Abstract
Dendritic cells (DCs) are key antigen-presenting cells with a potential role in tumor vaccines. We investigated the hypothesis that early reconstitution of DCs after allogeneic hematopoietic stem cell transplantation (SCT) improves survival. We also correlated DC reconstitution with complications of relapse and acute graft-versus-host disease (aGVHD). Fifty patients underwent transplantation between February 2000 and March 2003, with a median follow-up of 501 days (range, 136-1263 days). Most (92%) received blood stem cells, and the remainder received bone marrow from HLA-matched sibling donors for predominantly high-risk hematologic malignancies. Around the time of engraftment, peripheral blood underwent flow cytometry analysis for DCs, and the cells were divided as DC1 and DC2. Using Kaplan-Meier analysis, patients with lower DC counts (< 4.97 cells/μL) were found to have significantly worse survival (P = .002), increased incidence of relapse (P = .002), higher incidence of aGVHD onset (P = .0005), and a composite end point of relapse or death (P = .0017). A Cox proportional hazards multivariate model adjusted for important covariates confirmed that low DC count is independently associated with death (hazard ratio [HR], 3.8; P = .02), time to relapse (HR, 11.6; P = .001), and aGVHD (HR, 3.3; P = .04). Sensitivity and specificity rates for low DC count in predicting death or relapse are 73% and 75%, respectively. Low numbers of circulating DCs significantly increase the risk for relapse and acute GVHD and predict death after allogeneic SCT. (Blood. 2004;103:4330-4335)
Introduction
Allogeneic hematopoietic stem cell/bone marrow transplantation (SCT/BMT) is the treatment of choice for several hematologic malignancies. An advantage of SCT over conventional chemoradiotherapies is the reconstitution of the donor immune system in the recipient, leading to graft-versus-tumor effects and contributing to potential cure after SCT.2,3 However, a major limitation of SCT is acute graft-versus-host disease (aGVHD).4 Identifying key functional immune cells reconstituted after transplantation may lead to limiting aGVHD and optimizing antitumor effects.
Dendritic cells (DCs) are the most potent antigen-presenting cells known and have a potential role as a cancer vaccine.5-7 Dendritic cells are divided into 2 functional phenotypes.7-9 Type 1 (DC1) expresses CD11c and polarizes naive T cells toward a T-helper 1 (TH1) phenotype.7,9 Type 2 (DC2) does not express CD11c but does express CD1238,10 and polarizes T cells toward a TH2 phenotype.7-9,11 We have previously demonstrated in a murine model that granulocyte colony-stimulating factor (G-CSF) administration to allogeneic donors alters DC function toward DC2 cytokine production.12,13 DC2 likely induces the formation of TH2 cells, which are known to reduce the incidence and severity of experimental aGVHD and to retain a graft-versus-leukemia effect in this model.14,15 The cellular effects of G-CSF on DC2 and TH2 in the human peripheral blood stem cell (PBSC) allograft have been confirmed in human studies.16,17 This DC2 and TH2 phenotype may explain the lack of increase in aGVHD after PBSCT compared with BMT despite a 10-fold increase in T cells in the PBSC allograft compared with the bone marrow allograft.18 The clinical significance of DC reconstitution after allogeneic SCT is unknown.
Given the central role of DCs in the immune system with potential antitumor effects, we hypothesized that the recovery of DCs after allogeneic SCT could be related to relapse and survival. Therefore, we measured circulating DCs in the peripheral blood at engraftment, with the rationale that this represents the earliest period of measurable donor immune reconstitution and could also be a clinically useful early indicator for outcomes after SCT.
Patients, materials, and methods
Patients
Fifty patients with hematologic disorders were evaluable after undergoing allogeneic SCT from matched sibling donors between February 2000 and March 2003. The University of Florida institutional review board approved the study, and informed consent was obtained. Patients for whom there were no engraftment samples for flow cytometry or who died before engraftment were inevaluable. One patient with persistent myeloproliferative disorder at the time of engraftment and poor donor cell chimerism (29%) 4 weeks after nonmyeloablative transplantation, necessitating subsequent myeloablative transplantation, was excluded. Patients were treated with myeloablative (73%) or nonmyeloablative (27%) conditioning and received aGVHD prophylaxis based on institutional guidelines and protocols. Most patients (92%) underwent PBSCT, and the remainder underwent BMT. Clinical data including survival, relapse, and grades II to IV aGVHD were obtained from Shands Hospital/University of Florida patient records.
Immunophenotypic analysis
Blood samples were obtained from donor stem cell products and from the recipients before conditioning. After transplantation, blood samples were drawn within a median of 1 day after engraftment (range, -4 to +8 days), defined as a sustained recovery of absolute neutrophil count (ANC) of more than 0.5 × 109/L (500/μL) for 3 days or an increase after a nadir in the case of nonmyeloablative SCT. Dendritic cells were identified and enumerated by 4-color flow cytometric analysis using a FACScalibur flow cytometer (Becton Dickinson, Mountain View, CA), as previously described.19-22 To minimize cell loss, whole blood staining with no wash was used, followed by red cell lysis with ammonium chloride. Dendritic cells were negative for lineage markers (CD3, CD14, CD16, CD19, CD20, CD56) and HLA-DR+. DC1 were CD11c+, and DC2 were CD123+. Absolute number (DC/μL) was determined by multiplying the percentage of DC1 and DC2 with the total number of white blood cells per μL. To confirm our technique, we repeated our analysis on several additional samples in triplicate, which reproduced DC counts within the range of 1 in 10 000 cells, confirming the validity and reproducibility of our analysis for DC counts. CD34+ cells, T cells (CD3+) infused, and CD3+ cells engrafted were also measured by flow cytometry.
Statistical methods
Patients had a minimum of 136 days of possible follow-up time and were monitored until death or the last study follow-up date of August 6, 2003. Additional study end points included relapse, grades II to IV aGVHD, and a composite end point of death or relapse. Recipients' engrafted DC counts were the main variable of interest to be tested for their predictive properties with regard to the study end points. We chose to divide the patients according to high and low DC counts. To designate the cutoff level for high versus low DC counts, a univariate disjoint clustering method, based on Euclidean squared distances, was used for the transformed variables. Along with the main response variable of engrafted DC counts, we also analyzed the 2 components of these, DC1 and DC2, separately. Groupings for DC1 and DC2 were created using the same clustering methodology.
Several other peripheral blood cell counts of interest were measured at baseline and immediately after engraftment, as was the amount of infused cells. Some cell counts were denoted as the original number of cells per a given volume, whereas some values had to be transformed to reduce variability and to account for nonlinear relationships among variables. All transformations were monotonic (preserving the order of values) and were selected based on graphic assessments and diagnostics of the transformed values. Logarithmic transformations were generally used for this purpose because many of the raw counts displayed an exponential distribution. On several occasions, a positive shift in location of the values had to be made to adequately use a logarithmic transformation for extremely small or null values.
We tested the effects of high versus low engraftment DC count on outcomes using univariate Kaplan-Meier models. Log-rank tests were used to measure significant differences between strata. These models were generated to display the univariate relationship of variables with outcomes and to test the significance of potential covariates to be introduced in multivariate models. To confirm outcomes and to adjust for potential confounding factors, multivariate Cox proportional hazards models were generated. Cox models were visually assessed for the proportional hazards assumption and for testing interaction terms with a time-dependent covariate. We initially constructed the Cox model with demographic information and DC count levels and included additional covariates that demonstrated independent univariate significance for the applicable outcomes or that showed significance when included in the multivariate model. Additional variables that were considered but were found to be insignificant and thus not used in the multivariate analyses were log (white blood cells) engrafted, CD3+ and DC infused, log ratio (DC1/DC2) cells, patient DC count before transplantation, and DC difference (log DCs before and after transplantation). To test the predictive value of the engrafted DC count for the outcomes tested, we evaluated the status of patients at 136 days (the minimum follow-up period) and used this as the applicable outcome measurement. From the level of DC count (high vs low), we tabulated the observations that had reached or failed to reach the various outcomes. With this information we calculated the sensitivity, specificity, positive predictive value, and negative predictive value for each outcome. Because positive and negative predictive values are traditionally used on naive cohorts, their values cannot be directly translated to future studies. To further ascertain the viability of the group delineation by the cluster technique, we produced a receiver operating characteristic (ROC) curve plot of the transformed DC count. A probability type 1 error, α = .05, was considered the threshold of statistical significance. All analyses were performed on SAS software (version 8.02; SAS Institute, Cary, NC).
Results
Patient and graft characteristics
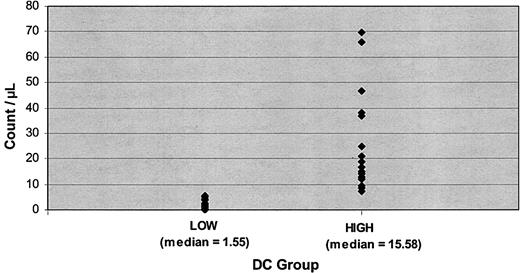
Patient, graft, and posttransplantation reconstitution characteristics are given in Table 1. Most patients (92%) underwent transplantation with peripheral blood stem cells. Patients were divided according to low and high engraftment DC counts based on the clustering technique that produced 23 observations in the low group and 27 in the high group. The median number of DCs for all patients was 6.9 cells/μL. The median number of DCs for the patients in the low group was 1.55, and for the high group it was 15.58 (Figure 1). Transformed cutoff criteria were equivalent to a nontransformed cell count of 4.97. Patients with a DC count equal to or higher than 4.97/μL were assigned to the high group, whereas patients with counts lower than 4.97/μL were assigned to the low group.
Patient and graft characteristics of low DC and high DC patient groups
Characteristics . | Low DC group; n = 23 . | High DC group; n = 27 . |
|---|---|---|
| Patients | ||
| Recipient male, % | 60.9 | 55.6 |
| Donor male, % | 39.1 | 40.7 |
| Recipient age, y | 45.6 ± 10.5 | 44.8 ± 14.4 |
| Donor age, y | 46.0 ± 12.0 | 43.8 ± 16.6 |
| Transplantation | ||
| Myeloablative, % | 82.6 | 63.0 |
| High disease risk, %* | 87.0 | 85.2 |
| Donor CMV+, % | 52.2 | 70.4 |
| Patient DC count before, median (range, lower to upper quartiles)† | 5.8 (4.4-15.6) | 10.1 (6.5-14.9) |
| Graft source | ||
| Peripheral blood stem cells, no. of patients | 23 | 23 |
| Bone marrow, no. of patients | 0 | 4 |
| Graft | ||
| CD34+ infused, × 106/kg | 5.6 ± 1.9 | 5.3 ± 2.1 |
| CD3+ infused, × 108/kg‡ | 3.1 ± 2.2 | 2.4 ± 1.8 |
| DCs infused, × 106/kg§ | 7.6 ± 5.5 | 7.1 ± 4.6 |
| Engraftment parameters | ||
| White blood cell count, 1 × 109/L | 5.6 ± 6.4 | 6.0 ± 7.6 |
| Median ANC, 1 × 109/L (mean ± SD) | 1.95 (4.2 ± 4.0) | 3.47 (4.2 ± 6.1) |
| CD3+ cells, 1 × 109/L | 0.47 ± 0.60 | 0.54 ± 0.64 |
Characteristics . | Low DC group; n = 23 . | High DC group; n = 27 . |
|---|---|---|
| Patients | ||
| Recipient male, % | 60.9 | 55.6 |
| Donor male, % | 39.1 | 40.7 |
| Recipient age, y | 45.6 ± 10.5 | 44.8 ± 14.4 |
| Donor age, y | 46.0 ± 12.0 | 43.8 ± 16.6 |
| Transplantation | ||
| Myeloablative, % | 82.6 | 63.0 |
| High disease risk, %* | 87.0 | 85.2 |
| Donor CMV+, % | 52.2 | 70.4 |
| Patient DC count before, median (range, lower to upper quartiles)† | 5.8 (4.4-15.6) | 10.1 (6.5-14.9) |
| Graft source | ||
| Peripheral blood stem cells, no. of patients | 23 | 23 |
| Bone marrow, no. of patients | 0 | 4 |
| Graft | ||
| CD34+ infused, × 106/kg | 5.6 ± 1.9 | 5.3 ± 2.1 |
| CD3+ infused, × 108/kg‡ | 3.1 ± 2.2 | 2.4 ± 1.8 |
| DCs infused, × 106/kg§ | 7.6 ± 5.5 | 7.1 ± 4.6 |
| Engraftment parameters | ||
| White blood cell count, 1 × 109/L | 5.6 ± 6.4 | 6.0 ± 7.6 |
| Median ANC, 1 × 109/L (mean ± SD) | 1.95 (4.2 ± 4.0) | 3.47 (4.2 ± 6.1) |
| CD3+ cells, 1 × 109/L | 0.47 ± 0.60 | 0.54 ± 0.64 |
Unless otherwise indicated, values are mean ± SD.
High disease risk: acute myelogenous leukemia (AML) and acute lymphoblastic leukemia (ALL) other than first complete remission (CR1), primary induction failure; acute leukemia from antecedent hematologic disorder; chronic myelogenous leukemia not in first chronic phase (CML not CP1); chronic lymphocytic leukemia (CLL); acute bilineage leukemia (ABIL); non-Hodgkin lymphoma (NHL); Hodgkin disease (HD); myelodysplastic syndrome (MDS) not refractory anemia (RA) or ringed sideroblasts (RARS); multiple myeloma (MM); eosinophilic leukemia (n = 1). Low disease risk: ALL CR1; AML CR1; CML CP1; MDS RA, RARS.
Known for 47 of 50 patients who underwent transplantation.
Known for 49 of 50 patients who underwent transplantation.
Known for 32 of 50 patients who underwent transplantation.
Dendritic cell group distributions. Values represent low and high DC counts.
Dendritic cell count and patient survival
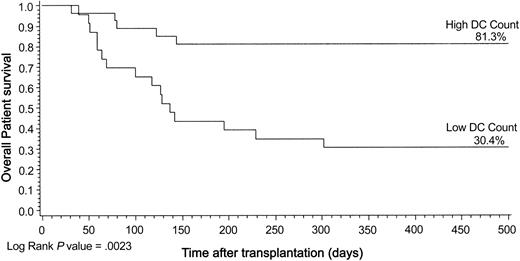
The median follow-up for patients alive is 501 days (range, 136-1263 days). Seven (26%) of 27 patients died in the high DC group compared with 16 (70%) of 23 patients in the low DC group, creating a significant impact on the study end point of patient death using Kaplan-Meier analysis (log rank; P = .002) (Figure 2). Similarly, low DC counts at engraftment were associated with significantly higher risk for patient death by univariate Cox model (relative risk [RR], 3.7; confidence interval [CI], 1.5-8.9) (Table 2). Low DC count was confirmed in the multivariate analysis as a significant risk factor for patient death (RR, 3.8; 95% CI, 1.3-11.3; P = .02) (Table 3). We repeated the same Kaplan-Meier models demonstrating significant results for PBSCT (P = .01) and myeloablative transplantation (P = .01) (Table 2).
Kaplan-Meier plot of patient survival by DC group illustrated for 500 days after transplantation. Statistical results in the text are given for the entire transplantation follow-up period. Log rank, P = .0023.
Kaplan-Meier plot of patient survival by DC group illustrated for 500 days after transplantation. Statistical results in the text are given for the entire transplantation follow-up period. Log rank, P = .0023.
Univariate relative risk analyses for survival, relapse, composite relapse or death, and aGVHD in low DC group
Outcome . | RR . | 95% CI . | P . |
|---|---|---|---|
| Death | |||
| All | 3.67 | 1.50-8.94 | .0023 |
| PBSCT | 3.06 | 1.26-7.47 | .0097 |
| Myeloablative | 3.93 | 1.28-12.09 | .0100 |
| Nonmyeloablative | 4.61 | 0.76-27.94 | .0766 |
| Relapse | |||
| All | 5.07 | 1.62-15.89 | .0021 |
| PBSCT | 4.19 | 1.34-13.12 | .0078 |
| Myeloablative | 11.89 | 1.50-94.57 | .0031 |
| Nonmyeloablative | 3.49 | 0.68-17.85 | .1116 |
| Relapse or death | |||
| All | 3.40 | 1.52-7.64 | .0017 |
| PBSCT | 2.80 | 1.25-6.29 | .0093 |
| Myeloablative | 4.80 | 1.57-14.72 | .0284 |
| Nonmyeloablative | 3.37 | 0.82-13.82 | .0745 |
| aGVHD | |||
| All | 3.21 | 1.61-6.40 | .0005 |
| PBSCT | 2.86 | 1.41-5.79 | .0024 |
| Myeloablative | 2.75 | 1.24-6.13 | .0096 |
| Nonmyeloablative | 3.66 | 0.89-14.97 | .0540 |
Outcome . | RR . | 95% CI . | P . |
|---|---|---|---|
| Death | |||
| All | 3.67 | 1.50-8.94 | .0023 |
| PBSCT | 3.06 | 1.26-7.47 | .0097 |
| Myeloablative | 3.93 | 1.28-12.09 | .0100 |
| Nonmyeloablative | 4.61 | 0.76-27.94 | .0766 |
| Relapse | |||
| All | 5.07 | 1.62-15.89 | .0021 |
| PBSCT | 4.19 | 1.34-13.12 | .0078 |
| Myeloablative | 11.89 | 1.50-94.57 | .0031 |
| Nonmyeloablative | 3.49 | 0.68-17.85 | .1116 |
| Relapse or death | |||
| All | 3.40 | 1.52-7.64 | .0017 |
| PBSCT | 2.80 | 1.25-6.29 | .0093 |
| Myeloablative | 4.80 | 1.57-14.72 | .0284 |
| Nonmyeloablative | 3.37 | 0.82-13.82 | .0745 |
| aGVHD | |||
| All | 3.21 | 1.61-6.40 | .0005 |
| PBSCT | 2.86 | 1.41-5.79 | .0024 |
| Myeloablative | 2.75 | 1.24-6.13 | .0096 |
| Nonmyeloablative | 3.66 | 0.89-14.97 | .0540 |
Cox proportional hazards model: time to patient death in low DC group
Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Low DC group | 3.77 | 1.26-11.27 | .02 |
| CD34 infused, × 106/kg | 1.36 | 0.98-1.88 | .07 |
| Log,ANC | 1.84 | 1.03-3.30 | .04 |
| Patient age | 0.97 | 0.90-1.05 | .43 |
| Patient sex male | 1.07 | 0.38-2.99 | .90 |
| Donor age | 1.00 | 0.94-1.07 | .94 |
| Donor sex male | 1.31 | 0.48-3.56 | .60 |
| High disease risk | 3.38 | 0.38-30.41 | .28 |
| Nonmyeloablative conditioning | 0.74 | 0.16-3.52 | .71 |
Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Low DC group | 3.77 | 1.26-11.27 | .02 |
| CD34 infused, × 106/kg | 1.36 | 0.98-1.88 | .07 |
| Log,ANC | 1.84 | 1.03-3.30 | .04 |
| Patient age | 0.97 | 0.90-1.05 | .43 |
| Patient sex male | 1.07 | 0.38-2.99 | .90 |
| Donor age | 1.00 | 0.94-1.07 | .94 |
| Donor sex male | 1.31 | 0.48-3.56 | .60 |
| High disease risk | 3.38 | 0.38-30.41 | .28 |
| Nonmyeloablative conditioning | 0.74 | 0.16-3.52 | .71 |
P values determined by χ2 analysis.
Dendritic cell count and relapse-free survival
Low DC count was significantly associated with more and earlier relapse after transplantation when analyzed using the Kaplan-Meier model (P = .002) (Table 2). We also examined this outcome in a multivariate Cox model and found a significant association for low DC count with death-censored relapse (RR, 11.6; 95% CI, 2.9-47.5; P = .001) (Table 4). In addition, for the composite end point of relapse or death, the univariate Kaplan-Meier model demonstrated a significant association between low DC counts and higher failure rates (P = .002) (Figure 3); these were significant in recipients of PBSCT (P = .01) and myeloablative conditioning (P = .03) (Table 2). The multivariate model for this outcome confirmed the significance of the DC count effect, and the hazard ratio (HR) for the low DC count group was 5.1 (CI, 2.0-13.3) (Table 5). Pretransplantation DC counts before conditioning for 47 patients (94%) and graft DC content for 32 patients (64%) were not significantly associated with relapse or death.
Cox proportional hazards model: time to patient relapse in low DC group
Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Low DC group | 11.63 | 2.86-47.46 | .001 |
| CD34 infused, × 106/kg | 1.47 | 1.01-2.14 | .043 |
| Log, ANC | 0.94 | 0.52-1.68 | .823 |
| Patient age | 1.01 | 0.93-1.10 | .742 |
| Patient sex male | 2.56 | 0.71-9.29 | .153 |
| Donor age | 0.99 | 0.91-1.06 | .681 |
| Donor sex male | 1.19 | 0.30-4.72 | .804 |
| Nonmyeloablative conditioning | 1.27 | 0.25-6.41 | .771 |
Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Low DC group | 11.63 | 2.86-47.46 | .001 |
| CD34 infused, × 106/kg | 1.47 | 1.01-2.14 | .043 |
| Log, ANC | 0.94 | 0.52-1.68 | .823 |
| Patient age | 1.01 | 0.93-1.10 | .742 |
| Patient sex male | 2.56 | 0.71-9.29 | .153 |
| Donor age | 0.99 | 0.91-1.06 | .681 |
| Donor sex male | 1.19 | 0.30-4.72 | .804 |
| Nonmyeloablative conditioning | 1.27 | 0.25-6.41 | .771 |
P values determined by χ2 analysis.
Kaplan-Meier plot of composite patient/relapse-free survival by DC group illustrated for 500 days after transplantation. Statistical results in the text are given for the entire transplantation follow-up period. Log rank, P = .0017.
Kaplan-Meier plot of composite patient/relapse-free survival by DC group illustrated for 500 days after transplantation. Statistical results in the text are given for the entire transplantation follow-up period. Log rank, P = .0017.
Cox proportional hazards model: time to patient composite relapse or death in low DC group
Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Low DC group | 5.14 | 2.00-13.26 | .001 |
| CD34 infused, × 106/kg | 1.26 | 0.96-1.65 | .100 |
| Log, ANC | 1.31 | 0.81-2.10 | .275 |
| Patient age | 0.98 | 0.92-1.04 | .470 |
| Patient sex male | 1.11 | 0.45-2.71 | .827 |
| Donor age | 1.00 | 0.95-1.05 | .993 |
| Donor sex male | 1.13 | 0.44-2.89 | .798 |
| High disease risk | 4.71 | 0.56-39.83 | .154 |
| Nonmyeloablative conditioning | 1.99 | 0.51-7.72 | .319 |
Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Low DC group | 5.14 | 2.00-13.26 | .001 |
| CD34 infused, × 106/kg | 1.26 | 0.96-1.65 | .100 |
| Log, ANC | 1.31 | 0.81-2.10 | .275 |
| Patient age | 0.98 | 0.92-1.04 | .470 |
| Patient sex male | 1.11 | 0.45-2.71 | .827 |
| Donor age | 1.00 | 0.95-1.05 | .993 |
| Donor sex male | 1.13 | 0.44-2.89 | .798 |
| High disease risk | 4.71 | 0.56-39.83 | .154 |
| Nonmyeloablative conditioning | 1.99 | 0.51-7.72 | .319 |
P values determined by χ2 analysis.
Dendritic cell count and aGVHD
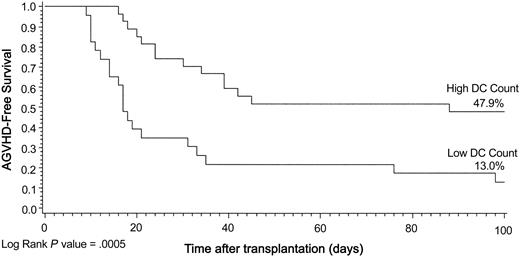
We also investigated the impact of the DC count on the occurrence of grades II to IV aGVHD within the first 100 days after transplantation. The Kaplan-Meier model showed a significant relationship between GVHD-free survival and DC count group (P = .0005) (Figure 4). The univariate proportional hazards estimate for the low DC group for the entire population was 3.2 (CI, 1.6-6.4) (Table 2). Results were also highly significant when evaluated for PBSCT (P = .002) and for myeloablative conditioning (P = .01). The multivariate model further confirmed the risk for low DC count (RR, 3.3; 95% CI, 1.5-7.4) (Table 6). Additionally, in this model, a higher ANC was associated with a significant risk for aGVHD (RR, 1.6; 95% CI, 1.1-2.5).
Kaplan-Meier plot of aGVHD-free survival by DC group illustrated for 100 days after transplantation. Data are in keeping with the definition of aGVHD. Log rank, P = .0005.
Kaplan-Meier plot of aGVHD-free survival by DC group illustrated for 100 days after transplantation. Data are in keeping with the definition of aGVHD. Log rank, P = .0005.
Cox proportional hazards model: time to acute GVHD in low DC group
Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Low DC group | 3.32 | 1.48-7.45 | .04 |
| CD34 infused, × 106/kg | 0.95 | 0.78-1.16 | .62 |
| Log, ANC | 1.65 | 1.08-2.53 | .02 |
| Patient age | 0.97 | 0.92-1.03 | .32 |
| Patient sex male | 1.27 | 0.56-2.88 | .56 |
| Donor age | 1.02 | 0.97-1.06 | .49 |
| Donor sex male | 0.71 | 0.32-1.56 | .39 |
| High disease risk | 0.87 | 0.20-3.82 | .85 |
| Nonmyeloablative conditioning | 0.85 | 0.30-2.45 | .76 |
Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Low DC group | 3.32 | 1.48-7.45 | .04 |
| CD34 infused, × 106/kg | 0.95 | 0.78-1.16 | .62 |
| Log, ANC | 1.65 | 1.08-2.53 | .02 |
| Patient age | 0.97 | 0.92-1.03 | .32 |
| Patient sex male | 1.27 | 0.56-2.88 | .56 |
| Donor age | 1.02 | 0.97-1.06 | .49 |
| Donor sex male | 0.71 | 0.32-1.56 | .39 |
| High disease risk | 0.87 | 0.20-3.82 | .85 |
| Nonmyeloablative conditioning | 0.85 | 0.30-2.45 | .76 |
P values determined by χ2 analysis.
Dendritic cell phenotypes DC1 and DC2 and posttransplantation outcome
To further understand the engrafted DC effect, we tested the 2 DC phenotypes, DC1 and DC2, separately. For DC1, the failure rates remained higher in the low-count group; log rank P values for the outcomes of death, relapse, and GVHD onset, respectively, were .01, .04, and .0002 (Table 7). Repeating the procedures for DC2, similar trends were noted with a significant correlation for aGVHD (log rank, P = .01). Neither the DC1 nor the DC2 low-count group showed a statistically significant effect independently in the multivariate model, but each did show a trend toward hazard in the low-count group.
Patient death, relapse, and aGVHD for DC1 and DC2
Outcome and DC level . | No. of patients . | No. of treatment failures . | Survival after transplantation, % . | P . |
|---|---|---|---|---|
| Death | ||||
| DC1 | .0130 | |||
| High | 25 | 7 | 52.4* | |
| Low | 25 | 16 | 35.6* | |
| DC2 | .0740 | |||
| High | 18 | 5 | 58.0* | |
| Low | 32 | 18 | 40.1* | |
| Relapse | ||||
| DC1 | .0434 | |||
| High | 25 | 5 | 79.3† | |
| Low | 25 | 11 | 44.3† | |
| DC2 | .0925 | |||
| High | 18 | 3 | 82.6† | |
| Low | 32 | 13 | 50.8† | |
| aGVHD | ||||
| DC1 | .0002 | |||
| High | 25 | 12 | 51.7‡ | |
| Low | 25 | 22 | 12.0‡ | |
| DC2 | .0137 | |||
| High | 18 | 9 | 49.4‡ | |
| Low | 32 | 25 | 21.9‡ |
Outcome and DC level . | No. of patients . | No. of treatment failures . | Survival after transplantation, % . | P . |
|---|---|---|---|---|
| Death | ||||
| DC1 | .0130 | |||
| High | 25 | 7 | 52.4* | |
| Low | 25 | 16 | 35.6* | |
| DC2 | .0740 | |||
| High | 18 | 5 | 58.0* | |
| Low | 32 | 18 | 40.1* | |
| Relapse | ||||
| DC1 | .0434 | |||
| High | 25 | 5 | 79.3† | |
| Low | 25 | 11 | 44.3† | |
| DC2 | .0925 | |||
| High | 18 | 3 | 82.6† | |
| Low | 32 | 13 | 50.8† | |
| aGVHD | ||||
| DC1 | .0002 | |||
| High | 25 | 12 | 51.7‡ | |
| Low | 25 | 22 | 12.0‡ | |
| DC2 | .0137 | |||
| High | 18 | 9 | 49.4‡ | |
| Low | 32 | 25 | 21.9‡ |
P values determined by log rank.
Patient survival 1000 days after transplantation.
Relapse-free survival 1000 days after transplantation.
aGVHD-free survival 100 days after transplantation.
Predictive value of posttransplantation dendritic cell count
To assess the viability of using the engrafted DC count group as a predictive classification, we used only information available for patients at 136 days, which was the minimum follow-up period. Eleven (48%) of 23 patents died in the low DC group, whereas only 4 (15%) of 27 patients died in the high DC group. Sensitivity and specificity for the composite outcome of death or relapse using cell count as the test for the condition were 73% and 75%, respectively. Positive predictive value of the test (the conditional probability that the outcome occurred given a risk test level) was 70%, and negative predictive value (the conditional probability that the outcome did not occur given a non-risk test level) was 78%. We repeated the tabulations and calculated the same statistics for the other study end points (Table 8).
Clinical predictive value of low DC count after transplantation
Outcome . | No. of patients with outcome . | No. of patients without outcome . | Sensitivity, % . | Specificity, % . | Positive predictive value, % . | Negative predictive value, % . |
|---|---|---|---|---|---|---|
| Death | 15 | 35 | 73 | 66 | 48 | 85 |
| Relapse | 14 | 36 | 71 | 64 | 43 | 85 |
| Relapse or death | 22 | 28 | 73 | 75 | 70 | 78 |
| Acute GVHD | 34 | 16 | 59 | 81 | 87 | 48 |
Outcome . | No. of patients with outcome . | No. of patients without outcome . | Sensitivity, % . | Specificity, % . | Positive predictive value, % . | Negative predictive value, % . |
|---|---|---|---|---|---|---|
| Death | 15 | 35 | 73 | 66 | 48 | 85 |
| Relapse | 14 | 36 | 71 | 64 | 43 | 85 |
| Relapse or death | 22 | 28 | 73 | 75 | 70 | 78 |
| Acute GVHD | 34 | 16 | 59 | 81 | 87 | 48 |
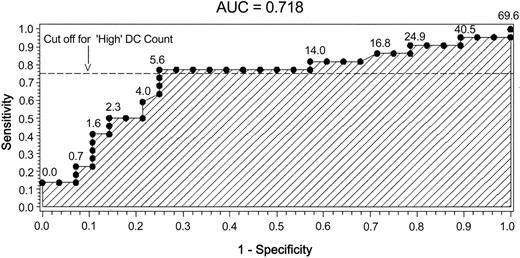
To retrospectively evaluate the cutoff level for the DC count groups and the overall predictive value of the model for the composite outcome of relapse or death, we constructed an ROC plot. The plot of sensitivity compared with 1-specificity revealed an area under the curve of 0.718. Also of note was that the chosen cutoff value was in proximity to an “elbow” of the plot, as depicted in Figure 5.
ROC plot for composite outcome of relapse or death. Predictive sensitivity and specificity testing of DC count after transplantation. Values on ROC plot indicate original DC engraftment cell count.
ROC plot for composite outcome of relapse or death. Predictive sensitivity and specificity testing of DC count after transplantation. Values on ROC plot indicate original DC engraftment cell count.
Discussion
Our data show for the first time that the reconstitution of absolute numbers of circulating DCs after allogeneic hematopoietic SCT is an independent predictor of posttransplantation survival, relapse, and aGVHD. We measured DCs in the peripheral blood around the time of engraftment based on the rationale that this represents the earliest period of measurable donor immune reconstitution. We hypothesized that early DC counts could have an important impact on transplantation outcomes given the central role for DCs in the immune system and specifically the potential for antitumor activity. It is important to emphasize that our low and high DC patient groups were comparable with regard to CD34+ and CD3+ cells infused, donor and recipient age and sex, disease risk status, and reconstitution of CD3+ T cells. We feel that the cutoff point of 4.97 cells/μL was a reasonable clinical tool for early patient assessment because it was generated by cluster analysis, with account taken for the absolute frequency of the event and with post hoc confirmation by ROC analysis to show actual correlation to clinical outcomes.
Our results indicate that higher numbers of DCs reconstituted after transplantation may reduce relapse (Tables 2 and 4; Figure 3). Although the mechanisms by which DCs decrease relapse requires further investigation, a recent study by Sato et al23 demonstrates that regulatory DCs administered after allogeneic BMT protected mice from relapse and acute GVHD. Mechanisms in this model include activating regulatory T cells through DCs. Other than DCs, we found using Cox proportional hazards models that higher numbers (> 5.5 × 106) of CD34 cells infused were significantly associated with relapse. Although the impact of CD34 cells was not our primary interest, we did not necessarily expect a significant risk associated with this. Recent observations suggest that higher numbers of CD34 cells affect posttransplantation survival detrimentally,24 though CD34 cells infused do not correlate with DC reconstitution.25 Furthermore, only DCs, but not CD34 cells, were found to be important for all the outcomes tested (ie, survival, relapse, and aGVHD).
Our results are striking in that DC count is an independent prognostic indicator for relapse and for the development of aGVHD, contrary to observations with graft T cells, the depletion of which decreases aGVHD occurrence but increases relapse.26 This lack of graft-versus-malignancy despite the development of GVHD may be explained by the predominance of high-risk malignancies (86%) in our patients, a group known for poor response to allogeneic donor lymphocyte infusion.27 Additional mechanisms for a protective impact of DCs against relapse and aGVHD could be extrapolated from the study by Sato et al,23 which suggests the role of immune regulatory cells. Evidence that DCs are involved in the pathophysiology of GVHD is based on murine models in which the lack of host DC activity prevented aGVHD development.28 Mixed chimerism and persistence of host DCs appear to be associated with aGVHD.29,30 Chimerism was not performed in our patients who underwent myeloablative transplantation. White blood cell chimerism analysis was performed in most (12 of 14) of our patients 4 weeks after nonmyeloablative transplantation; 11 of 12 had more than 95% donor cells. Fluorescence in situ hybridization analysis revealed 57% donor cells, 3.5% host cells, and 39% single chromosome cells in 1 patient. Although DC chimerism was not analyzed in our study, it is possible that most of our patients had full donor DC chimerism, as suggested by a recent study31 in which patients, after myeloablative and nonmyeloablative SCT, had donor-derived DCs by day 14.31 Chimerism analysis is difficult to perform because of the rarity (< 1%) of DCs32 and the requirement to propagate DCs in culture before analysis.33 This, however, is not practical for many transplantation centers. In addition, the clinical significance of DC chimerism and the association with relapse is unknown from previous studies. Therefore, measuring absolute DC count, as in our study, may be a simple and reproducible method to predict clinical outcomes of relapse, aGVHD, and survival after PBSCT and myeloablative transplantation. Determining whether posttransplantation DC counts are clinically relevant after nonmyeloablative transplantation requires additional studies with larger numbers of patients.
Identifying potential causes of low blood DC counts after SCT warrants further investigation. Potential causes include central and peripheral mechanisms, as postulated previously in HIV infection.34 Central causes include lack of DC production from bone marrow in the low DC group of patients, though inherently these patients had no impairment of DC production before transplantation. All our patients received related HLA-matched transplants, and this excludes HLA disparity for lack of DC production. Peripherally, DCs might have homed to lymphoid tissues or target organs of GVHD because of allogeneic stimuli given that 87% of patients with low DC counts acquired GVHD compared with 52% of patients with high DC counts (P = .0005) (Table 2.). Patients with low and high DC counts were clinically comparable for graft content of CD34+, CD3+, and DCs infused and for neutrophil and T-cell reconstitution, suggesting that DC engraftment was unique. It has been suggested that donor DC content in the bone marrow graft before transplantation is associated with outcome after BMT.35 Our data suggest that, at least for PBSCT, the number of DCs in the graft has no impact, whereas the numbers of DCs ultimately reconstituted in the recipient makes the difference. Importantly, for patients in whom DC content of the donor graft was known, the numbers of donor DCs infused had no impact on DC recovery after transplantation. Furthermore, in SCT, DC reconstitution may have other restrictions not yet fully understood because of the donor-host interactions unique to allogeneic SCT. To further delineate DCs functionally, we studied DC1 and DC2 phenotypes, which mirrored those of total DC recovery, suggesting that both subtypes are important for posttransplantation outcome. It is surprising that the adequate reconstitution of DC1 and DC2 appears to be important for improved posttransplantation outcomes because Waller et al35 suggest that higher numbers of DC2 are associated with relapse. However, there are key differences between that study and our observations. Our results concern the immune reconstitution of DCs after transplantation. In addition, most of our patients (92%) received peripheral blood instead of bone marrow as the stem cell source. We performed further subgroup analysis for patients specifically receiving peripheral blood stem cells, excluding those receiving bone marrow. Our results indicated that low numbers of DCs reconstituted after PBSCT were associated with adverse outcomes (Table 2).
In summary, our findings are unique in describing for the first time an early, easily reproducible clinical indicator for adverse clinical outcomes, such as relapse, death, and aGVHD, after hematopoietic SCT. This finding should allow for early classification of patients and potential therapeutic interventions. Future studies will have to be conducted to delineate the functional aspect of DCs in the disease process and to elucidate whether influencing the number of DCs at engraftment might improve outcomes.
Prepublished online as Blood First Edition Paper, February 12, 2004; DOI 10.1182/blood-2003-09-3325.
Supported by the University of Florida Opportunity Fund, The College of Medicine Incentive Fund, and Shands Hospital at the University of Florida.
Presented in part at the 43rd annual meeting of the American Society of Hematology, Orlando, FL, December 10, 2001.1
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Eugenia Martinez for secretarial assistance, Lili Tian for preliminary data analysis, Renee Boyette for protocol and sample collections, and Samantha Greene, Alex Pastos, and Pam Morgan for clinical updates and database information.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal