Abstract
Activation of the granulocyte-macrophage colony-stimulating factor (GM-CSF) family of receptors promotes the survival, proliferation, and differentiation of cells of the myeloid compartment. Several signaling pathways are activated downstream of the receptor, however it is not clear how these induce specific biologic outcomes. We have previously identified 2 classes of constitutively active mutants of the shared signaling subunit, human (h) βc, of the human GM-CSF/interleukin-3 (IL-3)/IL-5 receptors that exhibit different modes of signaling. In a factor-dependent bipotential myeloid cell line, FDB1, an activated mutant containing a substitution in the transmembrane domain (V449E) induces factor-independent proliferation and survival, while mutants in the extracellular domain induce factor-independent granulocyte-macrophage differentiation. Here we have used further mutational analysis to demonstrate that there are nonredundant functions for several regions of the cytoplasmic domain with regard to mediating proliferation, viability, and differentiation, which have not been revealed by previous studies with the wild-type GM-CSF receptor. This unique lack of redundancy has revealed an association of a conserved membrane-proximal region with viability signaling and a critical but distinct role for tyrosine 577 in the activities of each class of mutant.
Introduction
Interleukin 3 (IL-3), IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) are cytokines that affect the survival, growth, and differentiation of multiple cell lineages within the hematopoietic system. The functions of these factors are mediated by high-affinity membrane-bound receptors that are composed of specific ligand-binding subunits (α) and a common signal transducing subunit (βc).1-3 Several regions and motifs within human (h) βc have been identified that contribute to particular signaling pathways and biologic effects. The results of these studies have been previously reviewed,4,5 but some key observations are briefly summarized here. The membrane proximal region of the hβc contains conserved motifs including Box 1, which is essential for activation of Janus kinase 2 (JAK2) and induction of c-myc, events that are necessary for induction of proliferation and antiapoptotic effects of the activated receptor.6-9 Another conserved membrane proximal motif, Box 2, appears to make subtle contributions to long-term proliferation and c-fos promoter activation,7,8,10 however its specific mode of action in receptor signaling remains enigmatic. In addition, it is clear that phosphorylated tyrosine and serine residues are necessary for the association and activation of particular signaling molecules and pathways from the receptor.11-14
As well as uncovering specificity in the binding of particular adaptor and signaling molecules, mutational analysis has also demonstrated that considerable redundancy exists in the activation of signaling pathways from the GM-CSF receptor. For example, activation of the Ras/Map kinase (MAPK) pathway can be mediated through multiple tyrosine residues,8,15 as can activation of signal transducers and activators of transcription (STATs).12 The phosphatidylinositol 3-kinase (PI3-K)/Akt pathway can also be activated via multiple means: through an interaction of Ser585 and the 14-3-3 adaptor protein, through Tyr577 via Shc, and through Tyr612 via Src homology 2 domain–containing protein tyrosine phosphatase-2 (SHP2).15-17 Such redundancy of activation has made it difficult to assign particular signaling pathways to biologic outcomes. This is particularly true when considering the question of specificity of signaling from the GM-CSF and IL-3 receptors. These receptors share a common β subunit, and presumably activate common signaling pathways, yet are able to elicit the distinct biologic responses of differentiation and proliferation, respectively, in a number of myeloid cell lines.18,19 Clearly the cytokine-specific α subunits are excellent candidates for contributing, at least partially, to signaling specificity. This has been demonstrated by experiments that swapped either part or all of the cytoplasmic domains of the GM-CSF and IL-3 α subunits.19,20 However no distinct signaling events have yet been associated with these differential responses. The role of the common β subunit in mediating differentiative signaling has also been addressed. Expression of the human GM-CSF receptor complex (hGMR) in the M1, WEHI-3B D+, and WT19 cell lines induced macrophage differentiation in response to ligand.21,22 While regions of the hβc cytoplasmic domain were associated with particular aspects of differentiation, the regions required varied between the different cell lines.21,22 In addition, in WT19 cells, at least 2 redundant pathways signaling for macrophage differentiation from the hβc were detected.22 Thus studies aimed at addressing the mechanisms by which the IL-3/GM-CSF receptors induce proliferation and differentiation are still limited by the redundancy of signals that lead to common biologic outcomes. Moreover some of these cell lines show only limited cytokine requirements, for example M1 and WEHI-3B D+ cells, unlike normal myeloid progenitors, do not require cytokines for survival or growth.
We have addressed these questions here by developing a model system that uses activated mutants of the GM-CSF receptor in combination with a unique bipotential myeloid cell line (below). Previously, we have isolated several activated mutants of the hβc that are capable of stimulating factor-independent growth in a number of hematopoietic cell lines.23 Several of these mutants contain amino acid substitutions in the membrane-proximal extracellular (EC) domain of hβc, the best characterized being I374N (containing an isoleucine to asparagine substitution at position 374),24 and FIΔ (which contains a 37 amino acid duplication and exhibits similar properties).25 Another class of mutants (represented by the V449E valine to glutamic acid substitution) contains activating transmembrane (TM) substitutions.24
The EC and TM mutants were all isolated on the basis of their ability to confer factor-independent proliferation on FDC-P1 cells; however they clearly signal by distinct mechanisms and most likely activate overlapping signaling pathways that are a subset of those activated by the ligand-bound complex.26 Most notably there are obvious differences in receptor tyrosine phosphorylation and the phosphorylation of the adapter protein Shc, while no difference has been observed in phosphorylation of JAK2, extracellular signal-related kinase 1 (ERK1) and ERK2, orAkt, or with regard to STAT5 activation (Jenkins et al27 ; and T. Blake and T.J.G., unpublished data, October 1998).
Functional differences between the EC and TM activated mutants are clearly apparent in a novel murine hematopoietic cell line, FDB1.18 These cells are strictly factor-dependent for survival and proliferate continuously in the presence of IL-3, while in the presence of GM-CSF they undergo complete differentiation along the granulocyte and monocyte lineages. Importantly, IL-3 is “dominant” over GM-CSF as cells continue to proliferate without differentiation in a mixture of these 2 cytokines. FDB1 cells thus constitute a unique model of myeloid differentiation that permits dissection and association of signaling pathways contributing to myeloid cell proliferation, survival, and differentiation. Importantly, FDB1 cells also have differential responses to signaling via the 2 classes of activated mutants. When the EC mutants (FIΔ and I374N) are expressed in this cell line in the absence of growth factor, they deliver a differentiation signal (similar to GM-CSF), whereas the TM mutant, V449E, induces continuous factorindependent proliferation.18 This ability to mimic the cellular signaling outcomes of GM-CSF and IL-3 while triggering a reduced set of signaling events led us to propose that it would be possible to use these activated receptor mutants to associate receptor-associated events with particular cellular outcomes.
Materials and methods
Cytokines and antibodies
Recombinant murine (m) IL-3 and GM-CSF were produced from baculovirus vectors supplied by Dr Andrew Hapel (John Curtin School of Medical Research, Canberra, Australia). The anti-ERK1/2 and anti–phospho ERK1/2 antibodies were purchased from Cell Signaling Technology (Beverly, MA).
Cell lines
ψ2 producer cells transfected with retrovirus-containing receptor constructs were maintained as described previously.24 HEK 293T cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. FDB1 cells were maintained as described previously.18 Infected pools were maintained as described plus 1 μg/mL Puromycin (Sigma, St Louis, MO).
Construction of retroviral expression plasmids
The N′ fludarabine, cytarabine, and G-CSF (FLAG)–tagged full-length hβc, V449E, and FIΔ cDNAs in pRufPuro have been described previously.18 Cytoplasmic domain deletions were constructed by polymerase chain reaction (PCR) using the full-length clones as a template. All PCR products were generated by using common 5′ primer containing a BamHI site (5′ATCGGATCCGCCTGCCTGTCCAGAGC3′) and specific 3′ primers containing a stop codon and an EcoRI site as follows: 763 (5′CGGAATTCATGACCTGGGGGACCG3′); 626-(5′CGGAATTCACAGAGGGACCAGTTGCAC3′); 544 (5′CGGAATTCAATCTGAGG-CAGCTGGAGT3′); 517 (5′CGGAATTCACTCGCTGTCCCCGAATC3′). The tyrosine mutant hβc cDNAs (Y8F, Y577F, and Y612F) have been described previously28 and were a gift from Dr James Griffin (Dana-Farber Cancer Institute, Boston, MA). The cytoplasmic domains of the hβc containing the Y8F, Y577F, and Y612F mutations were each amplified using the primers 5′ (5′GCGATACGAACACATAGACC3′) and 3′ (5′CGGAATTCAACACACCTCC-CCAGG3′). The PCR products were restricted with FspI and EcoRI. Full-length V449E or FIΔ mutants in pRufPuro were digested with SnaBI and EcoRI to remove the cytoplasmic domain. These were then ligated to the digested Tyr mutant cytoplasmic domain PCR products to create V449E and FIΔ Tyr mutant constructs. Double tyrosine mutant cDNAs (Y577F/Y612F) were created by site-directed mutagenesis of Y612F cDNAs using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) using the 5′ primer (5′ GACTTCAATGGGCCCTTCCTAGGGCCGCCCCACAGC3′) and the 3′ primer (5′ GCTGTGGGGCGGCCCTAGGAAGGGCCCATTGAAGTC3′).
Transfection and infection
To make stable ψ2 producer cell lines, 30 μg retroviral construct was electroporated into 107 cells at 270V/975 μF with a Biorad Gene Pulser II (Hercules, CA). After overnight recovery, cells were selected with 4 μg/mL Puromycin (Sigma). To make transient viral producer lines, equal amounts of pEQEco ecotropic packaging vector (a gift from Dr Elio Vanin, St Jude Children's Research Hospital, Memphis, TN)29 and retroviral constructs were transfected into HEK 293T cells using Lipofectamine Plus Reagent per the manufacturer's instructions (Invitrogen Life Technologies, Carlsbad, CA). FDB1 cells were infected either by stable or transient viral producers as previously described.18 Cells were assessed for receptor cell surface expression by staining with a murine anti-FLAG antibody (Sigma) followed by an antimouse fluorescein isothiocyanate–conjugated antibody (Silenus, Hawthorn, Victoria, Australia) and analyzed by flow cytometry with the use of an Epics-Profile II analyzer (Coulter Electronics, Hialeah, FL). If necessary, cells were sorted for expression as previously described.24
Cell proliferation assays, viability assays, and differentiation assays
Cells were washed 3 times in Iscoves modified Dulbecco medium and cultured in 500 U/mL mIL-3 or mGM-CSF, or without factor. Cells were seeded at 5000/well in 96-well flat-bottom plates. Growth was assessed using CellTiter 96 Aqeous One Solution Cell Proliferation Assay per the manufacturer's instructions (Promega, Madison, WI). To assess differentiation, cells were cytocentrifuged and Jenner-Giemsa stained, and the proportion of differentiated cells was determined microscopically. To assess cell viability, the percentage of cells excluding trypan blue was determined microscopically using a hemocytometer.
Western blot analysis
For preparation of lysates for phospho-ERK analysis, 107 cells were initially starved for 6 hours in growth media without cytokine. Cells were left unstimulated or stimulated with 1000 bone marrow (BM) units/mL murine IL-3 for 5 minutes. Lysates were prepared and Western blot analysis was performed as described previously.27
Results
Construction of C-terminal truncations of the human βc activated mutants
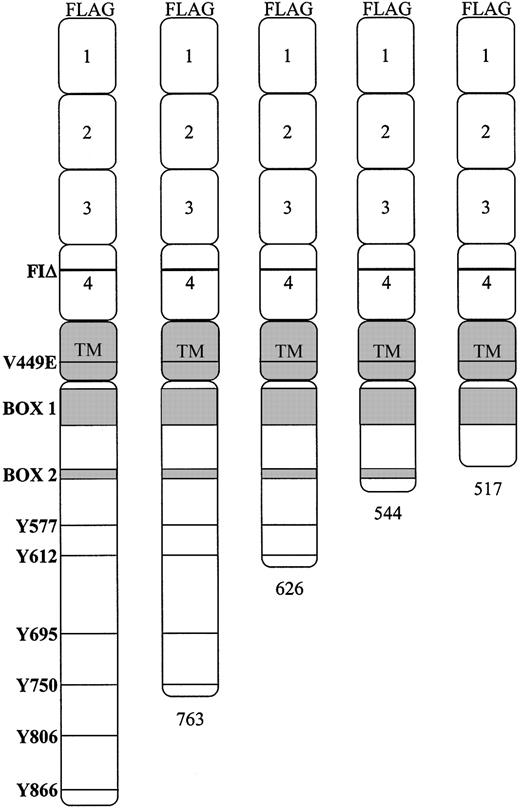
To examine functional regions of hβc necessary for inducing proliferative and differentiative signals we initially constructed a series of V449E and FIΔ mutants with sequential deletions of the cytoplasmic domain (Figure 1). Each was epitope-tagged at the N-terminus with the FLAG peptide to facilitate detection in the FDB1 cells. The numbering system that refers to the activated point mutation (V449E) includes the leader sequence of the hβcasinour previous publications; however, to maintain consistency with other studies that have used cytoplasmic truncations,10,30,31 our numbering system for truncations and cytoplasmic tyrosines does not include the leader sequence.
Schematic representation of the hβc. The positions of the activated mutants and the 6 distal cytoplasmic tyrosine residues are indicated. The cytoplasmic truncations, made in combination with each of the V449E and FIΔ activated mutants, are depicted relative to the full-length cytoplasmic domain.
Schematic representation of the hβc. The positions of the activated mutants and the 6 distal cytoplasmic tyrosine residues are indicated. The cytoplasmic truncations, made in combination with each of the V449E and FIΔ activated mutants, are depicted relative to the full-length cytoplasmic domain.
Ability of the V449E truncation mutants to induce factor-independent proliferation
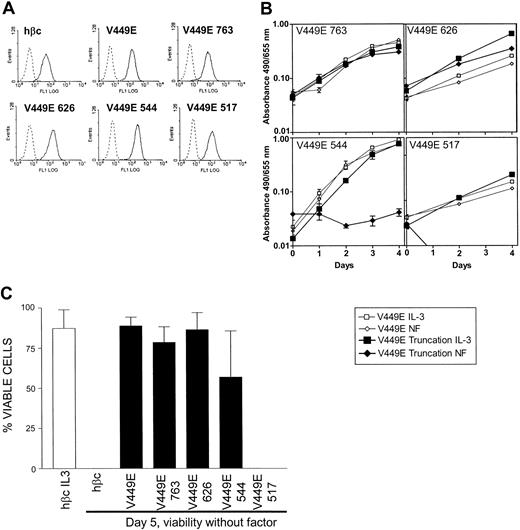
Pools of cells expressing full-length V449E and each of the 4 V449E cytoplasmic truncations were generated by retroviral infection. In all cases, expression by pools of infected cells was confirmed by flow cytometry (as shown in Figure 2A) and Western analysis (data not shown). As observed previously,18 full-length V449E generates a strong proliferative signal such that cells were able to proliferate without factor in a manner similar to cells in IL-3 (Figure 2B). Cells expressing each truncation mutant were compared with the full-length V449E in at least 3 independent experiments; representative results are shown in Figure 2B. Truncation of the cytoplasmic domain of the V449E mutant to amino acids 763 or 626 did not affect its ability to confer factor-independent proliferation on FDB1 cells when compared with the full-length V449E (Figure 2B). Not unexpectedly, these truncations were also able to maintain full cellular viability over a 5-day period in the absence of factor (Figure 2C). These results indicate that the residues distal of 626 are dispensable for proliferative and survival signals generated by this factorindependent mutant. In contrast, growth and viability were clearly affected by truncating the V449E cytoplasmic domain to amino acid 544. Factor-independent growth was abolished, however a substantial proportion of the cells were still viable, at day 5 without factor (Figure 2C). A further truncation of V449E to residue 517 completely abolished the ability of cells expressing this mutant to maintain growth or viability without factor (Figure 2B-C). These results indicate that an essential proliferation signal generated by V449E originates from the domain between amino acids 545 and 626 and that a viability signal emanates from the region between amino acids 518 and 544.
Effect of V449E cytoplasmic truncations on factor-independent growth and viability. (A) Cell surface expression of the indicated FLAG-tagged hβc proteins on FDB1 cells expressing cytoplasmic truncations of the V449E activated mutant. Infected cells (solid lines) were stained with an anti-FLAG monoclonal antibody and compared with identically stained uninfected cells (dotted lines). (B) Proliferation of FDB1 cells expressing cytoplasmic truncations of the V449E activated mutant. Growth without factor (NF indicates no factor) (diamonds) is shown compared with growth with the control cytokine IL-3 (squares). Results representative of at least 3 independent experiments are shown. Error bars indicate the SD of triplicate samples. (C) Viability of cells without factor at day 5 after factor withdrawal. Cell viability was determined by trypan blue exclusion, and the combined results of at least 3 independent experiments are shown. Error bars represent the SD of at least 3 independent experiments.
Effect of V449E cytoplasmic truncations on factor-independent growth and viability. (A) Cell surface expression of the indicated FLAG-tagged hβc proteins on FDB1 cells expressing cytoplasmic truncations of the V449E activated mutant. Infected cells (solid lines) were stained with an anti-FLAG monoclonal antibody and compared with identically stained uninfected cells (dotted lines). (B) Proliferation of FDB1 cells expressing cytoplasmic truncations of the V449E activated mutant. Growth without factor (NF indicates no factor) (diamonds) is shown compared with growth with the control cytokine IL-3 (squares). Results representative of at least 3 independent experiments are shown. Error bars indicate the SD of triplicate samples. (C) Viability of cells without factor at day 5 after factor withdrawal. Cell viability was determined by trypan blue exclusion, and the combined results of at least 3 independent experiments are shown. Error bars represent the SD of at least 3 independent experiments.
Differentiation of factor-independent cells expressing V449E C-terminal truncations
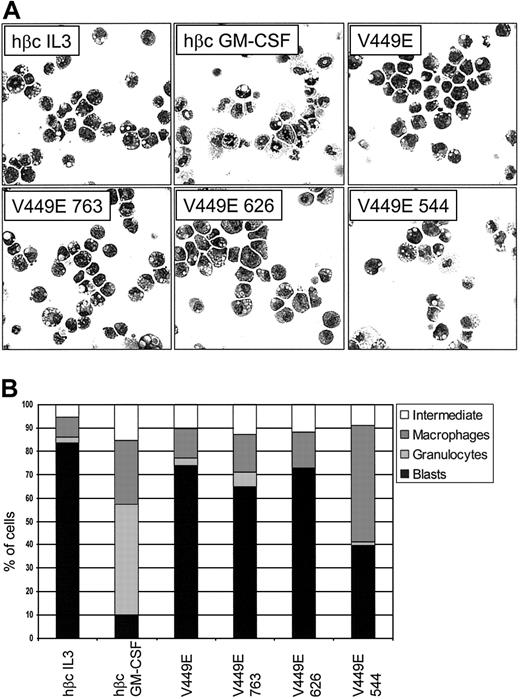
As FDB1 cells are capable of granulocyte-macrophage differentiation, we examined the morphology of factor-independent cells expressing the V449E truncations to see if altered signaling by these mutants was affecting the differentiation state of the cells. Control FDB1 cells (expressing only wild-type [wt] βc) were cultured in either mIL-3 or mGM-CSF for 5 days as a comparison. As expected, parental FDB1 cells cultured in mIL-3 were undifferentiated and those cultured in mGM-CSF, at day 5, had differentiated into neutrophils and macrophages (Figure 3A-B). At day 5 of culture without factor, cells expressing full-length V449E and the 763 and 626 truncations were also undifferentiated. This is consistent with the ability of these truncations to mediate factor-independent proliferation and viability. In contrast, cells expressing the V449E 544 truncation displayed increased differentiation along the monocyte/macrophage lineage, revealing the presence of a signal from the 545-626 region that is suppressing macrophage differentiation.
Morphology of cells expressing C-terminal truncations of the V449E activated mutant. (A) Control cells (expressing wt hβc) were cultured in the indicated growth factors for 5 days. Cells expressing truncations of the activated mutant were cultured in the absence of growth factor for 5 days. At day 5, cells were cytocentrifuged to assess morphology. Original magnification, × 400. (B) Differential counts from cytospins done at day 5. For each sample, 200 cells were scored microscopically for morphology. The combined data from a minimum of 3 independent experiments are shown. The standard error of the mean was less than 10% for all samples.
Morphology of cells expressing C-terminal truncations of the V449E activated mutant. (A) Control cells (expressing wt hβc) were cultured in the indicated growth factors for 5 days. Cells expressing truncations of the activated mutant were cultured in the absence of growth factor for 5 days. At day 5, cells were cytocentrifuged to assess morphology. Original magnification, × 400. (B) Differential counts from cytospins done at day 5. For each sample, 200 cells were scored microscopically for morphology. The combined data from a minimum of 3 independent experiments are shown. The standard error of the mean was less than 10% for all samples.
Tyrosine 577 is essential for proliferation of the V449E mutant but not for survival
To further delineate the signaling events evoked by the V449E mutant we next examined the importance of tyrosine residues in the cytoplasmic region. To this end, we constructed a mutant where all 6 distal cytoplasmic tyrosine residues were replaced with phenylalanine (Y6F; see Figure 1 for positions of tyrosines). In addition, because we had found that the 544-626 cytoplasmic region of V449E is important for proliferation and suppression of differentiation, we constructed mutants that replaced either or both of the 2 tyrosine residues in this region singly or together with phenylalanine (Y577F, Y612F, and Y2F, respectively).
Since we previously reported that the V449E activated mutant exhibits constitutive tyrosine phosphorylation in FDC-P1 cells,27 we predicted that at least some tyrosine residues may be important for its activity. This was confirmed by the inability of V449E Y6F to confer factor-independent proliferation on FDB1 cells and a marked reduction in its ability to sustain viability without factor at day 5 (Figure 4A-B). Mutation of tyrosine 577 to phenylalanine abolished the ability of V449E to proliferate without factor, consistent with a major proliferative signal emanating from this tyrosine residue (Figure 4A). Interestingly, however, this mutation was able to separate factor-independent proliferation from viability, as there was no reduction in viability at day 5 (Figure 4B). Mutation of tyrosine 612 did not have a measurable effect on proliferation or viability (Figure 4A-B), and, not surprisingly, mutation of both tyrosines together (Y2F) gave a phenotype identical to that observed for Y577F alone (Figure 4A-B). To determine whether the effects of Tyr577 in V449E were transduced by ERK 1/2 kinases, we performed phospho-ERK Western blots of lysates from the mutant cell lines (Figure 4C). V449E was able to constitutively activate ERK 1/2, however mutation of Tyr577 alone or Tyr577 and Tyr612 together (Y2F) did not abolish this activation. In contrast, removal of the 6 distal tyrosines by mutation (Y6F) or truncation (V449E 544) severely reduced constitutive ERK 1/2 activation. Stimulation of cells with mIL-3 was able to induce equal amounts of phospho-ERK 1/2 in all the cell lines, indicating that there was no intrinsic defect in this signaling pathway in any of the cells examined (data not shown).
Effects of V449E cytoplasmic tyrosine mutants on factor-independent growth and viability. (A) Growth without factor (diamonds) is shown compared with growth with the control cytokine IL-3 (squares). Results representative of at least 3 independent experiments are shown. Error bars represent the SD of triplicate samples. (B) Viability of cells without factor at day 5 after factor withdrawal. Cell viability was determined by trypan blue exclusion, and the combined results of at least 3 independent experiments are shown. Error bars represent the SD of at least 3 independent experiments. (C) Constitutive activation of ERK1/2. Lysates were made from FDB1 cells expressing mutant receptors and were blotted for phospho-ERK1/2 and total ERK1/2 as described in “Materials and methods.”
Effects of V449E cytoplasmic tyrosine mutants on factor-independent growth and viability. (A) Growth without factor (diamonds) is shown compared with growth with the control cytokine IL-3 (squares). Results representative of at least 3 independent experiments are shown. Error bars represent the SD of triplicate samples. (B) Viability of cells without factor at day 5 after factor withdrawal. Cell viability was determined by trypan blue exclusion, and the combined results of at least 3 independent experiments are shown. Error bars represent the SD of at least 3 independent experiments. (C) Constitutive activation of ERK1/2. Lysates were made from FDB1 cells expressing mutant receptors and were blotted for phospho-ERK1/2 and total ERK1/2 as described in “Materials and methods.”
Morphology of factor-independent cells expressing V449E cytoplasmic tyrosine mutants
We next assessed the effects of V449E cytoplasmic tyrosine mutants on the differentiation of FDB1 cells (Figure 5). Cells expressing the Y6F mutant (which has only 20% viability at day 5) had the morphologic appearance of macrophages with the presence of very few undifferentiated cells. Thus, in the absence of major proliferation and survival signaling macrophages are the predominant remaining cell type. Cells expressing the Y577F mutant, which do not proliferate but retain viability, interestingly showed a small increase in the number of macrophages compared with parental V449E (Figure 5A-B). As was seen with factor-independent viability and proliferation, the Y612F mutant had no effect on V449E suppression of differentiation (Figure 5A-B). However, mutation of tyrosines 577 and 612 together (in the Y2F mutant) resulted in a marked shift from the blast cell phenotype to macrophages, considerably more than observed by mutating Tyr577 alone (Figure 5A-B). Thus, Tyr612 and Tyr577 may each contribute to a signal that suppresses differentiation.
Morphology of cells expressing cytoplasmic tyrosine mutants of the V449E activated mutant. (A) Cells expressing the indicated cytoplasmic tyrosine substitutions were cultured in the absence of growth factor for 5 days and cytocentrifuged to assess morphology. Original magnification, × 400. (B) Differential counts from cytospins done at day 5. For each sample, 200 cells were scored microscopically for morphology. The combined data from a minimum of 3 independent experiments are shown. The standard error of the mean was less than 10% for all samples.
Morphology of cells expressing cytoplasmic tyrosine mutants of the V449E activated mutant. (A) Cells expressing the indicated cytoplasmic tyrosine substitutions were cultured in the absence of growth factor for 5 days and cytocentrifuged to assess morphology. Original magnification, × 400. (B) Differential counts from cytospins done at day 5. For each sample, 200 cells were scored microscopically for morphology. The combined data from a minimum of 3 independent experiments are shown. The standard error of the mean was less than 10% for all samples.
These experiments show that there are both tyrosine-dependent and -independent viability signals generated by V449E. Importantly we have also shown that Tyr577 is specifically required for factor-independent proliferation and that multiple tyrosines contribute to a signal that suppresses differentiation of the FDB1 cell line. Next, we wished to compare these results with those obtained using corresponding second site mutants of FIΔ, which induces factor-independent granulocyte-macrophage differentiation, rather than continued factor-independent proliferation.
Function of the FIΔ cytoplasmic deletion mutants in factor-independent proliferation
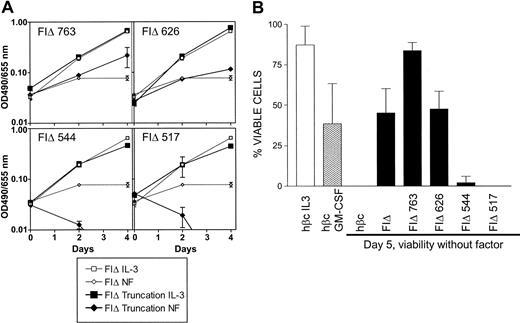
Pools of infected cells expressing each of the 4 cytoplasmic FIΔ truncations were cultured in the absence of factor and assays were performed to proliferation and viability. As previously reported,18 no significant proliferation was seen without factor in cells expressing the full-length FIΔ mutation (Figure 6A). In contrast, cells expressing the FIΔ 763 truncation appeared to be capable of some expansion without factor (Figure 6A) associated with improved viability over a 5-day period (Figure 6B). This may be due to the removal of a negative regulatory domain that has been previously reported to be in this region.21 The survival advantage of the FIΔ 763 truncation was not seen in the cells expressing the FIΔ 626 truncation, which behaved similarly to cells expressing the full-length FIΔ mutant (Figure 6A-B). In contrast, cells expressing FIΔ truncated to amino acid 544 lost all ability to maintain viability without factor as did cells expressing FIΔ 517. Thus in the FIΔ mutant, a major survival signal originates from the region between amino acids 545 and 626. The tyrosine-independent survival signal originating from between residues 518 and 544 of the V449E mutant is not generated by the FIΔ activated mutant, consistent with different modes of signaling for the EC and TM classes of mutants.
Effects of FIΔ cytoplasmic truncations on factor-independent growth and viability. (A) Proliferation of FDB1 cells infected with cytoplasmic truncations of the FIΔ activated mutant. Growth without factor (diamonds) is shown compared with growth with the control cytokine IL-3 (squares). Results representative of at least 3 independent experiments are shown. Error bars represent the SD of triplicate samples. (B) Viability of cells without factor at day 5 after factor withdrawal. Cell viability was determined by trypan blue exclusion, and the combined results of at least 3 independent experiments are shown. Error bars represent the SD.
Effects of FIΔ cytoplasmic truncations on factor-independent growth and viability. (A) Proliferation of FDB1 cells infected with cytoplasmic truncations of the FIΔ activated mutant. Growth without factor (diamonds) is shown compared with growth with the control cytokine IL-3 (squares). Results representative of at least 3 independent experiments are shown. Error bars represent the SD of triplicate samples. (B) Viability of cells without factor at day 5 after factor withdrawal. Cell viability was determined by trypan blue exclusion, and the combined results of at least 3 independent experiments are shown. Error bars represent the SD.
Differentiation of factor-independent cells expressing FIΔ cytoplasmic truncations
We next examined the morphology of cells expressing the C-terminal truncations after 5 days without factor. As expected, the full-length FIΔ mutant could induce the differentiation of FDB1 cells to both granulocytes and macrophages (Figure 7A-B). A slight increase in the number of undifferentiated cells at day 5 was observed with the FIΔ 763 expressing cells compared with those expressing full-length FIΔ. Cells expressing FIΔ 626 behaved similarly to full-length FIΔ, differentiating completely by day 5; however the cells showed a bias toward granulocytic differentiation at this time point (Figure 7B). In contrast, the lack of viable factor-independent cells expressing FIΔ 544 shows that survival of cells expressing FIΔ is strictly dependent on the 544-626 region. We cannot rule out that a similar survival signal emanates from the V449E mutant, as it may be masked by the presence of the secondary signal in the proximal region. However the FIΔ mutant clearly does not activate the proliferation pathway that originates in the 545-626 region of V449E.
Morphology of cells expressing cytoplasmic truncations of the FIΔ activated mutant. (A) Control cells (expressing wt hβc) were cultured in the indicated growth factors for 5 days. Cells expressing truncations of the activated mutant were cultured in the absence of growth factor for 5 days. At day 5, cells were cytocentrifuged to assess morphology. Original magnification, × 400. (B) Differential counts from cytospins done at day 5. For each sample, 200 cells were scored microscopically for morphology. The combined data from a minimum of 3 independent experiments is shown. The standard error of the mean was less than 10% for all samples.
Morphology of cells expressing cytoplasmic truncations of the FIΔ activated mutant. (A) Control cells (expressing wt hβc) were cultured in the indicated growth factors for 5 days. Cells expressing truncations of the activated mutant were cultured in the absence of growth factor for 5 days. At day 5, cells were cytocentrifuged to assess morphology. Original magnification, × 400. (B) Differential counts from cytospins done at day 5. For each sample, 200 cells were scored microscopically for morphology. The combined data from a minimum of 3 independent experiments is shown. The standard error of the mean was less than 10% for all samples.
FIΔ generates tyrosine-dependent survival signals
We have previously shown that the EC hβc mutants, I374N and FIΔ, which are capable of inducing factor-independent proliferation of FDC-P1 cells and differentiation of FDB1 cells, do not exhibit any detectable tyrosine phosphorylation in the absence of factor (McCormack and Gonda18 and Jenkins et al27 ). Despite this, mutational analysis has shown that replacement of all I374N cytoplasmic tyrosines with phenylalanine compromises its ability to induce factor-independent growth in FDC-P1 cells (T. Blake and T.J.G., unpublished data, July 1998). We therefore predicted that tyrosine residues might also be important for constitutive activity of the EC FIΔ duplication mutant. We introduced phenylalanine substitutions equivalent to those used to replace cytoplasmic tyrosine residues in V449E (ie, Y6F, Y577F, Y612F, and Y2F) and expressed these in FDB1 cells. Substitution of the 6 membrane distal cytoplasmic tyrosine residues with phenylalanine abolished factor-independent activity of FIΔ, as shown by the rapid loss of cellular viability. This was similar to the effect seen with cells expressing only wt hβc after factor withdrawal (Figure 8A-B). Substitution of tyrosine residues 577 or 612, separately or together, did not affect viability signals from FIΔ as these mutants were able to maintain survival without factor at least as well as parental FIΔ (Figure 8B). These data show an absolute requirement for at least some cytoplasmic tyrosines for the factor-independent activity of FIΔ. Any effect of the Y2F mutation on viability may be masked in the context of the full-length receptor, as the effects of the Y6F mutant show that viability signals must also be emanating from other cytoplasmic tyrosines. These results indicate that there are multiple viability signals generated in FIΔ, any of which may be sufficient, but none of which have been found to be essential for maintaining viability.
Effects of FIΔ cytoplasmic tyrosine mutants on factor-independent growth and viability.(A) Growth without factor (diamonds) is shown compared with growth with the control cytokine IL-3 (squares). Results representative of at least 3 independent experiments are shown. Error bars represent the SD of triplicate samples. (B) Viability of cells without factor at day 5 after factor withdrawal. Cell viability was determined by trypan blue exclusion, and the combined results of at least 3 independent experiments are shown. Error bars represent the SD.
Effects of FIΔ cytoplasmic tyrosine mutants on factor-independent growth and viability.(A) Growth without factor (diamonds) is shown compared with growth with the control cytokine IL-3 (squares). Results representative of at least 3 independent experiments are shown. Error bars represent the SD of triplicate samples. (B) Viability of cells without factor at day 5 after factor withdrawal. Cell viability was determined by trypan blue exclusion, and the combined results of at least 3 independent experiments are shown. Error bars represent the SD.
Cytoplasmic tyrosine mutations of FIΔ can modulate factor-independent differentiation of FDB1 cells
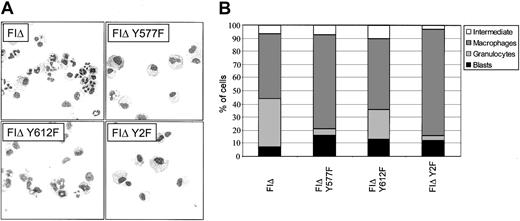
To investigate the potential effects of the FIΔ tyrosine mutations on differentiation, the morphology of cells expressing these mutants was assessed at day 5 of culture without factor. Substitution of tyrosine 577 with phenylalanine almost totally abolished neutrophil differentiation but did not affect macrophage differentiation (Figure 9A-B). Substitution of tyrosine 612 with phenylalanine was also able to shift the balance of differentiation to macrophage production, however the effect seen was not as dramatic. As might be expected, the Y2F mutant also showed almost total loss of neutrophil differentiation. Thus, the signals for neutrophil and macrophage differentiation induced by FIΔ are separable.
Morphology of cells expressing cytoplasmic tyrosine mutants of the FIΔ activated mutant. (A) Cells expressing the indicated cytoplasmic tyrosine substitutions were cultured in the absence of growth factor for 5 days and cytocentrifuged to assess morphology. Original magnification, × 400. (B) Differential counts from cytospins done at day 5. For each sample, 200 cells were scored microscopically for morphology. The combined data from a minimum of 3 independent experiments is shown. The standard error of the mean was less than 10% for all samples except for FIΔ Y577F blasts (SEM = 14.7%), and FIΔ Y577F macrophages (SEM = 18.4%).
Morphology of cells expressing cytoplasmic tyrosine mutants of the FIΔ activated mutant. (A) Cells expressing the indicated cytoplasmic tyrosine substitutions were cultured in the absence of growth factor for 5 days and cytocentrifuged to assess morphology. Original magnification, × 400. (B) Differential counts from cytospins done at day 5. For each sample, 200 cells were scored microscopically for morphology. The combined data from a minimum of 3 independent experiments is shown. The standard error of the mean was less than 10% for all samples except for FIΔ Y577F blasts (SEM = 14.7%), and FIΔ Y577F macrophages (SEM = 18.4%).
Discussion
Understanding the role of the GM-CSF receptor family in controlling myeloid progenitor cell survival, proliferation, and differentiation and the mechanisms through which these effects occur has proved to be complex and challenging. We have developed a system with 2 key features designed to help better address these issues. First, the FDB1 cell line has proved to be a powerful model system in which to study GM-CSF/IL-3 receptor signaling, as (like normal cells) it is strictly factor-dependent and shows the full spectrum of biologic responses, that is, proliferation, and differentiation in response to IL-3 and GM-CSF, respectively. Second, we have used FDB1 cells together with a set of constitutive mutants of hβc, which induce biologic effects similar to IL-3 and GM-CSF but do not activate all pathways downstream of the normal GM-CSF receptor, thus reducing the redundancy of pathways involved. This is of clear importance as one of the main complications in connecting signaling events to biologic outcomes is the presence of multiple, functionally equivalent pathways. That this occurs for the GM-CSF receptor family is clear from several previous studies.10,21,22,30-32 While multiple regions have been associated with survival, proliferation, and differentiation in different circumstances, a reduced level of redundancy in our system has allowed the association of new receptor features and signaling pathways with survival, proliferation, self-renewal, and modulation of differentiation (summarized in Figure 10).
Summary of the effects on FDB1 cells of V449E and FIΔ mutational analysis.
The membrane-proximal region (518-544) of the V449E mutant is required for a tyrosine-independent survival signal
This short region (518-544) of hβc is conserved in the cytokine receptor family and contains the Box 2 motif.33 Importantly, we have found this region to be active in generation of a constitutive survival signal from the V449E mutant. In contrast, an equivalent truncation of FIΔ was unable to support factor-independent survival. This differential signaling is most likely related to the nature of the complex formed by the constitutive receptors. The mode of activation of the V449E mutant is presumed to involve homodimerization, while that of the EC mutants involves a functional association with GMRα.23 The EC mutants, while able to activate JAK2, lack detectable receptor tyrosine phosphorylation, consistent with the failure to form appropriate hβc dimers that mediate transphosphorylation by associated JAK2.
While it is tempting to ascribe the survival function of the 518-544 region to the Box 2 motif, it has not been demonstrated to bind any specific signaling molecules. Moreover, internal deletion of Box 2 does not affect the ability of the GM-CSF receptor to induce short-term proliferation and JAK2 activation in Ba/F3 cells.7,8 Therefore we may be able to use our system to further define signaling activities associated with Box 2.
A tyrosine-dependent proliferation signal emanates from Tyr577 in V449E
A key aim of this study was to determine regions of the hβc cytoplasmic domain that are required for proliferative signaling by V449E. The region between amino acids 545 and 626 was essential for factor-independent proliferation, and further mutational analysis determined tyrosine 577 to be an essential requirement. Cells expressing the V449E 544 truncation or V449E Y577F did not proliferate but were still able to survive without factor, demonstrating that proliferative and survival signals can be separated. Previous work from several other laboratories has shown that the presence of Tyr577 is required for binding and phosphorylation of the Shc adaptor molecule.7,8,13 In fact Tyr577 is both necessary and sufficient for activation of Shc.34 This is in contrast to other signaling molecules associated with this tyrosine residue such as SHP2 and STAT5b, whose activation can also be mediated by several other tyrosine residues.12 Interestingly, in FDC-P1 cells, we have found that activation of Shc can be detected in response to signaling from V449E but not the I374N EC mutant (T. Blake and T.J.G., unpublished data, October 1998), further associating Shc with the proliferative activity of the V449E mutant. Activation of Shc has been linked to the MAPK (for a recent review see Ravichandran35 ), PI3-K,15 and Src family kinase pathways.36 Gab2 activation also occurs through tyrosine 577, while a dominant-negative Gab2 suppresses PI3-K activation and proliferation of Ba/F3 cells in response to IL-3.15 Importantly, mutation of this tyrosine to phenylalanine (Y577F) in the context of wild-type hβc (while abolishing detectable Shc activation) does not significantly affect proliferation or viability of Ba/F3 cells in response to hGM-CSF.13 This is not surprising as activation of both ERK1/2 and PI3-K/Akt can occur by Shc-independent routes.13,16,17 In agreement with this, we found that mutation of Tyr577 did not abolish V449E-induced constitutive activation of ERK1/2 and that mutation of several additional tyrosine residues was required to significantly affect ERK1/2 activation (Figure 4C). As activation of the ERK1/2 MAPK pathway is intact in the V449E Y577F mutant, it is likely that the loss of a different Shc-mediated signaling pathway may be responsible for the observed proliferation defect. Future biochemical studies in this system will confirm this and lead to the elucidation of this pathway.
Ablation of the major V449E proliferative signal also had significant effects on the differentiation status of cells. FDB1 cells expressing the mutant forms of V449E showed an increased propensity to differentiate to macrophages. This suggests the possibility that proliferation is associated with a block in the pathway mediating macrophage differentiation, at least (next paragraph). Macrophage differentiation was most pronounced when Tyr577 and Tyr612 were mutated together (in the Y2F mutant). Thus, although Tyr612 was not shown to affect V449E activity when mutated alone, it contributes to a signal that can block differentiation of FDB1 cells.
Little neutrophil differentiation was observed with V449E Y2F, even at time points earlier than day 5 (data not shown), suggesting that the mutant either retains the ability to suppress neutrophil differentiation and/or lacks the ability to induce it. We favor the former since our preliminary experiments have shown that V449E Y2F can suppress neutrophil differentiation induced by GM-CSF (data not shown). Thus V449E appears to generate a proliferation signal that is closely coupled to suppression of macrophage differentiation but independent of a separate, as-yet-undefined signal that suppresses granulocyte differentiation. The pathways that mediate such blocks in myeloid differentiation are not well understood but play an important role in leukemic transformation. These mutants may provide useful tools for dissecting such a pathway.
FIΔ requires tyrosine residues for viability despite its lack of detectable tyrosine phosphorylation
The FIΔ truncation series shows that factor-independent differentiation and viability signals originate from the region between amino acids 545 and 626. Given that mutation of all 6 membrane distal cytoplasmic tyrosines to phenylalanine abolished survival in the absence of factor, we tested whether the tyrosine residues in the 545-626 region were important for FIΔ-induced survival. However mutation of these 2 residues in full-length FIΔ did not affect viability, indicating that tyrosine residues downstream of amino acid 626 are contributing to an independently generated signal for viability. This result, demonstrating a tyrosine-dependent viability signal in FIΔ was of particular interest given that EC mutants have no detectable phosphorylation of tyrosine residues.27 We cannot rule out that the tyrosine residue/s responsible for FIΔ viability signaling are phosphorylated at a level that is sufficient for physiologic signaling via phosphotyrosine binding proteins but undetectable using phosphotyrosine antibodies. These results also raise the possibility that proteins that bind nonphosphorylated tyrosine residues are involved in this response (for review see Yaffe37 ).
Tyr577 is required for a signal that supports granulocyte differentiation induced by FIΔ
A previous study of the GM-CSF receptor family in myeloid cell differentiation has indicated a specific role for GMRα in induction of differentiation.19 Regions of the IL-3 and GM-CSF α subunit cytoplasmic domains have been identified that contribute to specifying a proliferation or differentiation response (see also Evans et al20 ); however, these studies did not explore the regions of the hβc required for the different responses. One of the aims of our study was to separate signals for granulocyte and macrophage differentiation emanating from FIΔ. This was problematic using truncations, as all signaling was abolished by the FIΔ 544 truncation. Importantly, however, a single amino acid substitution (Y577F) in the FIΔ-activated mutant specifically abolished granulocytic differentiation at day 5 following factor withdrawal. With this mutant, cells present at day 5 of differentiation were predominantly macrophages in contrast to normal FIΔ, which generated approximately equal numbers of granulocytes and macrophages. The presence of granulocytes (up to 20%) was noted at day 2 of factor withdrawal (compare with normal FIΔ, which had 38% granulocytes at day 2), but numbers dropped rapidly after this time point (data not shown). This result suggests that multiple signals are generated that contribute to the pathway that stimulates maximal granulocyte production and survival in the FDB1 cell line. The nature of the disruption in FIΔ signaling by the Y577F mutant is intriguing given that, as discussed in “FIΔ requires tyrosine residues for viability despite its lack of detectable tyrosine phosphorylation,” there is no detectable phosphorylation of the receptor on EC mutant tyrosine residues.27 The major signaling events that have been delineated as originating from Tyr577 thus far all involve activation of Shc or SHP2, both of which are thought to require binding to phosphotyrosine. As we have observed that EC mutants do not appear to activate Shc (T. Blake, unpublished observations, October 1998), it is unlikely that Y577F is having an effect on granulocyte differentiation via a Shc-dependent pathway. We are now exploring the possibility that activation of a novel signaling pathway is involved in mediating this differentiation.
In summary, our data are consistent with constitutive mutants of hβc activating only a subset of the pathways activated by the wild-type receptor in response to ligand.27 This has provided an opportunity to remove a significant level of redundancy from the GM-CSF receptor system and permitted simultaneous analysis of survival, proliferation, and the differentiation response. Follow-up studies using biochemical and gene expression profiling approaches will be informative with regard to associating signaling pathways and downstream genes with the different cellular outcomes. Ultimately, we predict that these studies will lead to a better understanding of the receptor-associated signaling and gene expression changes associated with myeloid cell behavior and leukemogenesis.
R.J.D. and T.J.G. contributed equally to this work.
Supported by research grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (R.J.D. and T.J.G.) and the National Health and Medical Research Council of Australia (T.J.G.). T.J.G. was a Senior Research Fellow of the National Health and Medical Research Council of Australia, and R.J.D. was a Henley Properties Principal Research Fellow and is currently a Peter Nelson Leukaemia Research Fellow of the Cancer Council of South Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-05-1435.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal