Abstract
The activity and safety profile of selective B-cell depletion with rituximab, an anti-CD20 monoclonal antibody, were evaluated in 10 patients with acquired hemophilia. Rituximab was given intravenously at the dose of 375 mg/m2 once weekly for 4 consecutive weeks. Infusion-related side effects were observed in 3 patients but were of mild intensity and did not require discontinuation of treatment. Eight patients with Factor VIII (FVIII) inhibitor titers between 4 and 96 Bethesda units per milliliter (BU/mL) achieved a complete remission, which was defined as a return to normal FVIII activity and undetectable FVIII inhibitor titers. Two more patients with inhibitor levels greater than 100 BU/mL experienced only a partial transient decrease of the inhibitor after rituximab alone, but they achieved a complete response after being challenged with a combination of rituximab plus pulse intravenous cyclophosphamide. With a median follow-up of 28.5 months (range, 12-41 months), 3 patients have thus far relapsed. Retreatment with the monoclonal antibody at the same dose and schedule resulted in a new sustained response in all these patients. In conclusion, rituximab appears an effective and well-tolerated treatment for patients with acquired hemophilia and low inhibitor titers. A reinforcement of therapy with other agents seems to be required to achieve a full and durable response in those patients with high inhibitor levels. (Blood. 2004;103:4424-4428)
Introduction
Acquired hemophilia is a rare disorder with an incidence of 0.2 to 1 per million persons per year.1,2 It occurs because of spontaneous development of autoantibodies against the factor VIII (FVIII) molecule, leading to inhibition of FVIII binding to von Willebrand factor, to activated factor IX, or to negatively charged phospholipids.3 It should be suspected in patients presenting with hemorrhage for which no apparent cause can be found and in the absence of previous history of bleeding diathesis. These patients predominantly have soft-tissue and systemic bleeding episodes and, unlike in congenital bleeding disorders, joint bleeds are relatively rare. The laboratory investigations show normal thrombin time (TT), prothrombin time (PT), and prolonged activated partial thromboplastin time (APTT), which is not corrected with the addition of normal plasma in 1:1 ratio, as seen in congenital FVIII deficiency. This disorder may be associated with pregnancy, malignancy, rheumatoid arthritis, and other autoimmune disorders; but in most cases no cause can be identified.4 Although up to 36% of patients who do not receive immunosuppressants experience a spontaneous resolution of their autoantibodies,2 this occurrence is unpredictable, and patients remain at great risk of fatal bleeding as long as the antibody persists.3 Accordingly, attempts to accelerate the disappearance of these inhibitors are warranted in the best interests of the patient.
Treatment strategies in patients with acquired FVIII inhibitors follow 2 major objectives. During the acute stage, effective control of bleeding manifestations is the foremost objective. The ultimate therapeutic goal during the subacute phase is the elimination of the inhibitor, thereby curing the disease. Control of bleeding manifestations can be obtained with plasma exchange, the administration of human or porcine FVIII, activated prothrombin complex concentrate (APCC) with FVIII inhibitor bypassing activity, deamino-D-arginine vasopressin, and, lately, recombinant human activated factor VII (rFVIIa). For the elimination of FVIII inhibitors, several treatment strategies are used. Some good responses have been reported following administration of high-dose immunoglobulin.5 However, immunosuppressive therapy with corticosteroids and cytotoxic drugs, alone or in combination, is regarded as the mainstay of therapy.3,4
Recently, selective B-cell depletion with rituximab, a chimeric anti-CD20 monoclonal antibody, has been shown to be quite effective in the treatment of immune disorders resulting from autoantibodies, including idiopathic thrombocytopenic purpura,6 nonfamilial thrombotic thrombocytopenic purpura,7 autoimmune hemolytic anemia,8 and mixed cryoglobulinemia.9 Recent reports also suggest a role for rituximab in the treatment of patients with acquired FVIII inhibitors.10,11 We present our experience with the management of patients with acquired hemophilia using an approach with rituximab.
Patients and methods
Patients
The 10 patients with acquired hemophilia of this study were treated at our institutions from June 1999 to October 2002. Their clinical and laboratory characteristics are reported in Table 1. Detailed clinical history and physical examination, history of pregnancy, joint pains, autoimmune disorders, and underlying malignancy were carefully recorded. In 6 patients, no underlying condition predisposing to the development of acquired FVIII inhibitors was detected. These were considered to be idiopathic. In 2 patients the development of acquired FVIII inhibitor was associated with malignancy (low-grade non-Hodgkin lymphoma, prostate carcinoma), in 1 patient with rheumatoid arthritis, and in 1 case with a recent pregnancy. Four patients had already received at least 2 lines of treatment. Previous treatments included standard dose (1-2 mg/kg/d) oral prednisone; oral cyclophosphamide (2 mg/kg/d); CVP (cyclophosphamide, vincristine, prednisone) protocol of vincristine (2 mg) in a single dose on day 1, cyclophosphamide 500 mg intravenously on day 1 and 200 mg/d orally from day 2 to day 5, along with oral prednisone (100 mg/d from day1 to day 5); plasma exchange; and rFVIIa.
Clinical and laboratory features of patients with acquired hemophilia
Case no. . | Age, y . | Sex . | Clinical presentation . | Associated conditions . | Disease duration . | Previous therapies . | LAC . | ANA . | FVIII activity . | FVIII inhibited titers, BU . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | F | GI bleeding, muscular hematomas, ecchymoses | Rheumatoid arthritis | 1 d | None | + | + | < 1% | 160 |
| 2 | 74 | M | Ecchymoses, prolonged bleeding after cuts | Low-grade NHL | 2 wk | None | − | − | < 1% | 4 |
| 3 | 61 | M | Muscular hematomas, epistaxis | None | 3 mo | PDN, CTX | − | − | < 1% | 28 |
| 4 | 49 | M | Ecchymoses, hematuria | None | 1 wk | None | − | − | < 1% | 32 |
| 5 | 30 | F | Ecchymoses | After pregnancy | 2 mo | None | − | − | < 1% | 16 |
| 6 | 27 | F | Epistaxis, prolonged bleeding after cuts | None | 10 d | None | + | + | < 1% | 96 |
| 7 | 78 | M | Ecchymoses, gingival bleeding | Prostate carcinoma | 3 mo | PDN, CTX | − | − | < 1% | 64 |
| 8 | 62 | F | Ecchymoses, muscular hematomas, gingival bleeding, hemarthroses | None | 13 mo | PDN, CVP, CTX, PE, rFVIIa | − | − | < 1% | 250 |
| 9 | 58 | F | Ecchymoses, menorrhagia | None | 5 d | None | + | − | < 1% | 96 |
| 10 | 68 | M | Ecchymoses, epistaxis | None | 6 mo | PDN, CTX | − | − | < 1% | 16 |
Case no. . | Age, y . | Sex . | Clinical presentation . | Associated conditions . | Disease duration . | Previous therapies . | LAC . | ANA . | FVIII activity . | FVIII inhibited titers, BU . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | F | GI bleeding, muscular hematomas, ecchymoses | Rheumatoid arthritis | 1 d | None | + | + | < 1% | 160 |
| 2 | 74 | M | Ecchymoses, prolonged bleeding after cuts | Low-grade NHL | 2 wk | None | − | − | < 1% | 4 |
| 3 | 61 | M | Muscular hematomas, epistaxis | None | 3 mo | PDN, CTX | − | − | < 1% | 28 |
| 4 | 49 | M | Ecchymoses, hematuria | None | 1 wk | None | − | − | < 1% | 32 |
| 5 | 30 | F | Ecchymoses | After pregnancy | 2 mo | None | − | − | < 1% | 16 |
| 6 | 27 | F | Epistaxis, prolonged bleeding after cuts | None | 10 d | None | + | + | < 1% | 96 |
| 7 | 78 | M | Ecchymoses, gingival bleeding | Prostate carcinoma | 3 mo | PDN, CTX | − | − | < 1% | 64 |
| 8 | 62 | F | Ecchymoses, muscular hematomas, gingival bleeding, hemarthroses | None | 13 mo | PDN, CVP, CTX, PE, rFVIIa | − | − | < 1% | 250 |
| 9 | 58 | F | Ecchymoses, menorrhagia | None | 5 d | None | + | − | < 1% | 96 |
| 10 | 68 | M | Ecchymoses, epistaxis | None | 6 mo | PDN, CTX | − | − | < 1% | 16 |
LAC indicates lupus anticoagulant; ANA, antinuclear antibodies; GI, gastrointestinal; NHL, non-Hodgkin lymphoma; PDN, prednisone; CTX, cyclophosphamide; CVP, cyclophosphamide, vincristine, prednisone; PE, plasma exchange; rFVIIa, recombinant human activated factor VII.
On study entry, patients had signed an institutional review board-approved informed consent.
Laboratory studies
Laboratory investigations included a complete hemogram and serum chemistry profiles. Tests for rheumatoid factor and antinuclear antibodies were performed to detect collagen vascular disease. Coagulation studies were carried out on venous blood samples drawn in 3.8% sodium citrate in a ratio of 9:1. Routine coagulation tests included PT, APTT, and fibrinogen assay. Screening tests for FVIII inhibitors included mixing studies of patient plasma (PP) and normal plasma (NP) according to the Bethesda method.12 The plasma samples were incubated at 37°C for 2 hours and then the APTT test was repeated. Marked prolongation of APTT in the mixture of NP and PP in comparison to that in PP alone was indicative of FVIII inhibitors. Single factor-activity determination (FI, FII, FV, FVIII, FIX, FX, and von Willebrand factor) was carried out by using commercially available factor-deficient plasma or test kits (Dade Behring, Marburg, Germany; Immuno, Vienna, Austria). The presence of a lupus anticoagulant was investigated as previously reported.13
Monitoring of patients included a weekly hemogram, determination of lymphocyte subsets, screening coagulation tests plus FVIII activity and FVIII inhibitor titers during rituximab treatment, biweekly determinations of the same tests for 3 months after rituximab treatment, and monthly determinations thereafter.
Treatment regimen
Rituximab (Mabthera; Roche, Milan, Italy) at a dose of 375 mg/m2 was administered intravenously once weekly for a total of 4 infusions (days 1, 8, 15, and 22). The drug was reconstituted in normal saline to a concentration of no more than 1 to 4 mg/mL. The initial infusion rate was 50 mg/h, with subsequent infusion-rate increase if no toxicity was seen. Premedication with oral acetaminophen 500 mg and diphenhydramine 50 mg was given to all patients. Patients who experienced any treatment-related nausea or vomiting with the first treatment received subsequent premedication with a serotonin receptor antagonist. One patient (case 10) received concomitant prednisone at 1 mg/kg/d and oral cyclophosphamide at 2 mg/kg/d.
Complete remission was defined as a clinical improvement associated with normalization of FVIII (> 70% of normal) and disappearance of FVIII inhibitors as measured by the Bethesda assay.
Results
Response
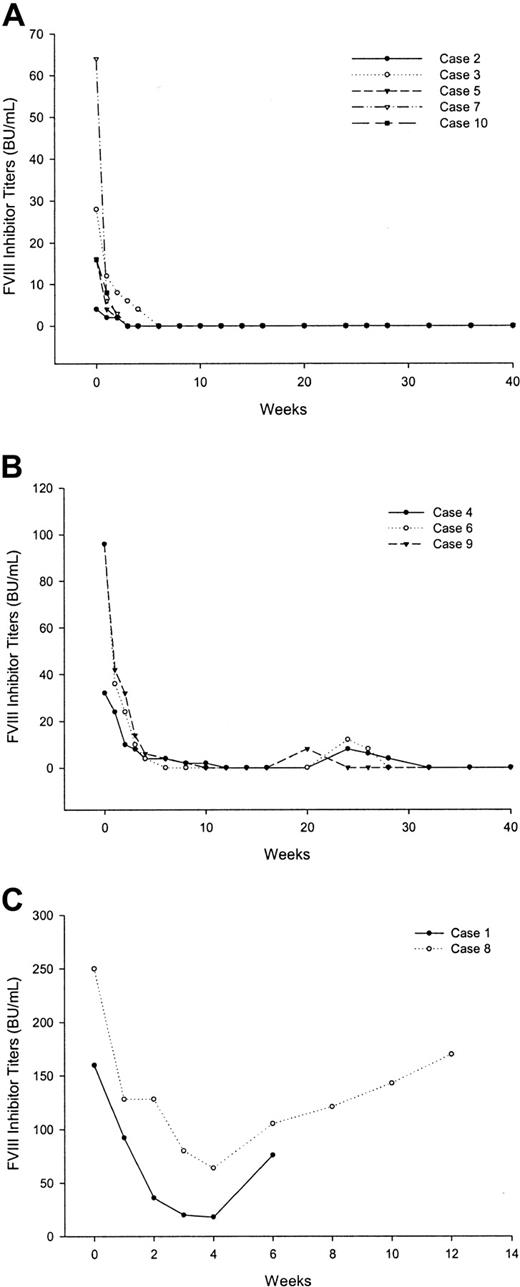
All patients were evaluable for response. Patients with critical bleeding of recent onset (cases 1 and 9) were managed with rFVIIa before receiving rituximab. All other patients experienced a rapid clinical improvement following the administration of rituximab. This improvement was most striking in patients 2 and 5, whose large ecchymoses resolved rapidly following the first dose of rituximab, their sole treatment. Except patients 1 and 8, FVIII levels normalized, and the inhibitor became undetectable, between 3 and 12 weeks from the start of rituximab (Figure 1A-B). Patients 1 and 8 did not respond to rituximab infusions, although inhibitor titers decreased by more than 50% relative to baseline (Figure 1C).
Behavior of factor VIII inhibitor titers in (A) patients with a continuous sustained response, (B) relapsed patients, and (C) nonresponders.
Behavior of factor VIII inhibitor titers in (A) patients with a continuous sustained response, (B) relapsed patients, and (C) nonresponders.
In patient 2, a 74-year-old man with a recent diagnosis of indolent non-Hodgkin lymphoma, rituximab treatment was associated with a partial clinical response of the lymphoma.
Duration of response
With a median follow-up of 28.5 months (range, 12-42 months), 3 patients have thus far relapsed (cases 4, 6, and 9). The time to relapse was 14 weeks, 20 weeks, and 10 weeks, respectively (Figure 1B). Patient 7 died of progression of his prostate carcinoma. At last follow-up, 12 months after the last rituximab infusion, he still retained a complete response.
Treatment of relapsed and nonresponding patients
Relapsed patients were rechallenged with rituximab at the same dose and schedule. Repeat treatment resulted in a new sustained response in all these patients (Figure 1B).
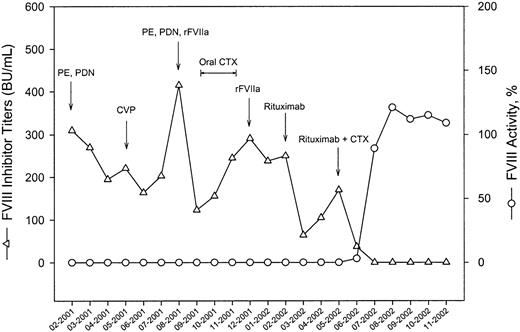
Patients 1 and 8, who had not responded to rituximab alone, were subsequently treated with an association of intravenous cyclophosphamide, 1 g/m2 on day 1, plus rituximab at the same dose and schedule used before. Combination therapy was initiated 8 and 6 weeks after the last infusion of single-agent rituximab, respectively. In both cases such therapy resulted in a complete clearance of inhibitor titers. Figure 2 illustrates the clinical course of patient 8. This patient, a 62-year-old woman, had a 13-month history of acquired hemophilia manifesting with the spontaneous development of giant ecchymoses, painful hematomas of the gluteal regions, hemarthrosis of the right knee, and gingival bleeding. Patient history included 5 years of essential hypertension treated with angiotensin-converting enzyme inhibitors but was otherwise unremarkable. There was no family history of autoimmune diseases, bleeding disorders, or neoplasias. FVIII inhibitor titers at the time of presentation were 310 Bethesda units per milliliter (BU/mL). Critical bleeding episodes were managed with plasma exchange and/or rFVIIa. Immunosuppressive therapy with prednisone, combination chemotherapy, and oral cyclophosphamide did not result in a sustained suppression of the inhibitor. FVIII inhibitor titers before rituximab administration were 250 BU/mL. Treatment with rituximab produced a decrease of inhibitor titers to as low as 64 BU/mL after the last rituximab infusion, but titers were already increasing 2 weeks later. After administration of cyclophosphamide plus rituximab, inhibitor titers rapidly declined and were no longer detectable 6 weeks after the last rituximab infusion.
Clinical course of a patient with acquired hemophilia and high FVIII inhibitor titers responding to the combination therapy rituximab plus cyclophosphamide. For details, see “Treatment of relapsed and nonresponding patients.”
Clinical course of a patient with acquired hemophilia and high FVIII inhibitor titers responding to the combination therapy rituximab plus cyclophosphamide. For details, see “Treatment of relapsed and nonresponding patients.”
Adverse events
Three patients experienced first-infusion reactions, consisting of fever (1 patient), chills (2 patients), and nausea (1 patient). Reactions were typically brief and of mild intensity (grade 1 according to the National Cancer Institute criteria) and did not require discontinuation of antibody infusion. No other acute or delayed relevant side effect or infectious complication occurred.
Monitoring of immunologic parameters
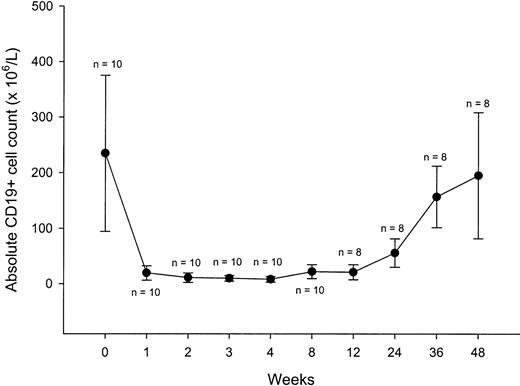
Peripheral blood B cells, evaluated by flow cytometry as CD19+ cells, had a median pretreatment count of 234 × 106/L (range, 63-461 × 106/L). This count sharply declined with treatment, to subnormal levels after the first dose for most of the patients (Figure 3). The B-cell counts fell to less than 30 × 106/L within 4 weeks in all patients, including nonresponders. Recovery of circulating B cells began between weeks 12 and 26; by week 48 the B-cell counts were comparable to baseline. Median absolute T-cell counts in peripheral blood, using CD3, CD4, and CD8 as well as natural killer cell counts, remained stable during the study period.
B-cell counts over the course of rituximab treatment and during follow-up. Each data point represents the mean (± SD) CD19+ cell count at a particular time.
B-cell counts over the course of rituximab treatment and during follow-up. Each data point represents the mean (± SD) CD19+ cell count at a particular time.
There were no significant changes in mean immunoglobulin G (IgG) or IgM levels after rituximab. Patients with normal IgM levels before treatment had their levels remain normal; 2 patients with low initial IgM levels (case 2 and 8) and one with a low initial IgG level (case 10) maintained their IgM and IgG levels. One patient (case 2) had his IgG level decrease from normal (910 mg/dL) to less than normal; the week 12 level was 713 mg/dL (normal, 800-1800 mg/dL) and week 52 was 689 mg/dL.
In 3 patients, the presence of FVIII inhibitors was associated with the presence of lupus anticoagulant activity (LAC); 2 of these patients also tested positive for antinuclear antibodies with a speckled pattern (Table 1). Both LAC and ANA were no longer detectable 8 weeks after the last rituximab infusion.
Discussion
Following the dramatic successes with the treatment of lymphoma, rituximab has become an appealing candidate for the treatment of nonmalignant diseases involving B cells. The central concept has been the removal of the cellular source of pathogenic autoantibodies, but effective targeting of B cells may also affect pathogenesis by removing the many functional contributions of B lymphocytes to the cell-to-cell interactions that drive the disease process.14 Idiopathic thrombocytopenic purpura (ITP), the most common hematologic autoimmune disease, has been useful for the development of innovative clinical trials, in part because of its perceived simple pathogenetic process; high titers of autoantibodies directed against platelet glycoproteins lead to depressed platelet counts and a bleeding diathesis.6,15 The promising results obtained in ITP have warranted the use of rituximab in several other disorders in which a pathogenic role for autoantibodies is generally accepted or demonstrated. The present study supports the efficacy and safety of rituximab therapy in patients with acquired FVIII inhibitors, in agreement with previous preliminary reports.10,11 Rituximab showed efficacy both as first-line therapy in patients with a recent diagnosis, and as rescue therapy in patients unresponsive to other immunosuppressive treatments. Not unexpectedly, peripheral B-cell depletion was found in all patients, irrespective of the type of response, and the pattern of B-cell depletion appeared comparable to that seen in other autoimmune disorders treated with this antibody.6,9 Responses were observed only in patients with inhibitor titers below 100 BU/mL, with complete disappearance of the inhibitor and normalization of FVIII activity occurring in 3 to 12 weeks. The 3 relapses observed during follow-up were recorded in those patients with higher baseline inhibitor titers, but rechallenge with the monoclonal antibody at the same dose and schedule resulted in a new sustained response in all patients. The 2 nonresponders had inhibitor levels greater than 100 BU/mL and experienced only a partial and transient decrease of the inhibitor following rituximab. However, they had a complete and sustained response to combination therapy with rituximab plus pulse intravenous cyclophosphamide. These data support the view that a reinforcement of conventional rituximab therapy is necessary to achieve a full response in those patients with high inhibitor levels, because it is likely that the dominant cellular source of disease-associated autoantibodies, especially IgG antibodies, are plasma cells that do not bear CD20.16 Thus, even after a course of rituximab has depleted susceptible mature B cells, plasma cells may still continue to produce disease-causing autoantibodies for months or even years.17 For this reason, the optimal treatment of diseases that have autoantibody-mediated pathology may require a regimen that also affects plasma cells. The same mechanism also accounts for the minimal changes in serum immunoglobulin levels, with only moderate declines in IgG and IgM concentrations in a few of our patients. The good results obtained by adding cyclophosphamide to rituximab do not necessarily imply that this combination should be considered the standard salvage strategy in patients unresponsive to rituximab alone. More prospective data are necessary, and agents other than cyclophosphamide or different combinations of drugs might well show a similar efficacy.
It appears that all our patients had inhibitors with a type I kinetics, because there was a linear relationship between the logarithm of residual Factor VIII level and plasma dilution at higher concentrations of plasma until complete inhibition occurred. In fact, in all our cases there was no residual Factor VIII activity. Although this is considered an unusual finding in acquired hemophilia, in which most inhibitors show a type II kinetics and a small amount of residual FVIII activity may be detected, other case series with no baseline residual Factor VIII activity have been reported in the literature.18 In the few cases with inhibitors showing a type II kinetics described thus far,11 treatment with rituximab resulted in complete responses with the same patterns as in our series.
In addition to the durability of responses, rituximab compares favorably to other therapies because of the substantial lack of significant toxicity. Mild side effects occurred only during the first infusion, and by the second and subsequent infusions no further infusion-related toxicities were recorded. Because this agent depletes normal B cells, it is also notable that no relevant infectious episodes were observed during the follow-up period. Conversely, immunosuppressive therapy bears potential complications such as neutropenic fever, herpes zoster, myelodysplasia, and cataracts4 ; a few deaths as a result of sepsis have also been reported.19,20
In conclusion, our results indicate that rituximab therapy has a valuable effect in patients with acquired hemophilia and low inhibitor titers. The combination with other agents seems to be required to achieve a response in patients with high inhibitor titers. In view of its mild toxicity, rituximab should be strongly considered in severely affected patients who do not respond to standard therapy or in those in whom cytotoxic therapy bears a high risk of morbidity. Some issues about this agent, such as the optimum dose and treatment schedule, the exact mechanisms of action, and long-term side effects, remain to be explored and warrant further investigation.
Prepublished online as Blood First Edition Paper, March 2, 2004; DOI 10.1182/blood-2003-11-4075.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal