nevertheless, there is not a linear relationship between thymic output and peripheral blood T cells, suggesting that there is another level of regulation. Adoptive transfer of cells into a lymphopenic recipient leads to proliferation of the transferred cells until a new set-point is reached. Proliferation of naive cells in lymphopenic murine models is associated with up-regulation of markers of activation and typically conversion to a memory phenotype.32-38 Memory cells derived through this process are not antigen experienced and may or may not have fully developed effector functions. The clinical significance of homeostatic expansion is that the T-cell pool is less diverse than a comparably sized T-cell pool derived from recent thymic emigrants. From the perspective of the chromosome 22q11.2 deletion syndrome patients, the peripheral blood T-cell counts may not accurately reflect the robustness of the T-cell compartment.
To address this question, we examined telomere length and TRECs as markers of replicative history within a CD4/CD45RA population. There are various ways of identifying naive T cells and none are wholly satisfactory.39 Furthermore, naive cells can acquire a memory phenotype during homeostatic proliferation without having engaged antigen.32,33,38 Thus, surface markers do not accurately identify all cells with naive or memory effector functions. Nevertheless, these events are thought to be infrequent in a nonmanipulated human. The magnitude of the differences, and the consistent results seen in these studies, make it unlikely that the results reflect contaminating memory cells within the CD4/CD45RA population.
The TREC and telomere analyses clearly demonstrate that the CD4/CD45RA population from patients has a more extensive replicative history than controls. The TREC assay suggested that this population had undergone 1 to 2 additional rounds of replication, while the telomere assay suggested that at least 5 additional rounds of replication had occurred in patient cells compared with controls. This likely reflects the fact that the telomere assay samples the population median, which is minimally affected by small subsets of cells. TRECs, on the other hand, represent an average of the entire population. The importance of the TREC data and the telomere data is that patients unquestionably have a more extensive replicative history than controls. This is extremely unlikely to be due to increased infections, as it was seen in purified CD4/CD45RA cells. Furthermore, in data not reported in this article, we have performed the same analyses on samples from boys with X-linked agammaglobulinemia. This represents another population with frequent minor infections, and their telomeres and TRECs were no different than controls. Replication within a functionally naive population could occur with limited homeostatic expansion or could be due to memory cells that have undergone many rounds of proliferation and have reverted to a CD4/CD45RA surface phenotype.40 A predicted consequence of peripheral proliferation to enhance the T-cell counts is that the repertoire will be less diverse than if the cells had all been generated in the thymus. Indeed, that was the case, with patients having both more oligoclonal peaks and Vβ family dropouts than controls. Vβ family dropout has been previously reported in several young patients with the deletion,41 suggesting that the T-cell repertoire alterations may begin very early in life. In our older population, the increase in oligoclonal peaks and Vβ family dropouts was comparable with what has been seen in HIV-infected patients.42 An additional prediction would be impaired proliferative capacity as cells reach the critical telomere length of 5 to 7 kb.22,43 Our data cannot determine the cause of diminished proliferation; however, patient proliferative responses to antigen were impaired.
Young adult patients with chromosome 22q11.2 deletion syndrome have more frequent infections than their peers, and the findings of this study would portend that the frequency of infection might accelerate with age. Furthermore, the lymphopenia could contribute to the increased susceptibility to autoimmune disease seen in this population such has been seen in other settings.44
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-08-2824.
Supported by The Immune Deficiency Foundation and a GCRC grant (MO1-RR00240).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors gratefully acknowledge Kathakali Addya for performing the Vβ CDR3 spectrotyping and Daniel Douek for providing the TREC standards and helpful discussions. The participation of the patients and their families was essential to this study. We also would like to acknowledge Frans Snell and Nick Souters.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal