Abstract
In order to investigate the biologic activity of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) on human erythropoiesis, glycophorin A (GPA)+ erythroid cells were generated in serum-free liquid phase from human cord blood (CB) CD34+ progenitor cells. The surface expression of TRAIL-R1 was weakly detectable in the early-intermediate phase of erythroid differentiation (days 4-6; dim-intermediate GPA expression), whereas a clear-cut expression of TRAIL-R2 was observed through the entire course of erythroid differentiation (up to days 12-14; bright GPA expression). On the other hand, surface TRAIL-R3 and -R4 were not detected at any culture time. Besides inducing a rapid but small increase of apoptotic cell death, which was abrogated by the pan-caspase inhibitor z-VAD-fmk, the addition of recombinant TRAIL at day 6 of culture inhibited the generation of morphologically mature erythroblasts. Among the intracellular pathways investigated, TRAIL significantly stimulated the extracellular signal-regulated kinase 1/2 (ERK1/2) but not the p38/mitogen-activated protein kinase (MAPK) or the c-Jun NH2-terminal kinase (JNK) pathway. Consistently with a key role of ERK1/2 in mediating the negative effects of TRAIL on erythroid maturation, PD98059, a pharmacologic inhibitor of the ERK pathway, but not z-VAD-fmk or SB203580, a pharmacologic inhibitor of p38/MAPK, reverted the antidifferentiative effect of TRAIL on CB-derived erythroblasts.
Introduction
Normal erythroid development involves not only cell division and differentiation but also programmed cell death. Although some studies have shown that the Fas/FasL system negatively regulates human fetal erythropoiesis,1,2 little is known of the potential role of other members of the tumor necrosis factor (TNF) family of cytokines, which are structurally related proteins involved in the regulation of cell death and inflammation.3 One important member of the TNF family is TNF-related apoptosis-inducing ligand (TRAIL)/Apo-2 ligand (L), which exists as either a type II membrane protein or as a soluble form.4-6 TRAIL interacts with 4 high-affinity membrane receptors belonging to the apoptosis-inducing TNF-receptor (R) family. TRAIL-R1 (DR4) and TRAIL-R2 (DR5) transduce apoptotic signals upon binding of TRAIL, while TRAIL-R3 (DcR1) and TRAIL-R4 (DcR2) are homologous to DR4 and DR5 in their cysteine-rich extracellular domain, but lack apoptosis-inducing capability.7
TRAIL shows the unique property to induce apoptosis in a variety of neoplastic cells, including several hematologic malignancies,8-15 displaying minimal or absent toxicity on most normal cells. In spite of its potential as an anticancer therapeutic agent both in vitro and in vivo,16,17 the wide expression of TRAIL and TRAIL receptors in many normal tissues3-5 suggests that the physiologic role of TRAIL is more complex than induction of apoptosis in cancer cells. In this respect, other groups and we have shown that TRAIL acts as a negative regulator of adult erythropoiesis in both normal and pathologic conditions.14,18,19 However, it was evident from these studies that induction of apoptosis only partially accounted for the ability of TRAIL to negatively regulate normal erythropoiesis. Therefore, the aim of the present study was to gain insights into the pattern of expression of TRAIL receptors in cord blood–derived erythroid cells and to explore/elucidate the signal transduction pathways underlining the biologic activity of TRAIL on normal erythroid development.
Materials and methods
Purification of the cells
Cord blood (CB) specimens were collected according to institutional guidelines. CB mononuclear cells were isolated by density gradient centrifugation (Ficoll/Histopaque 1077 g/mL) and left to adhere to plastic for at least 2 hours at 37°C. After removal of adherent cells, CD34+ cells were isolated using a magnetic cell-sorting program Mini-MACS and the CD34 isolation kit (Miltenyi Biotech, Auburn, CA) in accordance with the manufacturer's instructions. The purity of CD34-selected cells was determined by FACScan (Lysis II program; Becton Dickinson, San José, CA) using a fluorescein isothiocyanate (FITC)–conjugated monoclonal antibody (MoAb), which recognizes a separate epitope of the CD34 molecule (HPCA-2; Becton Dickinson). The purity of CD34+ preparations ranged between 93% to 98%.
In vitro generation of erythroid cells and culture treatments
CB CD34+ cells were cultured in Ex-vivo-20 (BioWhittaker, Walkersville, MD) serum-free medium supplemented with nucleosides (10 μg/mL each), 0.5% bovine serum albumin (BSA, Chon fraction V), 10–4 M BSA-adsorbed cholesterol, 10 μg/mL insulin, 200 μg/mL iron-saturated transferrin, and 5 × 10–5 M 2-β-mercaptoethanol (all purchased from Sigma Chemical, St Louis, MO). Cells were adjusted to an optimal cell density of 5 × 104/mL and seeded in culture in the presence of stem cell factor (SCF, 50 ng/mL) + interleukin-3 (IL-3, 10 ng/mL) + erythropoietin (EPO, 4U/mL) to induce erythroid differentiation. All cytokines were purchased from Genzyme (Cambridge, MA). Fresh cytokines were added every 2 to 3 days and the cell density was readjusted to 4 × 105/mL.
Erythroid differentiation was monitored by analysis of surface glycophorin A (GPA) and by cell morphology examination. Surface expression of GPA was evaluated by flow cytometry as detailed in “Flow cytometric analyses.” For cell morphology examination, cells were spun on coverslips, fixed, stained with May-Grunwald-Giemsa, and observed at light microscopy with an Axyophot Zeiss microscope, equipped with a CoolScan video camera (both from Lamda Photometrics, Batford Mill, United Kingdom).
Recombinant histidine 6–tagged TRAIL(114-281) was produced in bacteria, purified by chromatography as previously described.19,20 The absence of endotoxin contamination in the recombinant TRAIL preparation (< 0.1 endotoxin units/mL) was assessed by limulus amebocyte lysate (LAL) assay (BioWhittaker). The optimal TRAIL concentration (100 ng/mL), used in most experiments, was determined based on preliminary assays in which scalar TRAIL doses (ranging from 0.01 to 1 μg/mL) were tested. Pharmacologic inhibitors of the caspase (z-VAD-fmk), extracellular signal-regulated kinase 1/2 (ERK1/2, PD98059), p38/mitogen-activated protein kinase (MAPK, SB203580) pathways, and the inhibitor of lipopolysaccharide (polymyxin B) (all from Calbiochem, La Jolla, CA) were used at the concentrations indicated in “Results.”
Flow cytometric analyses
For flow cytometric analyses, surface cell staining was performed at 4°C for 40 minutes by incubating 3 × 105 cells in 200 μL phosphate-buffered saline (PBS, containing 1% BSA and 5% human plasma) with the indicated antibodies. CD34 and GPA expression were detected using FITC-conjugated anti-CD34 and phycoerythrin (PE)–conjugated anti-GPA MoAbs (BD Pharmingen, San Diego, CA), respectively. Nonspecific fluorescence was assessed by incubation with irrelevant isotype-matched conjugated MoAbs. GPA expression is reported in the text as percentage of positive cells and/or as mean fluorescence intensity (MFI). Surface expression of TRAIL receptors was evaluated by indirect staining with primary MoAbs antihuman TRAIL-R1, TRAIL-R2, TRAIL-R3, and TRAIL-R4 (all from Alexis Biochemicals, Lausen, Switzerland), followed by PE-conjugated antimouse secondary Abs (Immunotech, Marseille, France). Nonspecific fluorescence was assessed using normal mouse immunoglobulin G (IgG) followed by second layer Abs. In some experiments, double staining was performed by incubation with primary MoAbs antihuman TRAIL-R1, TRAIL-R2 (Alexis), followed by PE-conjugated antimouse secondary Ab. Then, after 2 washings, cells were incubated with Cy-chrome–conjugated anti-GPA MoAb (BD Pharmingen). Flow cytometric analyses were performed by FACScan (Becton Dickinson). For apoptosis evaluation, cells were stained with Annexin V–FITC and propidium iodide (PI) and analyzed by flow cytometry as previously detailed.19,20
[3H]Thymidine incorporation assay
Erythroid cells were plated onto 96-well plates at a density of 5 × 103 cell/well. The cells were then pretreated with inhibitors or vehicle for 1 hour and then incubated with TRAIL for 18 hours in the presence of [3H]thymidine (1 μCi [0.037 MBq]). [3H]thymidine-labeled DNA was then assayed by harvesting the cells using Brandel Harvester 96 (Brandel, Gaithersburg, MD). [3H]Thymidine levels were then measured using a Beckman model LS6000IC liquid scintillation counter (Beckman Coulter, Fullerton, CA).
Western blot analyses
Cells were harvested in lysis buffer containing 1% Triton X-100, Pefablock (1 mM), aprotinin (10 μg/mL), pepstatin (1 μg/mL), leupeptin (10 μg/mL), NaF (10 mM), and Na3VO4 (1 mM). Protein determination was performed by Bradford assay (Bio-Rad, Richmond, CA). Equal amounts of protein (50 μg) for each sample were migrated in acrylamide gels and blotted onto nitrocellulose filters. The following antibodies were used: rabbitAbs anti-ERK1/2, anti–phospho-ERK1/2, anti-p38, anti–phospho-p38, anti–c-Jun NH2-terminal kinase (JNK1), anti–phospho-JNK1 (all from New England Biolabs, Beverly, MA), and MoAb antitubulin (Sigma). Blotted filters were first probed with antibodies for the phosphorylated forms of ERK1/2, p38, and JNK1. After incubation with peroxidase-conjugated anti–rabbit or anti–mouse IgG (Sigma), specific reactions were revealed with the enhanced chemiluminescence (ECL) Western blotting detection reagent. Membranes were stripped by incubation in Re-Blot 1X Ab stripping solution (Chemicon International, Temecula, CA) and reprobed for the respective total protein kinase content or tubulin for verifying loading evenness. Densitometry values were estimated by the ImageQuant software (Molecular Dynamics, Sunnyvale, CA). Multiple film exposures were used to verify the linearity of the samples analyzed and to avoid saturation of the film.
Statistical analysis
The results were evaluated using analysis of variance with subsequent comparisons by Student t test for paired or nonpayer data, as appropriate. Statistical significance was defined as P < .05. Values are reported as means ± SD.
Results
CD34-derived erythroblasts show persistent expression of TRAIL-R2
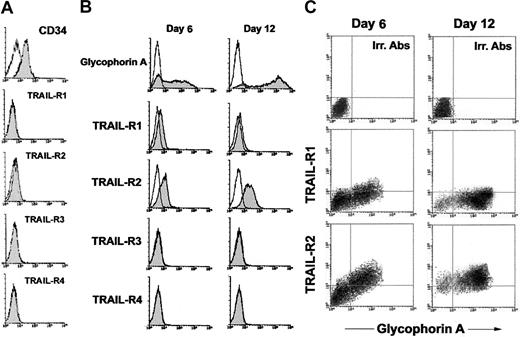
Having observed that TRAIL protein is expressed in human adult bone marrow19 and in human fetal livers at different ages of gestation (8-22 gestational weeks; data not shown) in the first group of experiments, we have analyzed the phenotypic surface expression of transmembrane TRAIL receptors (TRAIL-R1, R2, R3, and R4) on freshly purified CB CD34+ hematopoietic progenitor cells (day 0), as well as at early-intermediate (day 6) and late (days 12-14) culture times (Figure 1). As shown in Figure 1A, highly purified populations of CB CD34+ cells did not express any TRAIL receptors. On the other hand, after 6 days of serum-free liquid culture in the presence of SCF + IL-3 + EPO, when most of the cells showed a dim-intermediate expression of GPA, an intermediate surface expression of TRAIL-R2 and a dim surface expression of TRAIL-R1 became apparent (Figure 1B-C). At later culture times (days 12-14), when most erythroblasts showed a bright expression of GPA, surface TRAIL-R2 expression was increased with respect to earlier culture times, while the dim expression of surface TRAIL-R1, observed at earlier time points, declined, becoming barely detectable in most experiments (Figure 1B-C). On the other hand, TRAIL-R3 and TRAIL-R4 were never expressed on the surface of GPA+ erythroblasts (Figure 1B). The first conclusion of this group of analyses is that CB CD34+ cells are not affected by TRAIL, as previously shown by us and by others in adult CD34+ cells,18-20 due to the lack of expression of surface TRAIL receptors.
Surface expression of TRAIL receptors in erythroid cultures. Surface TRAIL receptor (TRAIL-R1, TRAIL-R2, TRAIL-R3, and TRAIL-R4) expression was evaluated by flow cytometry in CD34+ cells freshly purified (A) and in CD34+ cells cultured in the presence of EPO + IL-3 + SCF (B-C). In panels B-C, erythroid differentiation was monitored at 6 and 12 days of liquid culture by analysis of surface GPA expression. In panels A-B, shaded histograms represent cells stained with MoAbs specific for the indicated surface antigens (CD34, glycophorin A, TRAIL receptors), whereas unshaded histograms represent the background fluorescence obtained from the staining of the same cultures with isotype-matched control MoAbs. In panel C, surface TRAIL-R1 and TRAIL-R2 expression was analyzed in combination with surface glycophorin A at 6 and 12 days of culture. Horizontal axis indicates the relative surface glycophorin A expression detected by Cy-chrome fluorescence intensity. Vertical axis indicates the TRAIL-R1 or TRAIL-R2 expression detected by indirect PE fluorescence intensity. Representative negative controls, constituted by cells stained with irrelevant (Irr.) isotype-matched MoAbs, are shown in the top panels. A representative of 5 (A-B) and 3 (C) separate experiments is shown.
Surface expression of TRAIL receptors in erythroid cultures. Surface TRAIL receptor (TRAIL-R1, TRAIL-R2, TRAIL-R3, and TRAIL-R4) expression was evaluated by flow cytometry in CD34+ cells freshly purified (A) and in CD34+ cells cultured in the presence of EPO + IL-3 + SCF (B-C). In panels B-C, erythroid differentiation was monitored at 6 and 12 days of liquid culture by analysis of surface GPA expression. In panels A-B, shaded histograms represent cells stained with MoAbs specific for the indicated surface antigens (CD34, glycophorin A, TRAIL receptors), whereas unshaded histograms represent the background fluorescence obtained from the staining of the same cultures with isotype-matched control MoAbs. In panel C, surface TRAIL-R1 and TRAIL-R2 expression was analyzed in combination with surface glycophorin A at 6 and 12 days of culture. Horizontal axis indicates the relative surface glycophorin A expression detected by Cy-chrome fluorescence intensity. Vertical axis indicates the TRAIL-R1 or TRAIL-R2 expression detected by indirect PE fluorescence intensity. Representative negative controls, constituted by cells stained with irrelevant (Irr.) isotype-matched MoAbs, are shown in the top panels. A representative of 5 (A-B) and 3 (C) separate experiments is shown.
TRAIL exhibits antidifferentiative effects when added to immature erythroblasts
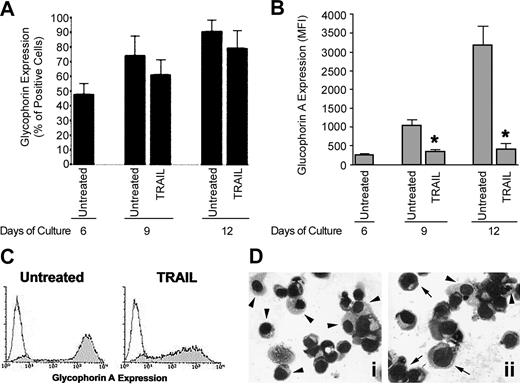
In order to characterize the biologic activity of TRAIL on CB erythropoiesis, recombinant TRAIL was added to erythroid cultures at day 6, when GPA+ immature erythroblasts showed an intermediated expression of TRAIL-R2 and a dim expression of TRAIL-R1 (Figure 1B). The potential interference of the prosurvival signal transduction pathways elicited by the cytokine cocktail used to induce erythroid differentiation21,22 was avoided by adding TRAIL 48 hours after the last addition of SCF + IL-3 + EPO. Every 3 days after TRAIL addition, cultures were monitored for cell viability, surface GPA expression levels, and morphology. TRAIL induced a small decrease in the total number of viable cells (data not shown) as well as of percentage of GPA+ erythroblasts (Figure 2A) evident at day 9, which, however, did not reach statistical significance. On the contrary, TRAIL induced a marked decrease in the mean fluorescence level of surface GPA (Figure 2B and 2C), observed after 3 days from the addition of TRAIL (day 9 of culture, P < .01) and persisting at later time points (day 12 of culture, P < .01) (Figure 2B). The possibility that TRAIL treatment was shifting erythropoiesis to another lineage was unlikely because the percentage of GPA+ cells generated in culture was not significantly affected by TRAIL. Moreover, the antidifferentiative activity of TRAIL was confirmed by morphologic analysis of cytospin samples, which showed decreased number of mature orthochromatic erythroblasts with respect to control cultures (Figure 2D), thus ruling out the possibility that TRAIL effect could just be on GPA cell surface expression. Of note, these biologic effects of TRAIL were not due to potential contaminating endotoxin, as polymyxin B did not affect TRAIL activity (data not shown).
Effect of TRAIL on erythroid maturation. CD34+ cells were cultured in the presence of EPO + IL-3 + SCF for 6 days and then cells were either left untreated or treated with TRAIL. At the indicated times, cultures were analyzed for glycophorin A expression and cell morphology. In panels A-B, surface glyophorin A expression, reported either as percentage of positive cells (A) or as mean fluorescence intensity (MFI) (B), was measured by flow cytometry. Data represent the means ± SDs of 4 independent experiments performed in duplicate; *P < .01. In panels C-D, representative cell phenotype and cell morphology, examined on day 12 of culture by flow cytometry and by light microscopy after May-Grunwald-Giemsa staining, respectively, are shown. In panel D, some mature (arrowheads) and immature (arrows) erythroblasts are indicated in the untreated (i) and TRAIL-treated (ii) cultures. Original magnification, × 40. Similar results were observed in 3 independent experiments performed in duplicate.
Effect of TRAIL on erythroid maturation. CD34+ cells were cultured in the presence of EPO + IL-3 + SCF for 6 days and then cells were either left untreated or treated with TRAIL. At the indicated times, cultures were analyzed for glycophorin A expression and cell morphology. In panels A-B, surface glyophorin A expression, reported either as percentage of positive cells (A) or as mean fluorescence intensity (MFI) (B), was measured by flow cytometry. Data represent the means ± SDs of 4 independent experiments performed in duplicate; *P < .01. In panels C-D, representative cell phenotype and cell morphology, examined on day 12 of culture by flow cytometry and by light microscopy after May-Grunwald-Giemsa staining, respectively, are shown. In panel D, some mature (arrowheads) and immature (arrows) erythroblasts are indicated in the untreated (i) and TRAIL-treated (ii) cultures. Original magnification, × 40. Similar results were observed in 3 independent experiments performed in duplicate.
In the next experiments, we investigated whether the adverse effect of TRAIL on CB erythroid maturation could be secondary to modulation of erythroid cell survival/proliferation or if it was due to a direct antidifferentiative effect of TRAIL on normal erythropoiesis. As shown in Figure 3A, exposure to TRAIL resulted in an increase of apoptosis, which peaked at 24 hours from TRAIL addition (P < .05) and was accompanied by a modest but significant (P < .05) decrease of thymidine uptake (Figure 3B). As expected on the basis of previous findings on adult erythropoiesis,18,19 the pan-caspase inhibitor z-VAD-fmk completely abrogated the proapoptotic activity of TRAIL (Figure 3A-B). Importantly, however, z-VAD-fmk did not revert the adverse effect of TRAIL on morphologic maturation of erythroblasts, evaluated 3 to 4 days (days 9-10 of culture) from the addition of TRAIL ± z-VAD-fmk in culture (Figure 3C). These data are consistent with a previous study showing that z-VAD-fmk rather arrests the maturation of erythroid progenitors at early stages of differentiation.23
Effect of TRAIL on erythroid cell survival/growth. CD34+ cells were cultured for 6 days in the presence of EPO + IL-3 + SCF. At this time point, cultures were preincubated with vehicle (0.1% dimethyl sulfoxide [DMSO]) or z-VAD-fmk (z-VAD, 20 μM) before treatment with TRAIL. After approximately 24 hours of treatment, apoptosis was assessed by annexin V–FITC binding and PI staining (A), whereas cell proliferation was assayed by measuring [3H]thymidine incorporation (B). In panel B, data represent the means ± SDs of 4 experiments; *P < .05. In panel C, representative cell morphology, examined on days 9 to 10 of culture by light microscopy after May-Grunwald-Giemsa staining, is shown. Most erythroblasts show an immature morphology in both TRAIL and TRAIL + z-VAD-fmk–treated cultures. Original magnification, × 40.
Effect of TRAIL on erythroid cell survival/growth. CD34+ cells were cultured for 6 days in the presence of EPO + IL-3 + SCF. At this time point, cultures were preincubated with vehicle (0.1% dimethyl sulfoxide [DMSO]) or z-VAD-fmk (z-VAD, 20 μM) before treatment with TRAIL. After approximately 24 hours of treatment, apoptosis was assessed by annexin V–FITC binding and PI staining (A), whereas cell proliferation was assayed by measuring [3H]thymidine incorporation (B). In panel B, data represent the means ± SDs of 4 experiments; *P < .05. In panel C, representative cell morphology, examined on days 9 to 10 of culture by light microscopy after May-Grunwald-Giemsa staining, is shown. Most erythroblasts show an immature morphology in both TRAIL and TRAIL + z-VAD-fmk–treated cultures. Original magnification, × 40.
The antidifferentiative effect of TRAIL on erythroblasts is mediated by activation of the ERK/MAPK pathway
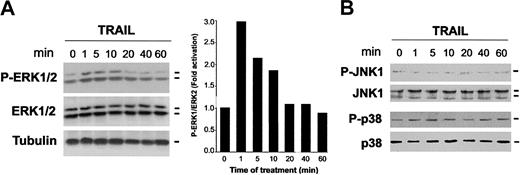
To date, at least 3 subgroups of mitogen-activated protein kinase (MAPK) family members have been involved in a wide range of cellular responses.24 The first subgroup includes 2 isoforms of the extracellular signal-regulated kinases, ERK1 and ERK2. The 2 other subgroups are SAPK1/JNK1 (stress-activated protein kinase-1/c-Jun NH2-terminal kinase) and SAPK2/p38.24 Of note, different family members of the MAPK family have been involved in the control of erythroid maturation.25-31 Therefore, we have investigated whether the MAPK pathways are engaged by the interaction between TRAIL and TRAIL receptors in primary erythroid cells, obtained after 6 days of culture. For this purpose, Western blot analyses were performed using antibodies specific for the residues that are phosphorylated in each kinase upon activation (Figure 4). After exposure to TRAIL, a rapid induction of phospho-ERK1/2 was observed at 1 to 10 minutes of treatment (Figure 4A). On the other hand, TRAIL did not activate either p38/MAPK or JNK1 at any time point examined (Figure 4B). Of note, TRAIL addition to 12-day erythroblasts still resulted in the rapid activation of ERK pathway (data not shown).
Time-course analyses of ERK1/2, p38/MAPK, and JNK1 phosphorylation in erythroid cells in response to TRAIL. CD34+ cells were cultured in the presence of EPO + IL-3 + SCF for 6 days and then were exposed to TRAIL for the indicated times. Equal amounts of cell lysates were analyzed for ERK1/2 (A), JNK1, and p38/MAPK (B) phosphorylation by Western blot analyses using antibodies specific for the native form of the kinases and for residues that are phosphorylated in each kinase upon activation. Tubulin staining is also shown as loading control. In panel A, protein bands were quantified by densitometry and level of P-ERK1/2 was calculated for each time point, after normalization to ERK1/2 in the same sample. Unstimulated basal expression was set as unity. A representative of 3 separate experiments is shown.
Time-course analyses of ERK1/2, p38/MAPK, and JNK1 phosphorylation in erythroid cells in response to TRAIL. CD34+ cells were cultured in the presence of EPO + IL-3 + SCF for 6 days and then were exposed to TRAIL for the indicated times. Equal amounts of cell lysates were analyzed for ERK1/2 (A), JNK1, and p38/MAPK (B) phosphorylation by Western blot analyses using antibodies specific for the native form of the kinases and for residues that are phosphorylated in each kinase upon activation. Tubulin staining is also shown as loading control. In panel A, protein bands were quantified by densitometry and level of P-ERK1/2 was calculated for each time point, after normalization to ERK1/2 in the same sample. Unstimulated basal expression was set as unity. A representative of 3 separate experiments is shown.
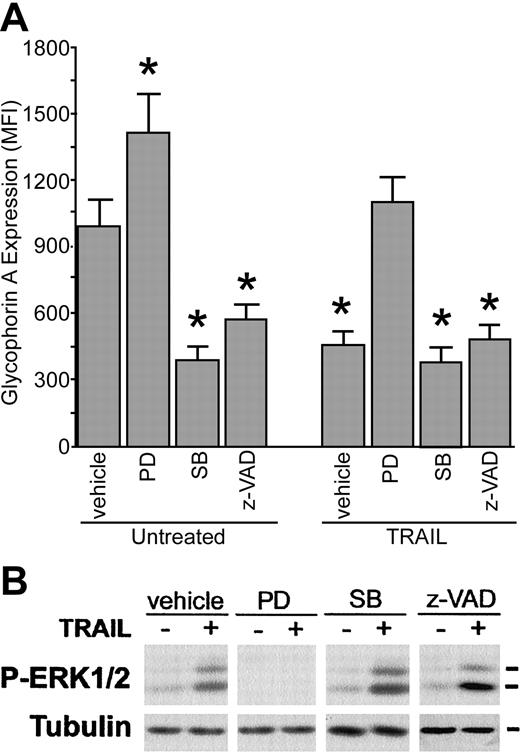
Since MAPK pathways have been suggested to play regulatory roles in erythroid differentiation,25-31 we have assessed the effect of specific cell-permeable inhibitors, such as PD98059 and SB203580, commonly used inhibitors of the ERK and p38/MAPK pathways, respectively, on 6-day erythroid cells, cultured with or without TRAIL. Treatment with PD98059 and SB203580, used alone, showed opposite effects on GPA expression evaluated after 3 additional days of culture, the former significantly (P < .05) increasing and the latter inhibiting (P < .05) the mean fluorescence intensity of surface GPA in erythroid cells (Figure 5A). The inhibitory effect of SB203580 on GPA surface expression was comparable with that of z-VAD-fmk (Figure 5A). Remarkably, preincubation with PD98059, used at concentrations that abrogated phospho-ERK activation (Figure 5B), totally abrogated the antidifferentiative effect of TRAIL (Figure 5A). On the contrary, preincubation with SB203580 or zVAD-fmk did not show significant effects on TRAIL-mediated inhibition of GPA surface expression (Figure 5A) and ERK phosphorylation (Figure 5B). U0126, an unrelated inhibitor of the ERK pathway, showed an effect similar to that of PD98059 (data not shown).
Effect of pharmacologic inhibitors on erythroid differentiation. CD34+ cells were cultured for 6 days in the presence of EPO + IL-3 + SCF. At this time point, cultures were preincubated with vehicle (0.1% DMSO), PD98059 (PD, 10 μM), SB203580 (SB, 10 μM), or z-VAD-fmk (z-VAD, 20 μM) before treatment with TRAIL. (A) Surface glyophorin A expression, reported as MFI, was measured by flow cytometry. Data represent the means ± SDs of 4 different experiments; *P < .05, compared with untreated culture. (B) The level of phosphorylated ERK1/2 was analyzed in cell lysates at 5 minutes of TRAIL treatment. Equal loading was confirmed by tubulin staining. This experiment is representative of 3 independent experiments that gave similar results.
Effect of pharmacologic inhibitors on erythroid differentiation. CD34+ cells were cultured for 6 days in the presence of EPO + IL-3 + SCF. At this time point, cultures were preincubated with vehicle (0.1% DMSO), PD98059 (PD, 10 μM), SB203580 (SB, 10 μM), or z-VAD-fmk (z-VAD, 20 μM) before treatment with TRAIL. (A) Surface glyophorin A expression, reported as MFI, was measured by flow cytometry. Data represent the means ± SDs of 4 different experiments; *P < .05, compared with untreated culture. (B) The level of phosphorylated ERK1/2 was analyzed in cell lysates at 5 minutes of TRAIL treatment. Equal loading was confirmed by tubulin staining. This experiment is representative of 3 independent experiments that gave similar results.
The effect of pharmacologic inhibitors on GPA expression in TRAIL-treated cultures could not be ascribed to modulation of cell survival/proliferation since both PD098059 and SB203580 strongly suppressed [3H]thymidine incorporation in cultures without TRAIL (untreated culture set at 100%; + PD098059: 54.2% ± 7%; + SB203580: 50.7% ± 6%; means of 4 independent experiments ± SD) as well as in TRAIL-treated cultures (+ TRAIL alone: 75% ± 7%; + PD098059 + TRAIL: 37.7% ± 4%; + SB203580 + TRAIL: 40.6% ± 5%; means of 4 independent experiments ± SD). These data confirm that both ERK and p38 pathways are involved in mediating erythroid proliferation, and, more importantly, they demonstrate that the differential effect of PD098059 and SB203580 inhibitors on GPA surface expression cannot be ascribed to a differential activity of cell proliferation.
Discussion
In this study, we found that a dim surface expression TRAIL-R1 was detectable only at early phases of erythroid development, while TRAIL-R2 surface expression was detected at early phases of erythroid differentiation and showed a progressive increase as erythroid maturation proceeded. These observations suggest that TRAIL-R2, rather than TRAIL-R1, likely plays a mechanistic role in erythroid development.
Among the members of the MAPK family investigated, TRAIL was effective in activating ERK1/2, but not JNK and p38 in erythroid cultures. Moreover, ERK1/2 activation by TRAIL was observed at both early (day 6) and late (days 12-14) culture times strongly implicating TRAIL-R2, the only receptor expressed at both time points, in triggering ERK1/2 phosphorylation. Although we cannot exclude the possibility that TRAIL/TRAIL-receptor interaction stimulates the ERK pathway indirectly, for instance by modulating the conformation of an integral transmembrane protein, this possibility is unlikely in light of the ability of caspase-8, a major downstream effector of TRAIL-R1/R2, to activate the ERK pathway in lymphoid cells.32
MAPKs form a large family of serine-threonine protein kinases conserved through evolution.24 In mammalian cells, 3 major MAP kinase cascades have been identified: extracellular signal regulated kinases (ERK), c-Jun amino-terminal kinases (JNK) or stress-activated protein kinases (SAPK), and p38 MAP kinase (p38). These kinases, which represent the end of pathways involving multiple serine-threonine kinases activated in a cascade, have become prototypes for the study of structurally related but functionally distinct pathways on cell development.
The classical MAP kinases (ERK1 and ERK2) are activated by a variety of cell growth and differentiation stimuli and play a central role in mitogenic signaling.24 The role of p38 and JNK pathways is more complex. In fact, various environmental stresses (osmotic shock, ultraviolet radiation, heat shock, X-ray radiation, hydrogen peroxide, and protein synthesis inhibitors)27 and proinflammatory cytokines, such as TNF-α and type I interferon (IFN), induce p38 and JNK activation.21,25 However, in hematopoiesis, p38 and JNK are also activated by survival/growth factors, such as SCF and EPO.25,26 In particular, it has been shown that JNK and p38 activities peaked 24 hours after EPO treatment, and decrease slowly, being detectable up to 48 hours from EPO withdrawal in the mouse EPO-dependent HCD57 erythroleukemia cell line.28 Consistently, ERK, p38, and JNK showed low basal phosphorylation levels in erythroid cultures examined 48 hours after the last addition of SCF + IL-3 + EPO.
The ability of TRAIL to activate ERK1/2 in primary normal CB erythroblasts is particularly noteworthy. In fact, it has been previously shown that inhibition of ERK1/2 and activation of p38/MAPK signal transduction pathway play a critical role in butyrate-induced erythroid differentiation of the K562 cell line,29 as well as in EPO-dependent differentiation of mouse erythroleukemia SKT6 cells26 and of primary erythroblasts.32 Conversely, it has been shown that the ERK1/2 pathway in erythroid cells is involved in the early proliferative phases of erythropoiesis31 and in the inhibition of terminal erythroid differentiation.26 In line with these findings, we have here demonstrated that PD098059, the pharmacologic inhibitor of the ERK1/2 pathway, significantly inhibited erythroid proliferation and up-regulated GPA surface expression in both control and TRAIL-treated cultures.
ERK1/2 activation has also been involved in mediating differentiation along the megakaryocytic and myeloid lineages.31,33 Thus, in agreement with previous studies showing that p38 plays an essential role in mediating the differentiation activity of EPO, while the ERK1/2 pathway antagonizes erythroid differentiation,25-31,33 our data indicate that the TRAIL-mediated activation of ERK1/2 is responsible for the antidifferentiative effect of TRAIL on normal erythroid development.
The potential physiologic significance of our present findings is underscored by the fact that TRAIL is expressed in fetal liver (data not shown) and adult human bone marrow.14,19 Moreover, although TRAIL protein offers great promise as a cancer therapeutic,34,35 its potent antidifferentiative effect on normal erythropoiesis adds a cautionary note to the prolonged treatment of cancer patients with pharmacologic concentrations of recombinant TRAIL protein.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-06-2137.
Supported by Fondo per l'Incentivazione della Ricerca di Base (FIRB) (P.S. and G.Z.), Associazione Italiana per la Ricerca sul Cancro (AIRC) (G.Z.), and “Juselius Foundation” (M.H.) grants.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 3. Effect of TRAIL on erythroid cell survival/growth. CD34+ cells were cultured for 6 days in the presence of EPO + IL-3 + SCF. At this time point, cultures were preincubated with vehicle (0.1% dimethyl sulfoxide [DMSO]) or z-VAD-fmk (z-VAD, 20 μM) before treatment with TRAIL. After approximately 24 hours of treatment, apoptosis was assessed by annexin V–FITC binding and PI staining (A), whereas cell proliferation was assayed by measuring [3H]thymidine incorporation (B). In panel B, data represent the means ± SDs of 4 experiments; *P < .05. In panel C, representative cell morphology, examined on days 9 to 10 of culture by light microscopy after May-Grunwald-Giemsa staining, is shown. Most erythroblasts show an immature morphology in both TRAIL and TRAIL + z-VAD-fmk–treated cultures. Original magnification, × 40.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/2/10.1182_blood-2003-06-2137/6/m_h80245513003.jpeg?Expires=1770378831&Signature=lC1tViP29xyURMovnpBiEkrEHGW2YN0epawVgMYX1lCCtlFxvYtIuDDHHMJayGzgXgCJA~uSB6sqZvQXvLyPme0v0SPWFXVMpAdtOTf9zkIpKiZoU7uRnPfFdtfuRZ4YltodLt0Vx-rh02ou91xWaY2SUFeMzWsTMWyrTTkbvfmIGurofC~OGMvWN9JzxdFAzXu38hE~sh4yhmB-BTYeuX-JTeJJXvhmjiTUrMvDT4KhTYd6wL~-mrJtJjCvmXPh~h-RT3hW5YSpiwP2faiq0fjjnrhFNv0BKt~kFM6WEL1~767~9wN4Qhyx~qj-eEE01GkJnT7nPcLdaw1WK1BE2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal