Abstract
In this prospective multicenter program, we investigated allogeneic stem cell transplantation (ASCT) from HLA-identical siblings following reduced-intensity conditioning (RIC) regimen for patients with refractory metastatic solid tumors (STs). Fifty-seven patients, of whom 39 had a progressive disease (PD) at time of ASCT, received an RIC ASCT combining fludarabine, antithymocyte globulin (ATG), and busulfan. Patients were analyzed in terms of engraftment, transplant-related mortality (TRM), disease response, and outcome. In this setting, RIC was associated with rapid engraftment and low overall TRM (9% [95% confidence interval (CI), 1%-16%]). The cumulative incidence of objective responses (ORs) reached 14% (95% CI, 6%-30%) with this being significantly higher in patients without PD (44% [95% CI, 21%-67%] versus 0; P < .0001) at time of ASCT. Achievement of OR translated into a significantly better overall survival (OS). In multivariate analysis, OS was significantly influenced by disease status at time of ASCT (odds ratio, 4.88; P < .001) and chronic graft-versus-host disease (GVHD) occurrence (odds ratio, 2.86; P < .01). Overall, these results showed that OR can occur after RIC ASCT for resistant ST with a relatively low TRM and potential benefit especially in patients with slowly progressive disease. Further studies are warranted in patients with less advanced ST.
Introduction
The graft-versus-leukemia (GVL) effect has been shown to be a major component of the antileukemic efficacy of allogeneic stem cell transplantation (ASCT).1 Moreover, T-lymphocyte depletion of the graft and the therapeutic use of donor lymphocyte infusions (DLIs)2 have led to a better appreciation of this GVL effect.2-4 In patients suffering from refractory solid malignancies, only anecdotal evidence of a graft-versus-tumor (GVT) effect had been reported thus far.5,6 Because the initial report suggesting some clinical and biologic clues supporting a GVT effect, at least in breast cancer,5 the overall experience of ASCT in patients with solid tumors (STs) remained scarce.7,8 In 2001, a total of 6413 ASCTs were reported to the European Group for Blood and Marrow Transplantation (EBMT) registry, but only 149 (2%) were performed in patients with STs.9 However, the benefit of immunotherapeutic approaches for selected STs is now widely documented in a nonallogeneic setting,10 indicating that some immune effectors are able to induce tumor regression. In addition, the latter has been further established with the development of dendritic cell vaccines.11 In the allogeneic setting, the importance of minor HLA antigen mismatch was shown to be an important determinant of immune tumor control.12 On the basis of the initial case reports, we started in 1996 to investigate the potential of ASCT in patients with advanced metastatic STs. Our main objective was to investigate the feasibility of ASCT defined as a procedure being acceptable for both patients and the medical oncology community. Our initial experience combined with that of others confirmed that standard-dose myeloablative ASCT is associated with a GVT effect, but also a prohibitive transplant-related toxicity rate.7,13 The introduction of reduced-intensity preparative regimens that could mediate a potent GVT effect in patients with hematologic malignancies14-16 offered an attractive tool for investigation in patients with STs. On the basis of these encouraging results, we investigated ASCT for STs with an antithymocyte globulin (ATG)–based reduced-intensity conditioning (RIC) regimen.15 Here, we report our experience in 57 consecutive patients with a diagnosis of various advanced STs treated in 8 transplantation centers in France. The patients were all included in the same cooperative program over a period of 3.5 years. The main objective of this trial was to determine the optimal transplantation procedure allowing for minimal transplant-related mortality (TRM) with maximum GVT effect and, therefore, included sequential modifications of the initial conditioning regimen and graft source.
Patients and methods
Patients and donors
Patients aged younger than 60 years with a diagnosis of a metastatic or refractory ST and who had an HLA-identical sibling donor were prospectively included in this trial from October 1998 through July 2002. Advanced disease was defined as a primary or secondary metastatic spread with failure of at least one line of appropriate treatment for the specific tumor. Measurable disease was not required before inclusion. HLA compatibility was determined by serologic typing for HLA class I antigens and molecular typing for HLA class II antigens. The protocol was approved by the Institut Paoli Calmettes Institutional Review Board, the local ethical committee of Marseille II (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale), the scientific committee of the Sociéte Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC), and the French agency for health products security (AFSSAPS). All patients and donors gave written informed consent prior to entry into this study. Five patients had previously been reported following a short follow-up.17 All data, obtained through January 2003, were analyzed.
Conditioning regimen
The conditioning regimen was adapted from the regimen reported by Slavin et al.15 It included fludarabine (Fludara; Schering, Lys-Lez-Lannoy, France) with a daily dose of 30 mg/m2 for 6 consecutive days (administered intravenously over 30 minutes), oral busulfan (BU) (4 mg/kg/d for 2 consecutive days) (GlaxoSmithKline, Marly-le-Roi, France), and ATG for 4 consecutive days (2.5 mg/kg/d administered intravenously over 6 to 8 hours) (SangStat, Lyon, France). This regimen was used unchanged for the first cohort of 9 patients. ATG was then reduced to a total dose of 7.5 mg/kg over 3 days in 8 patients, but 1 of them received only 5 mg/kg because of poor tolerance. Finally, ATG was further reduced to only 1 day (2.5 mg/kg) on day –3 for the remaining 40 patients.
Stem cell collection and infusion
Sixteen patients received a bone marrow (BM) graft, whereas 41 were infused with peripheral blood stem cells (PBSCs). BM was collected on day 0 under general anesthesia using conventional techniques with the goal to obtain 2 × 108 mononucleated cells per kilogram of recipient weight as previously reported.18 If PBSCs were to be collected, donors were treated with subcutaneous rh-granulocyte colony-stimulating factor (G-CSF; Neupogen; Amgen, Neuilly sur Seine, France) at a daily dose of 10 μg/kg. Apheresis was performed with the goal to harvest at least 4 × 106 CD34+ cells/kg of recipient weight in 2 to 4 consecutive sessions as previously reported.18 Unmanipulated BM or PBSC grafts were infused through central venous catheters on day 0. The stem cell product was analyzed in terms of hematopoietic progenitors (CD34+ cells) and CD3+ lymphoid cells by using standard flow cytometry procedures at each participating center as previously described.18 Per protocol, after each monthly evaluation after ASCT, a donor lymphocyte infusion (DLI) could be considered for patients with progressive disease. Cyclosporine A (CSA) was stopped at least 10 days before DLI, and patients had to be free of clinical graft-versus-host-disease (GVHD) at time of the DLI. The planned CD3+ cell dose of DLI was 1 × 10–7/kg, and a 2-month period was recommended between 2 infusions. A semi-log increase was recommended for the second dose.
Supportive care
Supportive care was performed according to standard procedures at each participating center and was expected to be the same for all patients for a given center.18 In each center, these procedures included laminar air flow or high-efficiency particular air (HEPA)–filtered rooms for initial hospitalization and oral intestinal decontamination with nonabsorbable antibiotics (colimycin and neomycin) from the beginning of conditioning until absolute neutrophil count (ANC) reached 0.5 × 109/L. Pneumocystis carinii prophylaxis included trimethoprim/sulfamethoxazole (10 mg/kg/d) administered before transplantation (days –10 to –1) and twice weekly (7 mg/kg/d trimethoprim) for at least 6 months, as soon as ANC exceeded 0.5 × 109/L. Prophylaxis against herpes simplex virus consisted of intravenous acyclovir 250 mg × 3 days, starting on day +2 until day +9. In addition, antibacterial and antifungal prophylaxis included intravenous vancomycin and amphotericin B. Broad-spectrum intravenous antibiotics were begun for temperatures greater than 38.5°C or for clinical signs of infection.
Hematologic recovery and GVHD assessment
Time to neutrophil engraftment was defined as the first of 3 consecutive days in which the ANC exceeded 0.5 × 109/L. Time to platelet engraftment was defined as the first of 3 days with 20 × 109/L without platelet transfusion during a 5-day period. GVHD prophylaxis included CSA alone without methotrexate. CSA was started intravenously on day –2 in all patients, at a dose of 2 mg/kg, and switched to oral formulation as soon as the patient was able to take oral medication after engraftment. The dosage was adjusted as previously reported.18 In case of tumor progression without evidence of GVHD, CSA was tapered usually over 10 to 15 days. Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were evaluated according to standard criteria.19,20
Disease response assessment
Tumor response was scored according to the international RECIST criteria.21 Investigations were chosen according to measurable sites of the disease at time of ASCT. Mostly, computer tomography (CT) scanning was performed on a monthly basis during the first year and then every third month. Tumor regression had to persist for at least 30 days after response for the patient to be considered as a responder. A complete response (CR) was defined as complete disappearance of all measurable disease. A partial response (PR) indicated at least a 30% decrease in the sum of the longest diameters of metastatic lesions compared with measurements before ASCT. Progressive disease (PD) was defined by at least a 20% increase in the same measurements or the occurrence of a new lesion. Stable disease (SD) was defined as changes not meeting the definitions of CR, PR, or PD.
Chimerism analysis
Patient–donor chimerism in unsorted peripheral blood and BM cells was assessed at days 15 to 30, 45 to 60, 90 to 120, and 150 to 180 after ASCT, using fluorescence in situ hybridization (FISH) to detect X and Y chromosomes for recipients of sex-mismatched transplants and polymerase chain reaction (PCR)–based analysis of polymorphic microsatellite regions for recipient of sex-matched transplants.22 Full donor chimerism was defined as the presence of more than 95% donor cells.
Patient accrual
Patients were studied in cohorts of 10. A new cohort of 10 patients was started if day-100 TRM was below 15% in the previous cohort.
Statistical analysis
All data were computed by using SEM for Windows (SILEX, Mirefleurs, France).23 The Mann-Whitney test was used for comparison of continuous variables. Categorical variables were compared by using the chi square test. Results were expressed as probabilities (%) with 95% confidence intervals (CIs). The cumulative incidence method was used to calculate the probabilities of developing aGVHD or cGVHD, the probabilities of achieving an objective response (OR) or dying of TRM.24 The probability of overall survival (OS) was calculated according to the Kaplan and Meier method, with surviving patients censored at the point of last follow-up, and differences in outcomes were computed by using the Mantel-Hanzel test.25 Because the occurrences of tumoral response or cGVHD were delayed after ASCT, OS was measured from a prespecified “landmark” time when analyzing effect of these 2 factors in a landmark analysis. The following factors were tested when appropriate to evaluate a potential predictive effect on incidence of OR and OS in a univariate analysis: diagnosis, age (age < 45 years versus age ≥ 45 years), disease status prior to ASCT (stable versus progressive), graft source (BM versus PBSC), ATG dose (1 day versus 2-4 days), aGVHD (grade 0-I versus grades II-IV), and cGVHD (yes versus no). A multivariate analysis was performed by using the Cox proportional hazard regression model, including in the model all the variables with P < .05 in the univariate analysis.26
Results
Patient characteristics
Patient characteristics are shown in Table 1. Four different diagnoses accounted for 84% of the population: renal cell carcinoma (RCC), breast carcinoma, malignant melanoma, and ovarian carcinoma. At time of ASCT, all patients had an evaluable disease and 39 of them (68%) had a PD. Patients had a median of 2 metastatic sites (range, 1-4) and received a median of 2 lines of treatment (range, 1-4) over a period of 33 months (range, 5-146 months) prior to ASCT. Fifteen patients (26%) had undergone autologous stem cell transplantation (Au-SCT) at a median of 27 months (range, 2-100 months) prior to ASCT.
Patient and donor characteristics
Patient age, y, mean ± SD | 45 ± 10 |
| No. of male patients (%) | 27 (47) |
| Donor age, y, mean ± SD | 43 ± 10 |
| Female donor, n (%) | 32 (56) |
| Diagnosis, n (%) | |
| Renal cell carcinoma | 25 (43) |
| Breast carcinoma | 12 (21) |
| Melanoma | 5 (9) |
| Ovarian carcinoma | 5 (9) |
| Soft tissue sarcoma | 4 (7) |
| Other diagnosis* | 6 (11) |
| Previous autologous transplantation, n (%) | 13 (23) |
| Disease status prior to transplantation, n (%) | |
| Progressive disease | 39 (68) |
| Stable disease | 18 (32) |
| ATG dose, n (%) | |
| 5-10 mg/kg | 17 (30) |
| 2.5 mg/kg | 40 (70) |
| Total ATG dose, mg/kg, mean ± SD | 267 ± 184 |
| Graft source, n (%) | |
| Bone marrow | 16 (28) |
| PBSC | 41 (72) |
| Day-90 donor chimerism | |
| Studied patients | 40 |
| Full donor chimera, n (%) | 37 (93) |
| Days to full chimera, mean ± SD | 52 ± 26 |
| Donor lymphocyte infusion | |
| Patients, n (%) | 20 (35) |
| Day of first DLI, mean ± SD | 151 ± 131 |
| Acute GVHD | |
| Grades I-IV, n (%) | 37 (65) |
| Grades II-IV, n (%) | 24 (42) |
| Day of onset grades II-IV acute GVHD, mean ± SD | 43 ± 23 |
| Chronic GVHD | |
| Evaluable, n | 42 |
| Limited/extensive, n | 17/8 |
| Day of onset, mean ± SD | 120 ± 30 |
Patient age, y, mean ± SD | 45 ± 10 |
| No. of male patients (%) | 27 (47) |
| Donor age, y, mean ± SD | 43 ± 10 |
| Female donor, n (%) | 32 (56) |
| Diagnosis, n (%) | |
| Renal cell carcinoma | 25 (43) |
| Breast carcinoma | 12 (21) |
| Melanoma | 5 (9) |
| Ovarian carcinoma | 5 (9) |
| Soft tissue sarcoma | 4 (7) |
| Other diagnosis* | 6 (11) |
| Previous autologous transplantation, n (%) | 13 (23) |
| Disease status prior to transplantation, n (%) | |
| Progressive disease | 39 (68) |
| Stable disease | 18 (32) |
| ATG dose, n (%) | |
| 5-10 mg/kg | 17 (30) |
| 2.5 mg/kg | 40 (70) |
| Total ATG dose, mg/kg, mean ± SD | 267 ± 184 |
| Graft source, n (%) | |
| Bone marrow | 16 (28) |
| PBSC | 41 (72) |
| Day-90 donor chimerism | |
| Studied patients | 40 |
| Full donor chimera, n (%) | 37 (93) |
| Days to full chimera, mean ± SD | 52 ± 26 |
| Donor lymphocyte infusion | |
| Patients, n (%) | 20 (35) |
| Day of first DLI, mean ± SD | 151 ± 131 |
| Acute GVHD | |
| Grades I-IV, n (%) | 37 (65) |
| Grades II-IV, n (%) | 24 (42) |
| Day of onset grades II-IV acute GVHD, mean ± SD | 43 ± 23 |
| Chronic GVHD | |
| Evaluable, n | 42 |
| Limited/extensive, n | 17/8 |
| Day of onset, mean ± SD | 120 ± 30 |
ATG indicates antithymocyte globulin; and GVHD, graft-versus-host disease.
Other diagnoses include colorectal carcinoma (n = 1), gastric carcinoma (n = 1), nephroblastoma (n = 1), primitive neuro-ectodermal tumor (n = 1), prostate carcinoma (n = 1), and uterus carcinoma (n = 1).
Transplant-related events
All patients engrafted and reached an ANC of 0.5 × 109/L and 20 × 109/L platelets at a median of 15 days (range, 7-22 days) and 13 days (range, 8-44 days). Platelet count did not decrease below 20 × 109/L in 17 patients (30%). Chimerism analysis was performed in 40 patients only (appropriate technique was not available in the first 6 patients, noninformative in 1 patient, and not performed in 10 patients with early major tumor progression and impaired clinical status according to the attending physician decision). Full donor chimerism (DC) was achieved in 36 (90%) of 40 patients at a median of 35 days (range, 28-120 days) after ASCT with no significant differences according to the different doses of ATG used during conditioning. One patient showed a progressive mixed DC (80% DC at day 60 and 90% DC at day 120) but died on day 129. Twenty patients (35%) received at least one DLI after ASCT (1 DLI, n = 14; 2 DLI, n = 2; 3 DLI, n = 4). The median time to the first DLI was day 115 (range, days 38-625) after ASCT. Only one patient developed grade III aGVHD after DLI and one patient had a limited cGVHD. The overall cumulative incidence of aGVHD was 65% (95% CI, 53%-77%). Thirteen patients developed grade I aGVHD. Thirteen patients developed grade II aGVHD, whereas 11 patients developed grades III to IV aGVHD. The cumulative incidence of cGVHD was 44% (95% CI, 31%-57%) with 17 patients presenting with a limited form and 8 with a clinically extensive form starting at a median of 110 days (range, 100-195 days) after ASCT. The incidence of cGVHD was statistically associated with the use of PBSC as stem cell source (cumulative incidence of clinical cGVHD [95% CI]: PBSC, 77% [60%-92%]; BM, 17% [0%-38%]; P = .001) and with the use of a lower dose of ATG (cumulative incidence of cGVHD [95% CI]: 2.5 mg/kg, 74% [59%-89%]; 5-10 mg/kg, 18% [0%-41%]; P = .002).
Disease progression and tumor response
Overall, 8 patients achieved an OR (CR, n = 2; PR, n = 6) according to RECIST criteria at a median of 90 days (range, 30-270 days) after ASCT (cumulative incidence of OR [95% CI], 14% [5%-23%]) (Table 2). Median tumor decrease was 58% (range, 45%-90%) in patients with PR, with only one patient presenting a response inferior to 50% (unique patient number [UPN] 31, 45%) (Table 2). OR was documented in 4 diagnoses: 3 of the 5 patients with ovarian carcinoma, 2 of the 12 patients with breast carcinoma, 2 of the 25 patients with RCC, and 1 of the patients with malignant melanoma. In case of OR, disease regression was seen when full DC was achieved and when or after the patient had developed cGVHD (P = .08). Only 1 patient (UPN 10) reached an OR without developing any form of GVHD. One patient with RCC (UPN 39) converted to CR after 4 months of progressive PR. Another patient (UPN 42) reached CR on day 90. All other responders had a PR, 2 of them still improving at last follow-up (UPN 43 and UPN 45, Table 2). Three of these 8 patients received DLI on days 140, 150, and 620, respectively. In the first case (UPN 10), DLI was performed for disease progression after an initial response of 56% but was unable to control the disease. UPN 45 received DLI on day 150 for absence of response, presented 1 month later with cGVHD and after 2 months achieved a PR, which is still improving at last follow-up. A second DLI was performed on day 450. The last patient (UPN 39) received DLI on day 620 after pulmonary relapse and is presently only 25 days after DLI. Disease status prior to ASCT was the only predictive factor significantly associated with the achievement of an OR. All patients presenting an OR were in SD at time of ASCT (OR probability, 44% [95% CI, 21%-67%]) as compared with none of the 39 patients with PD (P = .000 05;Figure 1). In addition, the use of DLI was not associated with an increased rate of OR.
Characteristics of patients with objective response
. | Sex/age, y . | . | No. prior systemic treatment/auto . | . | . | Preparative regimen/graft . | Day 15-30/60-90 chimera . | aGVHD grade/day . | cGVHD grade/day . | DL 1 day . | Response . | . | Secondary progression/day . | . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | . | Pathology . | . | Status . | Metastasis sites . | . | . | . | . | . | Onset/day . | Max/day . | . | Outcome . | |
| 10 | F/43 | Melanoma | 3/0 | Stable | Liver/pulmonary | 10 mg/kg ATG/BM | ND/100% | 0 | 0 | 140 | PR/60 | -56%/90 | Yes/140 | Death, 665 d | |
| 31 | F/60 | Ovarian Ca | 3/1 | Stable | Peritoneal/CA12.5 | 2.5 mg/kg ATG/PBSC | 100%/100% | III/34 | Lim/100 | NA | PR/30 | -45%/30 | Yes/120 | Death, 332 d | |
| 34 | M/45 | RCC | 1/0 | Stable | Pulmonary | 2.5 mg/kg ATG/PBSC | 100%/100% | IV/38 | Ext/100 | NA | PR/90 | -50%/120 | Yes/330 | Death, 360 d | |
| 39 | F/53 | RCC | 1/0 | Stable | Pulmonary | 2.5 mg/kg ATG/PBSC | ND/100% | II/76 | Lim/100 | 620 | PR/150 | CR/270 | Yes/605 | Alive, 645 d | |
| 42 | F/54 | Breast Ca | 3/1 | Stable | Bone/CA15.3 | 2.5 mg/kg ATG/PBSC | ND/100% | 0 | Lim/100 | NA | CR/90 | CR/90 | Yes/310 | Alive, 615 d | |
| 43 | F/53 | Ovarian Ca | 4/1 | Stable | ADP/CA12.5 | 2.5 mg/kg ATG/PBSC | 100%/100% | 0 | Lim/140 | NA | PR/90 | -90%/270 | No | Alive in PR (ADP -90%; CA12.5, NL), 617 d | |
| 45 | F/32 | Breast Ca | 3/0 | Stable | Liver/mediastinal ADP | 2.5 mg/kg ATG/PBSC | >95%/100% | II/17 | Lim/180 | 150 | PR/30 | -60%/240 | No | Alive in PR (liver -60%), 591 d | |
| 51 | F/36 | Ovarian Ca | 4/2 | Stable | Pulmonary/parietal/CA12.5 | 2.5 mg/kg ATG/PBSC | ND/100% | 0 | Lim/110 | NA | PR/60 | -75%/150 | Yes/262 | Alive, 428 d | |
. | Sex/age, y . | . | No. prior systemic treatment/auto . | . | . | Preparative regimen/graft . | Day 15-30/60-90 chimera . | aGVHD grade/day . | cGVHD grade/day . | DL 1 day . | Response . | . | Secondary progression/day . | . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | . | Pathology . | . | Status . | Metastasis sites . | . | . | . | . | . | Onset/day . | Max/day . | . | Outcome . | |
| 10 | F/43 | Melanoma | 3/0 | Stable | Liver/pulmonary | 10 mg/kg ATG/BM | ND/100% | 0 | 0 | 140 | PR/60 | -56%/90 | Yes/140 | Death, 665 d | |
| 31 | F/60 | Ovarian Ca | 3/1 | Stable | Peritoneal/CA12.5 | 2.5 mg/kg ATG/PBSC | 100%/100% | III/34 | Lim/100 | NA | PR/30 | -45%/30 | Yes/120 | Death, 332 d | |
| 34 | M/45 | RCC | 1/0 | Stable | Pulmonary | 2.5 mg/kg ATG/PBSC | 100%/100% | IV/38 | Ext/100 | NA | PR/90 | -50%/120 | Yes/330 | Death, 360 d | |
| 39 | F/53 | RCC | 1/0 | Stable | Pulmonary | 2.5 mg/kg ATG/PBSC | ND/100% | II/76 | Lim/100 | 620 | PR/150 | CR/270 | Yes/605 | Alive, 645 d | |
| 42 | F/54 | Breast Ca | 3/1 | Stable | Bone/CA15.3 | 2.5 mg/kg ATG/PBSC | ND/100% | 0 | Lim/100 | NA | CR/90 | CR/90 | Yes/310 | Alive, 615 d | |
| 43 | F/53 | Ovarian Ca | 4/1 | Stable | ADP/CA12.5 | 2.5 mg/kg ATG/PBSC | 100%/100% | 0 | Lim/140 | NA | PR/90 | -90%/270 | No | Alive in PR (ADP -90%; CA12.5, NL), 617 d | |
| 45 | F/32 | Breast Ca | 3/0 | Stable | Liver/mediastinal ADP | 2.5 mg/kg ATG/PBSC | >95%/100% | II/17 | Lim/180 | 150 | PR/30 | -60%/240 | No | Alive in PR (liver -60%), 591 d | |
| 51 | F/36 | Ovarian Ca | 4/2 | Stable | Pulmonary/parietal/CA12.5 | 2.5 mg/kg ATG/PBSC | ND/100% | 0 | Lim/110 | NA | PR/60 | -75%/150 | Yes/262 | Alive, 428 d | |
UPN indicates unique patient number; DLI, donor lymphocyte infusion; ATG, antithymocyte globulin; BM, bone marrow; ND, not done; PR, partial response; Ca, carcinoma; PBSC, peripheral blood stem cell; Lim, limited; NA, not applicable; RCC, renal cell carcinoma; Ext, extensive; CR, complete response; ADP, adenopathy; and NL, normal.
Cumulative incidence of tumor response according to disease status at time of ASCT. Overall population is depicted by a dotted line. Patients with stable disease (solid line) are compared with patients with progressive disease (large dashed line) at time of transplantation (P = .000 05).
Cumulative incidence of tumor response according to disease status at time of ASCT. Overall population is depicted by a dotted line. Patients with stable disease (solid line) are compared with patients with progressive disease (large dashed line) at time of transplantation (P = .000 05).
Forty-eight patients did not achieve any response and progressed at a median of 60 days (range, 10-380 days) after ASCT. One patient died on day 67 from GVHD while in minimal response (tumoral regression, 23%). Although no single factor was found to be significantly associated with disease progression, time to progression was shorter in patients who received transplants in PD (median, 30 days [range, 10-380 days]) as compared with the remaining patients from the cohort (median, 180 days [range, 25-617 days]) (P = .002). In 6 of the 8 patients with OR, secondary progression occurred on day 335 (range, days 120-617) ie, 245 days (range, days 90-437) after an initial response achievement (Table 2).
Outcome
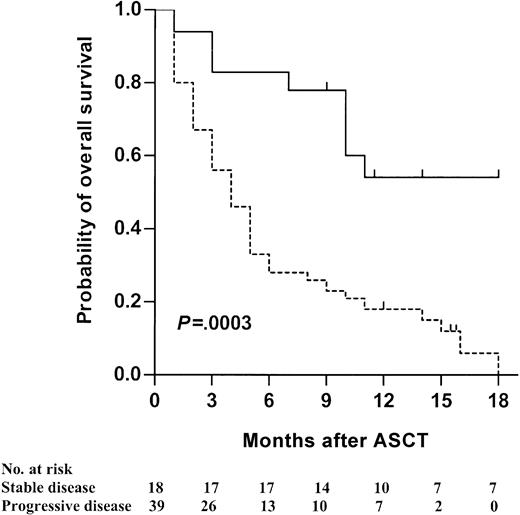
Overall, 5 patients died of procedure-related toxicity, giving a TRM incidence of 9% (95% CI, 1%-16%). Two of these 5 patients died on days 130 and 170, respectively, of disease progression and concomitant GVHD induced by voluntary CSA withdrawal in an attempt to stimulate a possible GVT effect. The 3 others died of GVHD. Forty-one other patients (72%) died of disease progression, 14 of them during the first 3 months. With a median follow-up of 16 months (range, 10-21 months), 11 patients were still alive. The 18-month OS was 19% (95% CI, 11%-33%). Univariate analysis of risk factors influencing OS is presented in Table 3. In a multivariate analysis, either taking into account the whole population or restricted to patients surviving beyond 3 months (landmark analysis) after ASCT, only 2 factors remained independently associated with a better survival (Table 4). OS was statistically improved when patients received transplants in SD (Figure 2) or when patients experienced cGVHD (Figure 3).
Univariate analysis of risk factors for tumor regression and survival
. | . | Objective response . | . | 18-month overall survival . | . | ||
|---|---|---|---|---|---|---|---|
. | No. of evaluable cases . | Cumulative incidence, % (95% CI) . | P . | Probability, % (95% CI) . | P . | ||
| Diagnosis | NS | NS | |||||
| Renal cell carcinoma | 25 | 8 (0-19) | 20 (9-41) | ||||
| Breast carcinoma | 12 | 17 (0-38) | 28 (9-62) | ||||
| Ovarian carcinoma | 5 | 60 (17-100) | 30 (6-75) | ||||
| Melanoma | 5 | 20 (0-55) | 20 (3-62) | ||||
| Others | 10 | 0 | 0 | ||||
| Patient age | NS | NS | |||||
| Younger than 45 y | 30 | 13 (1-25) | 13 (4-34) | ||||
| Older than 45 y | 27 | 15 (2-28) | 25 (12-44) | ||||
| Donor sex | NS | .02 | |||||
| Female | 31 | 13 (1-25) | 6 (2-20) | ||||
| Male | 26 | 15 (1-29) | 40 (23-60) | ||||
| Disease status | .00005 | .0003 | |||||
| Progressive | 39 | 0 | 0 | ||||
| Stable | 18 | 44 (21-67) | 54 (32-74) | ||||
| Stem cell source | NS | NS | |||||
| Bone marrow | 16 | 6 (0-18) | 13 (4-36) | ||||
| Peripheral blood stem cell | 41 | 17 (6-28) | 23 (12-39) | ||||
| ATG dose infused during conditioning | NS | .02 | |||||
| 2.5 mg/kg | 40 | 18 (6-30) | 26 (14-43) | ||||
| 5-10 mg/kg | 17 | 6 (0-17) | 6 (1-27) | ||||
| Acute GVHD | NS | NS | |||||
| Grades 0-I | 33 | 12 (1-23) | 23 (11-42) | ||||
| Grades II-IV | 24 | 17 (2-32) | 14 (5-34) | ||||
| Chronic GVHD | NS | .05 | |||||
| No | 17 | 6 (0-18) | 21 (8-46) | ||||
| Yes | 25 | 28 (10-46) | 35 (13-51) | ||||
. | . | Objective response . | . | 18-month overall survival . | . | ||
|---|---|---|---|---|---|---|---|
. | No. of evaluable cases . | Cumulative incidence, % (95% CI) . | P . | Probability, % (95% CI) . | P . | ||
| Diagnosis | NS | NS | |||||
| Renal cell carcinoma | 25 | 8 (0-19) | 20 (9-41) | ||||
| Breast carcinoma | 12 | 17 (0-38) | 28 (9-62) | ||||
| Ovarian carcinoma | 5 | 60 (17-100) | 30 (6-75) | ||||
| Melanoma | 5 | 20 (0-55) | 20 (3-62) | ||||
| Others | 10 | 0 | 0 | ||||
| Patient age | NS | NS | |||||
| Younger than 45 y | 30 | 13 (1-25) | 13 (4-34) | ||||
| Older than 45 y | 27 | 15 (2-28) | 25 (12-44) | ||||
| Donor sex | NS | .02 | |||||
| Female | 31 | 13 (1-25) | 6 (2-20) | ||||
| Male | 26 | 15 (1-29) | 40 (23-60) | ||||
| Disease status | .00005 | .0003 | |||||
| Progressive | 39 | 0 | 0 | ||||
| Stable | 18 | 44 (21-67) | 54 (32-74) | ||||
| Stem cell source | NS | NS | |||||
| Bone marrow | 16 | 6 (0-18) | 13 (4-36) | ||||
| Peripheral blood stem cell | 41 | 17 (6-28) | 23 (12-39) | ||||
| ATG dose infused during conditioning | NS | .02 | |||||
| 2.5 mg/kg | 40 | 18 (6-30) | 26 (14-43) | ||||
| 5-10 mg/kg | 17 | 6 (0-17) | 6 (1-27) | ||||
| Acute GVHD | NS | NS | |||||
| Grades 0-I | 33 | 12 (1-23) | 23 (11-42) | ||||
| Grades II-IV | 24 | 17 (2-32) | 14 (5-34) | ||||
| Chronic GVHD | NS | .05 | |||||
| No | 17 | 6 (0-18) | 21 (8-46) | ||||
| Yes | 25 | 28 (10-46) | 35 (13-51) | ||||
NS indicates not significant.
Multivariate analysis of risk factors for overall survival
. | Odds ratio . | Confidence interval . | P . |
|---|---|---|---|
| Overall population, n = 57 | |||
| Patients with stable disease | 4.36 | 1.98-9.61 | < .001 |
| Patients alive beyond 3 mo, n = 42 | |||
| Patients with stable disease | 4.88 | 1.94-12.29 | < .001 |
| Patients with cGVHD | 2.86 | 1.32-6.22 | < .01 |
. | Odds ratio . | Confidence interval . | P . |
|---|---|---|---|
| Overall population, n = 57 | |||
| Patients with stable disease | 4.36 | 1.98-9.61 | < .001 |
| Patients alive beyond 3 mo, n = 42 | |||
| Patients with stable disease | 4.88 | 1.94-12.29 | < .001 |
| Patients with cGVHD | 2.86 | 1.32-6.22 | < .01 |
Kaplan-Meier estimate of overall survival according to disease status at time of transplantation. Patients with stable disease (solid line) are compared with patients with progressive disease (dotted line) (P = .0003).
Kaplan-Meier estimate of overall survival according to disease status at time of transplantation. Patients with stable disease (solid line) are compared with patients with progressive disease (dotted line) (P = .0003).
Kaplan-Meier estimate of overall survival according to cGVHD occurrence. Patients with chronic GVHD (solid line) are compared with patients without chronic GVHD (dotted line) in a landmark analysis starting on day 100 (P = .05).
Kaplan-Meier estimate of overall survival according to cGVHD occurrence. Patients with chronic GVHD (solid line) are compared with patients without chronic GVHD (dotted line) in a landmark analysis starting on day 100 (P = .05).
Discussion
In this study we investigated the potential of ASCT in patients suffering from advanced ST. On the basis of the observations made in the allogeneic transplantation treatment of hematologic malignancies, our aim was to determine whether ASCT could induce clinically meaningful immunologic tumor control with an acceptable risk of TRM. These 2 objectives resulted in a study design in which we sequentially modified the preparative regimen and the stem cell source. The ATG-based RIC described by Slavin et al15 was appealing. It was designed as a conditioning platform to be suitable for all patients regardless of diagnosis. As this program was primarily defined as an allogeneic cellular immunotherapy, the purpose of the conditioning regimen was solely to allow engraftment of donor immune and hematopoietic cells. Furthermore, this regimen could be easily modified in an attempt to decrease transplant-related secondary infections while modulating alloreactivity.27,28 When PBSC transplantation was shown to be associated with a higher incidence of long-term cGVHD,18,29,30 we switched the stem cell source from BM to PBSCs. These changes were not associated with loss of engraftment or increase in TRM. Only 5 of the 57 patients died of transplant-related toxicity, 3 of them with GVHD as a unique cause.
In addition to the low TRM, tumor regression was observed in 8 patients. Interestingly, 3 of the 5 patients treated for ovarian carcinoma and 2 of 12 patients with breast cancer experienced an OR. We were the first to report such a GVT effect in ovarian carcinoma.13,17 This finding is in line with previous reports suggesting that an allogeneic GVT effect in breast carcinoma can be achieved.5,31,32 The RIC regimen we used included a limited dose of busulfan, and it may be argued that this chemotherapy might account for disease regression in these 5 patients. Although we cannot completely exclude some effect of this agent on disease evolution, it should be noted that all these patients have previously received high-dose chemotherapy, including high-dose alkylating agents with autologous stem cell rescue in 3 of these 5 patients. All of them have either progressed or showed no tumor regression after these treatments. In addition, time of onset, duration of response and the importance of tumor response were closely associated with the expression of clinical GVHD, arguing in favor of an allogeneic GVT effect. In 2000, Childs et al33 reported a seminal investigation in patients with RCC showing that RIC followed by ASCT can provide a significant antitumoral effect in patients with RCC. Among 19 patients with RCC, a disease sensitive to cytokines acting on T cells,34 they observed a tumor regression in 10 patients. Results from our series seem to be inferior to those initial observations. This finding might be due to a deleterious effect of our ATG-containing RIC or a different selection of patients. Both conditioning regimens included fludarabine, but we used a higher dose that was combined to ATG. It is possible that in patients with progressive RCC, a more potent immunosuppressive ATG-containing regimen might accelerate tumor progression prior to onset of an allogeneic GVT effect. Although we could not document an effect of the ATG dose on disease progression or time to progression for any of the tumor investigated, we cannot exclude such a possibility given the relatively small number of patients. However, strategies to decrease the tumor burden prior to ASCT may be of importance, at least for this tumor.33 Finally, we could document an OR in a patient with malignant melanoma. This patient did not develop any form of GVHD, complicating the interpretation of the potential GVT effect in this disease. Although it has been shown that rare tumor regression is possible in patients without clinical GVHD,33 the effect of busulfan contained in the preparative regimen or a spontaneous regression cannot be discarded in the case of this patient. The latter is in line with a recent report analyzing the outcome of 27 patients treated for malignant melanoma in different institutions worldwide.35
In this multicenter trial, we were able to document an overall 14% probability of objective tumor regression which increased to 18% in the last cohort of patients treated with only 1 day of ATG and PBSC transplantation. However, the depth of tumor regression was usually limited as only 2 patients has reached CR and 6 of 8 patients subsequently progressed with median response duration of 245 days (range, 90-437 days). Considering the status of the disease at time of ASCT, this result may not be unexpected and may even compare favorably with the usual results of anticancer drug phase 1 studies36 or immunotherapy.37 This is also an established fact in hematologic malignancies when ASCT is performed in blast crisis chronic myeloid leukemia, the most sensitive disease to the GVL effect, 50% of the patients will progress within 365 days compared with less than 10% of such patients who received transplants in chronic phase. In this series of patients, all had significant tumor masses, and we cannot compare our results with those expected in patients in CR or with minimal residual disease, as they were excluded from this protocol. However, 18 of the 57 patients received transplants while their disease was stable, and disease progression kinetic appeared to be a major factor to increase sensitivity to the allogeneic effect, which, for these patients, translated into tumor regression and survival. Although this effect may also be due to specific characteristics of the tumors at time of relative stability,38 a slow tumor kinetic might allow sufficient time for the allogeneic effect to develop. Indeed, median time to achieve OR was 90 days, whereas the median time to progression was 60 days. Furthermore, the close association between GVHD and tumor regression, as it has been already suggested in previous reports,5,32,33 argues clearly in favor of a long-term allogeneic GVT. Nevertheless, the relatively low number of patients included in this study must be taken into account because it might have not provided sufficient statistical power to determine other factors statistically associated with tumor response and survival. In addition this pilot study does not allow us to really estimate the negative effect of immunosuppression on tumor growth and survival, effect that might only be assessed prospectively in comparison with conventional alternatives.
In conclusion, our results show that a low-dose ATG-based RIC offers a low transplant-related mortality and can be associated with some degree of GVT effect in patients with advanced STs. These encouraging results provide a framework for other studies especially in situations in which tumor responses to ASCT were observed, such as ovarian cancer, breast cancer, and RCC. However, before being widely applicable, the ASCT strategy for solid tumors needs to be more refined to obtain a higher rate of complete remission that is mandatory to achieve cure in patients.10 This strategy may be assessed by selecting patients with less advanced diseases and by optimizing the postgraft immunomodulation. If the hypothesis of a benefit of earlier ASCT is confirmed, the optimal timing for ASCT will have to be redefined and incorporated into the upfront treatment strategies to offer patients the best chance of curability.
Prepublished online as Blood First Edition Paper, August 28, 2003; DOI 10.1182/blood-2003-07-2236.
Supported in part by a grant from the French Ministry of Health (PHRC 2000) and Laboratoires Amgen France, Neuilly sur Seine, France. Dr C. Cailliot provided some support for this study but did not participate in either the definition or data analysis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Finn Bo Petersen, University of Utah, for his critical and helpful reading of the manuscript and Daniel Olive and Beatrice Gaugler, INSERM U119, Marseille, for helpful discussions. We also thank patients and families for their support and confidence.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal