Abstract
The NF1 tumor suppressor gene encodes a guanosine triphosphotase (GTPase)-activating protein that negatively regulates Ras signaling and is inactivated in a subset of juvenile myelomonocytic leukemias (JMMLs). Adoptive transfer of fetal liver cells from Nf1 mutant mice models JMML; however, this system has important limitations as a platform for performing biologic and preclinical studies. We have exploited the interferon-inducible Mx1-Cre transgene to ablate a conditional mutant Nf1 allele in hematopoietic cells. Somatic inactivation of Nf1 induces a myeloproliferative disorder with 100% penetrance that is associated with a sub-acute clinical course, tissue infiltration by myeloid cells, hypersensitivity to granulocyte-macrophage colony stimulating factor, hyperproliferation, and resistance to apoptosis. These Mx1-Cre, Nf1flox/flox mice establish a tractable experimental model for testing therapeutics and for identifying mutations that cooperate with hyperactive Ras in myeloid leukemogenesis. (Blood. 2004;103:4243-4250)
Introduction
Juvenile myelomonocytic leukemia (JMML) is an aggressive myeloproliferative disease (MPD) characterized by monocytosis, thrombocytopenia, splenomegaly, and malignant infiltration of the skin, lymph nodes, lungs, liver, and other organs (reviewed in Emanuel et al1 and Arico et al2 ). The clinical course is relentless, and bone marrow transplantation is the only treatment that cures more than 10% of patients. Selective hypersensitivity of granulocyte-macrophage colony-forming unit (CFUGM) progenitors to granulocyte-macrophage colony-stimulating factor (GM-CSF) is an in vitro hallmark of JMML.3,4 The incidence of JMML is increased more than 200-fold in children with neurofibromatosis type 1 (NF1)5,6 ; this observation provided a starting point for elucidating the molecular basis of aberrant myeloid growth in this disorder. The NF1 gene encodes neurofibromin, a guanosine triphosphotase (GTPase)-activating protein (GAP) that negatively regulates p21ras (Ras) output by accelerating GTP hydrolysis (reviewed in Boguski and McCormick,7 Bernards,8 and Donovan et al9 ). Analysis of JMML cells from children with NF1 revealed homozygous NF1 inactivation because of somatic loss of the normal allele, which is associated with hyperactive Ras.10-13
Two groups used homologous recombination in embryonic stem cells to disrupt Nf1, the murine homolog of NF1.14,15 Approximately 10% of heterozygous (Nf1+/-) mutant mice spontaneously develop a MPD that resembles JMML during the second year of life.14 Homozygous mutant (Nf1-/-) embryos fail around embryonic day 13 (E13) with cardiovascular defects14,15 ; however, CFU-GM colonies derived from mutant fetal livers show hypersensitive growth in response to GM-CSF that is similar to human JMML cells.11,16 Importantly, adoptive transfer of Nf1-/- fetal liver cells consistently induces a JMML-like MPD in irradiated recipient mice.16 Nf1 inactivation leads to deregulated growth in multiple hematopoietic compartments and confers a durable proliferative advantage in competitive repopulation assays.17,18 In addition, a cross between Nf1 and Gmcsf mutant mice demonstrated that aberrant GM-CSF signaling plays a central role in initiating and maintaining the JMML-like MPD in vivo.19
In a previous study, irradiated recipient mice that received transplants with Nf1-/- fetal liver cells were harnessed to evaluate the efficacy of an inhibitor of the Ras processing enzyme farnesyltransferase, which included pharmacodynamic monitoring in primary hematopoietic cells.20 However, this model is both expensive and cumbersome because it requires maintaining a large breeding colony, performing multiple timed matings followed by embryo dissections around E13, and injecting fetal liver cells into irradiated hosts. A “second-generation” mutant strain was recently engineered by introducing loxP sites into the Nf1 locus by homologous recombination.21 Somatic inactivation of this Nf1flox allele, which is functionally wild type in the basal state, can be achieved by expressing Cre recombinase in specific cell types.21,22 We have exploited the interferon-inducible Mx1-Cre strain23 to ablate Nf1 in hematopoietic cells, and we find that this consistently results in an MPD that is associated with leukocytosis, splenomegaly, hyperproliferation, impaired apoptosis, and in vitro hypersensitivity to GM-CSF. We further describe the consequences of Nf1 inactivation on proliferation, survival, and Ras signaling in these mice.
Materials and methods
Breeding and treatment with polyinosinic-polycytidylic acid (pI-pC)
Nf1flox and Mx1-Cre mice were produced and characterized as described elsewhere.21,23 Compound mutant (Mx1-Cre, Nf1flox/flox) animals were generated on a mixed 129/Sv × C57BL/6 background. Pups received a single intraperitoneal injection at 3 to 5 days of age with 50 μL pI-pC (Sigma, St Louis, MO) at a concentration of 10 μg/μL diluted in sterile phosphate-buffered saline (PBS). Mice were maintained in the sterile animal care facility at the University of California, San Francisco (UCSF) and were fed pelleted chow and acidified water ad libitum. The experimental procedures were approved by the UCSF Committee on Animal Research.
Genotyping
We isolated DNA from blood samples using the GFX Genomic Blood DNA Purification Kit (Amersham, Arlington Heights, IL). Genotyping of the Nf1flox and recombined allele was carried out by using the primer sequence and polymerase chain reaction (PCR) conditions as described by Zhu et al.21 Genotyping for the Mx1-Cre allele was performed by using primers Cre1 (5′-CTG CAT TAC CGG TCG ATG CAA C-3′) and Cre2 (5′-GCA TTG CTG TCA CTT GGT CGT G-3′). Thermal cycle conditions were 94°C for 5 minutes and then 32 cycles of 94°C for 30 seconds and 70°C for 1 minute. The presence of a 300-bp band is indicative of the Mx1-Cre transgene.
Monitoring and isolation of hematopoietic cells
Mice were bled at 1.5, 3, and 5 months of age by nicking the dorsal tail veins with a surgical blade. Tail-vein blood samples (50-100 μL) were obtained for complete blood counts (CBCs). Samples were analyzed by using a Hemavet 850FS (DREW Scientific, Oxford, CT). Automated differential cell counts were used to monitor differentiated leukocyte populations over time. Criteria for killing mice by CO2 inhalation included a disheveled appearance, hunching, abnormal gait, and pallor. Bone marrow cells were collected by removing tibias and femurs and flushing out the marrow cavity with Iscove modified Dulbecco medium (IMDM; GIBCOBRL, Gaithersburg, MD) supplemented with 20% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT). Single cell suspensions were prepared by gently drawing the cells through a 25-gauge needle. Cell viabilities were ascertained by trypan blue exclusion.
Pathologic analysis and flow cytometry
Blood smears and cytospins were stained with Wright Giemsa (Sigma). Spleens and sternums were collected and sent for sectioning to the Mouse Pathology Shared Resource at the UCSF Comprehensive Cancer Center. CBCs were measured in peripheral blood, and manual differential counts (100-400 cells) were determined from Wright Giemsa-stained smears (blood) or cytospins (bone marrow or spleen) by using published recommendations.24 Fluorescence activated cell sorting (FACS) analysis was performed on peripheral blood, bone marrow, and splenocytes. Peripheral blood cells were incubated in red blood cell lysis buffer (0.16 M NH4Cl, 0.1 M KHCO3, 0.1 mM EDTA [ethylenediaminetetraacetic acid]) for 10 minutes at room temperature. The cells were washed twice in PBS and resuspended in PBS/0.1% bovine serum albumin (BSA), divided into aliquots, and placed in tubes; antibodies were added on ice to prevent capping. Isotype control antibodies conjugated to the fluorochromes fluorescein isothiocyanate (FITC) or phycoerythrin (PE) were used to determine background fluorescence, and a panel of lineage-specific antibodies was used, including CD3, B220, Gr-1, Mac1, c-kit, and Sca-1 (all antibodies from Pharmingen). Analysis was performed using FlowJo (Tree Star, San Carlos, CA), and data were collected using CellQuest software (Becton Dickinson, San Jose, CA).
Spectral karyotyping (SKY) analysis
Cytogenetic analysis was performed on spleen cells from mice with MPD. The initiation of short-term (24 hours) cultures, metaphase cell preparation, and spectral karyotyping were performed as described previously.25 A minimum of 10 metaphase cells were analyzed per case.
Adoptive transfer protocol
Recipient mice were lethally irradiated with a single fraction of 900 cGy by using a cesium source that delivered irradiation at 227 rad/min. Sublethal irradiation was administered at a dose of 450 cGy. Recipient mice (8 weeks old) were injected with donor cells immediately after irradiation. Mice were warmed for 5 minutes under a heat lamp to induce tail-vein dilation. Hematopoietic cells (2 × 106 per recipient) were suspended in 500 μL IMDM with 20% FCS and injected through a 28-gauge needle into the dorsal tail vein. Recipients received prophylactic oral antibiotics, consisting of polymyxin sulfate and neomycin sulfate for 2 weeks after irradiation. CBCs were measured serially between 7 and 24 weeks after transplantation.
Progenitor growth
CFU-GM colonies were grown from bone marrow cells and splenocytes. These assays were performed in methylcellulose medium (Stem Cell Technologies, Vancouver, BC, Canada) containing glutamine, penicillin/streptomycin, β-mercaptoethanol (BME), and varying doses of recombinant murine GM-CSF. Bone marrow and spleen cells were cultured at 5 × 104 and 1 × 105 cells/mL, respectively. Duplicate plates containing 1 mL medium were established and incubated at 37°C in 5% CO2 for 8 days. The colonies were scored through a binocular light microscope under 4 × magnification. To assess the effects of 13 cis retinoic acid (13cRA) on CFU-GM growth, various concentrations of the drug (10-4 M to 10-6 M) diluted in dimethyl sulfoxide (DMSO) were added to the medium immediately before plating.
Proliferation assay
The incorporation of 5-bromo-2-deoxyuridine (BrdU; Sigma) was measured in vivo in mice that were injected intraperitoneally with 1 mg BrdU/6 g body weight. The mice were killed 6 hours later, and marrow cells and splenocytes were collected and resuspended in IMDM supplemented with 20% FCS. Red cell lysis was performed as described in “Monitoring and isolation of hematopoietic cells,” and the cells were washed with PBS. Cells were incubated overnight with 1% paraformaldehyde and 0.01% Tween-20 at 4°C, washed twice with PBS, and resuspended in DNAse (Sigma) for 30 minutes at 37°C. After 2 additional washes with PBS, cells were stained with BrdU-FITC (Pharmingen) for 20 minutes. The cells were washed twice with PBS and then analyzed by flow cytometry.
Apoptosis assay
Bone marrow cells were harvested in IMDM supplemented with 20% FCS, and the red cells were lysed as described in “Proliferation assay.” The cells were resuspended in PBS with 2% FCS and 1.5 mM calcium chloride before staining with Annexin-GFP (a gift from Dr Joel Ernst, University of California, San Francisco) for 15 minutes at 4°C. The labeled cells were washed once in PBS and then analyzed by flow cytometry.
Isolation of Mac1-positive bone marrow cells
Bone marrow cells were harvested from tibias and femurs in 0.1% serum without growth factors and were labeled with an antimouse CD11b (Mac1)-PE antibody (1:50; Pharmingen) after red cell lysis. The stained cells were incubated with paramagnetic anti-PE microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) for 15 minutes at 4°C. The cells were passed through a 40-μ filter and were separated on an AutoMACS instrument using program Possel (Miltenyi Biotech). This procedure consistently yields more than 98% Mac1-positive cells.
Western blotting and Ras-GTP assay
Unfractionated bone marrow or Mac1-positive cells were incubated in 0.1% serum without growth factors for 4 hours, then stimulated with 0 or 10 ng/mL GM-CSF for 5 minutes. The cells were washed once with PBS containing 1 mM sodium orthovanadate and lysed in 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.5, 150 mM NaCl, 1% NP-40, 0.25% Na deoxycholate, 10% glycerol, 10 mM MgCl2, 25 mM NaF, 1 mM Na orthovanadate, and COMPLETE protease inhibitors (Amersham). Protein concentrations were quantitated and equalized for loading by using the Bio-Rad protein assay (Bio-Rad, Hercules, CA). Samples were boiled for 5 minutes in 1 × Laemli buffer, run on a 10% Tris (tris(hydroxymethyl)aminomethane)-HCl Criterion Precast Gel (Bio-Rad), and transferred onto a nitrocellulose membrane. The membranes were blocked for 1 hour in TBS-Tween containing 5% milk prior to overnight incubation at 4°C with antiphospho-MEK 1/2 (mitogen-activated protein kinase kinase 1 and 2; 1:500; Cell Signaling Technologies, Beverly, MA), antiphospho-Akt (1:5000; a gift from Dr David Stokoe, University of California, San Francisco), anti-MEK 1/2 (1:5000; Cell Signaling Technologies), and anti-Akt (1:1000; Cell Signaling Technologies). The blots were developed with a horseradish peroxidase-conjugated secondary antirabbit antibody (Amersham). Proteins were visualized by enhanced chemiluminescence (Amersham). Ras-GTP levels were measured as described elsewhere by precipitating GTP-bound Ras with a fusion protein that includes the Ras binding domain of Raf.26
Results
Mx1-Cre, Nf1flox/flox, and Nf1flox/flox littermates received a single injection of pI-pC (500 μg in 50 μL) at 3 to 5 days of age and were genotyped at weaning by analyzing DNA prepared from tail clips. As an additional control, some litters that were not treated with pI-pC were observed for signs of systemic illness. PCR analysis of DNA extracted from peripheral blood leukocytes at 6 weeks of age demonstrated complete excision of exons 31 and 32 in Mx1-Cre, Nf1flox/flox mice that received pI-pC (Figure 1A). Somatic inactivation of Nf1 was dependent on inheritance of the Mx1-Cre transgene (Figure 1A). Consistent with previous data, the Mx1-Cre transgene was active in other tissues, and we detected partial inactivation of Nf1 in kidney, lung, and other tissues (data not shown).
Blood leukocyte values and survival in Mx1-Cre, Nf1 flox/flox and Nf1 flox/flox mice. (A) PCR analysis of leukocyte DNA from 6-week-old pups that received a single injection of pI-pC shortly after birth. PCR amplification of the unrearranged Nf1flox allele yields a 350-bp product. A 280-bp fragment corresponding to a deletion of exon 31 (Δ31) is visible in 2 pups that inherited the Mx1-Cre transgene (+) but not in 3 pups that did not (-). Absence of the unrearranged allele in lanes 3 and 4 confirms a high efficiency of somatic recombination. (B) White blood cell counts (WBCs) in 3-month-old pI-pC-treated Mx1-Cre, Nf1flox/flox (Cre+)(n = 21) and control Nf1flox/flox littermates that did not inherit the Mx1-Cre transgene (Cre-) (n = 18). The abbreviations are LY, lymphocytes; NE, neutrophils; MO, monocytes; APC, absolute phagocyte count (neutrophils + monocytes). Leukocyte counts are expressed as ± SEM. Asterisks indicate significant differences (P < .05 by Student t test) between the Cre+ and Cre- animals. (C) A composite photomicrograph (original magnification × 400) of peripheral blood from a Cre+ mouse shows mature neutrophils (top left), intermediate forms (top right), a monocyte and a mature neutrophil (bottom left), and an intermediate form, which is likely in the monocytic lineage (bottom right). (D) Kaplan-Meier analysis demonstrates a significant reduction in survival in Cre+ (n = 59) versus Cre- (n = 72) littermates (P < .0001).
Blood leukocyte values and survival in Mx1-Cre, Nf1 flox/flox and Nf1 flox/flox mice. (A) PCR analysis of leukocyte DNA from 6-week-old pups that received a single injection of pI-pC shortly after birth. PCR amplification of the unrearranged Nf1flox allele yields a 350-bp product. A 280-bp fragment corresponding to a deletion of exon 31 (Δ31) is visible in 2 pups that inherited the Mx1-Cre transgene (+) but not in 3 pups that did not (-). Absence of the unrearranged allele in lanes 3 and 4 confirms a high efficiency of somatic recombination. (B) White blood cell counts (WBCs) in 3-month-old pI-pC-treated Mx1-Cre, Nf1flox/flox (Cre+)(n = 21) and control Nf1flox/flox littermates that did not inherit the Mx1-Cre transgene (Cre-) (n = 18). The abbreviations are LY, lymphocytes; NE, neutrophils; MO, monocytes; APC, absolute phagocyte count (neutrophils + monocytes). Leukocyte counts are expressed as ± SEM. Asterisks indicate significant differences (P < .05 by Student t test) between the Cre+ and Cre- animals. (C) A composite photomicrograph (original magnification × 400) of peripheral blood from a Cre+ mouse shows mature neutrophils (top left), intermediate forms (top right), a monocyte and a mature neutrophil (bottom left), and an intermediate form, which is likely in the monocytic lineage (bottom right). (D) Kaplan-Meier analysis demonstrates a significant reduction in survival in Cre+ (n = 59) versus Cre- (n = 72) littermates (P < .0001).
Cohorts of mice were observed for evidence of disease and by performing serial blood counts. Leukocyte counts and the numbers of differentiated myeloid and lymphoid cells were significantly elevated in the Mx1-Cre, Nf1flox/flox animals by 3 months of age (Figure 1B). Blood smears revealed increased numbers of morphologically normal lymphocytes, monocytes, and neutrophils. Animals with marked leukocytosis showed occasional intermediate myeloid forms (Figure 1C). Hemoglobin concentrations as well as red blood cell and platelet counts remained within the normal range (data not shown). Mx1-Cre, Nf1flox/flox mice developed overt signs of disease beginning between 5 and 6 months of age, which was characterized by hunching, abnormal gait, and a disheveled appearance, and 50% of the animals succumbed by 7.5 months (Figure 1D). This clinical syndrome was generally not accompanied by dramatic changes in peripheral blood counts, and transformation to acute leukemia did not occur. The development of MPD in Nf1flox/flox mice was dependent on the presence of the Mx1-Cre transgene. Interestingly, Mx1-Cre, Nf1flox/flox mice that were not injected with pI-pC occasionally developed MPD. These animals showed somatic inactivation of Nf1 in blood and bone marrow, which was likely because of endogenous interferon production in response to a subclinical infection or another stimulus (data not shown).
Pathologic analysis of sick Mx1-Cre, Nf1flox/flox mice revealed progressive splenomegaly with extensive infiltration of myeloid cells at various stages of maturation (Figure 2A-C). There was periportal invasion within the liver (Figure 2B) but not in other tissues. The bone marrow was highly cellular and comprised myeloid cells at various stages of differentiation (Figure 2C-D). FACS analysis confirmed the presence of a high percentage of myeloid cells (Mac1+ and Gr1+ cells) (Figure 2E). Increased numbers of Mac-1+, Gr-1lo cells, which are likely to represent immature monocytic cells, were identified consistently. Cytogenetic and spectral karyotype analysis of spleen samples from 6 diseased mice did not reveal clonal karyotypic abnormalities. According to guidelines published by the Hematopathology Subcommittee of the Mouse Models of Human Cancer Consortium (MMHCC),24 this disorder is classified as a myeloproliferative disease. This MPD models many features of human JMML, including increased numbers of differentiated granulocytic and monocytic cells, hypersensitivity to GM-CSF (see later in this section), and a subacute course. It is similar to the MPD that arises in lethally irradiated mice that are repopulated with homozygous Nf1-deficient fetal liver cells16,19 ; however, the course is somewhat more indolent. A complete histologic and FACS panel on the model has been posted on the MMHCC website (http://emice.nci.nih.gov/emice/mouse_models/organ_models/hema_models/hema_mouse_class/myelopro_disease/mpd).
Pathologic analysis of tissues from Mx1-Cre, Nf1 flox/flox mice. (A) Cre+ mice demonstrate progressive splenomegaly. Data are presented as the mean ± SEM. (A,C) Asterisks indicate significant differences (P < .05) between the Cre+ and Cre- animals. (B) Spleen (top) sections from Cre+ mice demonstrate expansion of the red pulp with myeloid infiltration. Liver sections (bottom) show periportal infiltration. (C) Manual differential counts of the percentage of nonerythroid cells in blood, bone marrow (BM), and spleen from Cre+ and Cre- mice. Note that the bone marrow myeloid cells show a normal pattern of differentiation and that the spleen shows a massive increase in myelopoiesis. The data are shown as the mean ± SD. (D) Cytospins of bone marrow and spleen in a Cre+ mouse with MPD show maturing neutrophilic and monocytic elements (original magnification × 200). (E) Representative FACS data from Cre- (left) and Cre+ (right) bone marrows. Cre+ mice with MPD demonstrate an increased percentage of Gr1+/Mac1+ cells (51% versus 22%). There is marked expansion in the Mac-1+/Gr-1lo subset (11.5% in Cre+ versus 2.13% in Cre- mice), which is consistent with an increase in monocytic cells. The numbers shown indicate the percentage of cells within each quadrant.
Pathologic analysis of tissues from Mx1-Cre, Nf1 flox/flox mice. (A) Cre+ mice demonstrate progressive splenomegaly. Data are presented as the mean ± SEM. (A,C) Asterisks indicate significant differences (P < .05) between the Cre+ and Cre- animals. (B) Spleen (top) sections from Cre+ mice demonstrate expansion of the red pulp with myeloid infiltration. Liver sections (bottom) show periportal infiltration. (C) Manual differential counts of the percentage of nonerythroid cells in blood, bone marrow (BM), and spleen from Cre+ and Cre- mice. Note that the bone marrow myeloid cells show a normal pattern of differentiation and that the spleen shows a massive increase in myelopoiesis. The data are shown as the mean ± SD. (D) Cytospins of bone marrow and spleen in a Cre+ mouse with MPD show maturing neutrophilic and monocytic elements (original magnification × 200). (E) Representative FACS data from Cre- (left) and Cre+ (right) bone marrows. Cre+ mice with MPD demonstrate an increased percentage of Gr1+/Mac1+ cells (51% versus 22%). There is marked expansion in the Mac-1+/Gr-1lo subset (11.5% in Cre+ versus 2.13% in Cre- mice), which is consistent with an increase in monocytic cells. The numbers shown indicate the percentage of cells within each quadrant.
A recent study in which a Krox20-Cre transgene was used to ablate Nf1 in Schwann cells found that the incidence and penetrance of benign neurofibromas was markedly increased in a heterozygous mutant background (ie, in Krox20-Cre, Nf1flox/- versus Krox20-Cre, Nf1flox/flox mice).22 On the basis of these data, we generated Mx1-Cre, Nf1flox/- mice to determine whether heterozygous inactivation of Nf1 in nonhematopoietic cells present in the bone marrow microenvironment might modulate the subacute course of the MPD. In this experiment, Mx1-Cre, Nf1flox/- pups that were injected with pI-pC at 3 to 5 days of age developed MPD with similar blood counts, disease latency, and survival as Mx1-Cre, Nf1flox/flox mice (data not shown).
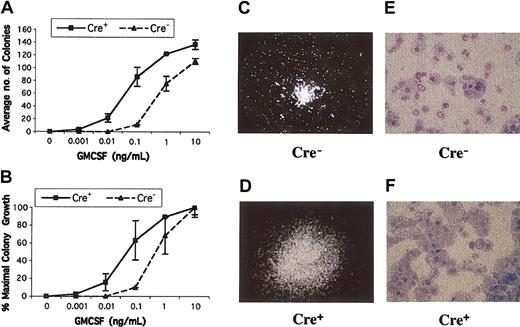
JMML bone marrows and Nf1-/- fetal liver cells form increased numbers of CFU-GM colonies in the presence of nonsaturating concentrations of recombinant murine GM-CSF.3,11,16,17 We detected elevated numbers of CFU-GM in the bone marrows of Mx1-Cre, Nf1flox/flox mice that were hypersensitive to GM-CSF (Figure 3A-B). In addition, mutant CFU-GM colonies were larger than normal and showed an abnormal spreading morphology (Figure 3C-D). Increased proliferation of myeloid progenitors from Mx1-Cre, Nf1flox/flox mice was also reflected by a 2- to 3-fold expansion in the number of cells recovered from methylcellulose cultures stimulated with 10 ng/mL recombinant murine GM-CSF. Wright-Giemsa staining revealed a higher percentage of monocyte-macrophage cells in Mx1-Cre, Nf1flox/flox cultures than in the control cultures (Figure 3E-F). Consistent with the myeloid infiltration visible in pathologic sections, the spleens of Mx1-Cre, Nf1flox/flox mice contained large numbers of CFU-GM, which formed colonies that were similar in size and morphology to those found in the bone marrow. By contrast, no CFU-GM colonies were obtained from the spleens of control mice (data not shown).
CFU-GM colony growth from Mx1-Cre, Nf1flox/flox and Cre- mice. (A-B) CFU-GM colony growth at various concentrations of GM-CSF. Bone marrow mononuclear cells were plated in duplicate in methylcellulose. Cre+ bone marrow from mice with MPD show a left shift in the GM-CSF dose-response curve when expressed in terms of total numbers of colonies (A) or the calculated percentage of maximal colony growth (B). Colony numbers in duplicate 1-mL plates are shown with ranges from a representative experiment. (C-D) CFU-GM colonies grown from Mx1-Cre, Nf1flox/flox and control mice photographed at 40 × magnification. A typical CFU-GM morphology from a normal mouse is shown in panel C at 40 × magnification. The colonies grown from Cre+ mice with MPD are larger and show abnormal spreading (D). (E-F) Cytospins of CFU-GM colonies stained with Wright-Giemsa from a wild-type mouse (E) contain approximately 70% neutrophils compared with 93% monocyte-macrophage cells in Cre+ mice. Original magnification, × 500 for panels E and F.
CFU-GM colony growth from Mx1-Cre, Nf1flox/flox and Cre- mice. (A-B) CFU-GM colony growth at various concentrations of GM-CSF. Bone marrow mononuclear cells were plated in duplicate in methylcellulose. Cre+ bone marrow from mice with MPD show a left shift in the GM-CSF dose-response curve when expressed in terms of total numbers of colonies (A) or the calculated percentage of maximal colony growth (B). Colony numbers in duplicate 1-mL plates are shown with ranges from a representative experiment. (C-D) CFU-GM colonies grown from Mx1-Cre, Nf1flox/flox and control mice photographed at 40 × magnification. A typical CFU-GM morphology from a normal mouse is shown in panel C at 40 × magnification. The colonies grown from Cre+ mice with MPD are larger and show abnormal spreading (D). (E-F) Cytospins of CFU-GM colonies stained with Wright-Giemsa from a wild-type mouse (E) contain approximately 70% neutrophils compared with 93% monocyte-macrophage cells in Cre+ mice. Original magnification, × 500 for panels E and F.
Retinoids partially correct the abnormal pattern of CFU-GM colony growth that is a hallmark of JMML,1,27 and 13cRA induces transient clinical and hematologic improvement in some patients.28 We, therefore, compared the effects of 13cRA on CFU-GM growth from Mx1-Cre, Nf1flox/flox and control bone marrows. As shown in Figure 3, Mx1-Cre, Nf1flox/flox marrows contained elevated numbers of CFU-GM, which were larger than wild-type colonies. Addition of 10-6 to 10-4 M 13cRA to methylcellulose cultures stimulated with 10 ng/mL GM-CSF induced proliferation of background cells as well as a dose-dependent reduction in the size of CFU-GM colonies. These effects were not selective but were observed in both mutant and wild-type cultures. Furthermore, 13cRA treatment was not associated with a reduction in the abnormally high percentage of monocyte-macrophage cells in CFU-GM grown from Mx1-Cre, Nf1flox/flox mice (data not shown).
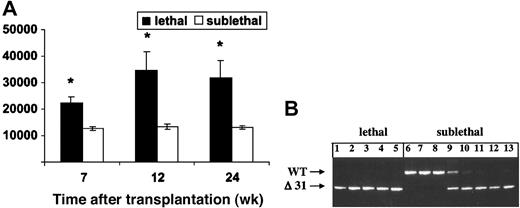
Bone marrow mononuclear cells from Mx1-Cre, Nf1flox/flox mice that had been treated with pI-pC were injected into lethally (n = 10) or sublethally (n = 10) irradiated recipients to ascertain whether the MPD could be transferred to secondary hosts. Each lethally irradiated recipient was repopulated with mutant cells and developed MPD (Figure 4A). By contrast, the leukocyte counts of mice that received a sublethal dose of irradiation remained normal over 6 months of observation (Figure 4A). Analysis of DNA extracted from blood leukocytes 2 to 4 months after transplantation revealed both host and donor-derived cells in all recipients (data not shown). Nf1-deficient cells made a sustained contribution to hematopoiesis in many animals, including some in which the wild-type band disappeared from the blood after 6 months (Figure 4B). Interestingly, pathologic evaluation of sublethally irradiated mice that were killed 8 months after injection of Nf1-/- cells revealed normal spleen size and architecture and no splenic CFU-GM despite a substantial contribution of mutant cells to the peripheral blood (data not shown).
Adoptive transfer of marrow cells from Mx1-Cre, Nf1 flox/flox mice with MPD. (A) WBCs in recipient mice that received a lethal or sublethal dose of radiation over time. Cells from 2 Cre+ mice were injected into equal numbers of lethally and sublethally irradiated mice. Data represent the mean ± SEM. Asterisks indicate significant differences between recipients that received lethal versus sublethal radiation (P < .05). (B) PCR analysis of blood leukocytes from lethally irradiated recipients demonstrates reconstitution with mutant (Δ31) cells with absence of a signal from the wild-type host (lanes 1-5). By contrast, some sublethally irradiated recipients that were analyzed 6 months after adoptive transfer showed absence of mutant Δ31 cells (lanes 6-8), some showed repopulation with donor cells (lanes 11-13), and others demonstrated the presence of both wild-type and mutant cells (lanes 9,10). None of these sublethally irradiated recipients had developed evidence of MPD by 8 months after adoptive transfer.
Adoptive transfer of marrow cells from Mx1-Cre, Nf1 flox/flox mice with MPD. (A) WBCs in recipient mice that received a lethal or sublethal dose of radiation over time. Cells from 2 Cre+ mice were injected into equal numbers of lethally and sublethally irradiated mice. Data represent the mean ± SEM. Asterisks indicate significant differences between recipients that received lethal versus sublethal radiation (P < .05). (B) PCR analysis of blood leukocytes from lethally irradiated recipients demonstrates reconstitution with mutant (Δ31) cells with absence of a signal from the wild-type host (lanes 1-5). By contrast, some sublethally irradiated recipients that were analyzed 6 months after adoptive transfer showed absence of mutant Δ31 cells (lanes 6-8), some showed repopulation with donor cells (lanes 11-13), and others demonstrated the presence of both wild-type and mutant cells (lanes 9,10). None of these sublethally irradiated recipients had developed evidence of MPD by 8 months after adoptive transfer.
We measured BrdU incorporation and performed annexin V staining to ascertain the effects of Nf1 inactivation on the proliferation and survival of primary hematopoietic cells. Mx1-Cre, Nf1flox/flox mice with MPD and controls were killed 6 hours after a single injection of BrdU, and the percentages of bone marrow and spleen cells that had incorporated the label were determined by flow cytometry. Whereas the fraction of BrdU-positive cells was similar in the bone marrows of diseased and control animals, proliferation was substantially greater within the spleens of Mx1-Cre, Nf1flox/flox mice (Figure 5A). Because the percentages of erythroid and myeloid cells in bone marrow and spleen differ markedly between Mx1-Cre, Nf1flox/flox and control mice, we also assessed BrdU incorporation in Mac1+ cells. Interestingly, proliferation was comparable in bone marrow-derived cells; however, a higher percentage of splenic Mac1+ cells was labeled in the Mx1-Cre, Nf1flox/flox mice (Figure 5B). Apoptosis was assessed by performing flow cytometry on bone marrow cells that had been labeled with an annexin V-green fluorescent protein (GFP). The percentage of freshly isolated cells stained by annexin-V was similar in Mx1-Cre, Nf1flox/flox and normal mice (data not shown); however, the mutant cells displayed greatly enhanced survival after 24 hours in culture without exogenous growth factors (Figure 5C).
Proliferation and apoptosis in Mx1-Cre, Nf1 flox/flox and Cre- mice. (A) BrdU incorporation in vivo by Cre+ and Cre- bone marrow cells and splenocytes. Increased proliferation is seen in the spleens of Cre+ mice (14.6% versus 4.5% labeled cells). (B) BrdU labeling of Mac1+ cells from Cre+ and Cre- mice. A higher percentage of splenic Mac1+ cells were labeled (20% versus 6.6%). (C) Annexin V staining of bone marrow mononuclear cells from Cre- and Cre+ mice showing greatly reduced apoptosis of Cre+ cells after 24 hours in culture without exogenous growth factors (27.9% versus 74.5% in the controls). The histograms present representative data from an analysis of 3 mice of each genotype. The numbers shown in each panel indicate the percentage of positive cells.
Proliferation and apoptosis in Mx1-Cre, Nf1 flox/flox and Cre- mice. (A) BrdU incorporation in vivo by Cre+ and Cre- bone marrow cells and splenocytes. Increased proliferation is seen in the spleens of Cre+ mice (14.6% versus 4.5% labeled cells). (B) BrdU labeling of Mac1+ cells from Cre+ and Cre- mice. A higher percentage of splenic Mac1+ cells were labeled (20% versus 6.6%). (C) Annexin V staining of bone marrow mononuclear cells from Cre- and Cre+ mice showing greatly reduced apoptosis of Cre+ cells after 24 hours in culture without exogenous growth factors (27.9% versus 74.5% in the controls). The histograms present representative data from an analysis of 3 mice of each genotype. The numbers shown in each panel indicate the percentage of positive cells.
We compared the percentage of Ras in the active GTP-bound conformation and assayed activation of the Ras effectors Akt and MEK in bone marrow mononuclear cells isolated from Mx1-Cre, Nf1flox/flox and normal mice. Unstimulated mutant cells showed a modest increase in baseline Ras-GTP levels (Figure 6A,C), but phosphorylation of the downstream effectors MEK and Akt were similar in unfractionated mutant and control bone marrows (Figure 6B-C). Exposure to GM-CSF induced robust Ras-GTP, MEK, and Akt activation from baseline levels that was equivalent in both genotypes (Figure 6B-C). Because Nf1-/- and wild-type bone marrows showed marked differences with respect to the relative proportions of different cell types, we isolated Mac1-positive cells and compared MEK activation in this myeloid subpopulation. In these experiments, Nf1-/- cells showed sustained activation of MEK after exposure to GM-CSF (Figure 6D).
Signal transduction in bone marrow cells from Mx1-Cre, Nf1 flox/flox and Cre- mice. (A) Bone marrow cells from mutant and control mice showing Ras-GTP levels in unstimulated cells and 5 minutes after GM-CSF. (B) Bone marrow cells from mutant and control mice showing basal and growth factor-induced levels of phosphorylated (p)-Akt and p-MEK in response to GM-CSF (at 5 minutes). (C) Ras-GTP, p-MEK, and p-Akt from panels A and B were quantified by densitometry. (D) Mac1-positive bone marrow cells from mutant and control mice showing basal and growth factor-induced activation of p-MEK in response to GM-CSF. The time course is shown from 5 to 60 minutes. Relative p-MEK activity is shown in the right panel. Levels in mutant and control cells were normalized for background and loading, with the unstimulated sample assigned arbitrary units of 100.
Signal transduction in bone marrow cells from Mx1-Cre, Nf1 flox/flox and Cre- mice. (A) Bone marrow cells from mutant and control mice showing Ras-GTP levels in unstimulated cells and 5 minutes after GM-CSF. (B) Bone marrow cells from mutant and control mice showing basal and growth factor-induced levels of phosphorylated (p)-Akt and p-MEK in response to GM-CSF (at 5 minutes). (C) Ras-GTP, p-MEK, and p-Akt from panels A and B were quantified by densitometry. (D) Mac1-positive bone marrow cells from mutant and control mice showing basal and growth factor-induced activation of p-MEK in response to GM-CSF. The time course is shown from 5 to 60 minutes. Relative p-MEK activity is shown in the right panel. Levels in mutant and control cells were normalized for background and loading, with the unstimulated sample assigned arbitrary units of 100.
Discussion
We have developed a robust and tractable model of MPD by harnessing the Mx1-Cre transgene to induce somatic inactivation of the Nf1 tumor suppressor and have used these Mx1-Cre, Nf1flox/flox mice to investigate how loss of Nf1 perturbs the in vivo growth of primary hematopoietic cells. This work extends previous studies of cultured Nf1-deficient fetal liver cells and of recipient mice repopulated with these cells.11,16-19
We selected the Mx1-Cre strain on the basis of data from other laboratories that imply that this Cre recombinase is active in both hematopoietic stem cells and in differentiated progeny.29-31 By inducing biallelic excision of a targeted locus, we could readily monitor the relative levels of recombined and unrearranged Nf1 in blood and bone marrow. We found that a single injection of pI-pC resulted in highly efficient Nf1 inactivation in all lineages. These results and our data showing that bone marrow from Mx1-Cre, Nf1flox/flox mice mediate long-term reconstitution in lethally irradiated recipients provide strong evidence that Nf1 is inactivated in the stem-cell compartment. Furthermore, although the ultimate contribution of cells that have undergone somatic recombination to hematopoiesis will be influenced by both the structure of the loxP-targeted allele and by how rearranging the locus modulates cell survival and proliferation, our data underscore the value of the Mx1-Cre strain for modifying conditional mutant alleles in hematopoietic cells. Importantly, although we detected significant levels of somatic Nf1 inactivation in some nonhematopoietic tissues, this was not associated with any overt phenotypes in mice observed for 9 to 12 months. However, this lack of specificity could be problematic for analyzing other conditional mutations.
Somatic inactivation of Nf1 results in progressive splenomegaly with a shift in hematopoiesis from the marrow to the spleen, and we identified proliferating myeloid cells within the spleens of Mx1-Cre, Nf1flox/flox mice. Splenomegaly is a prominent feature of JMML, and many centers perform splenectomies before transplantation. Although the benefits of this procedure are unclear, our data suggest that the spleen is an important site of disease that contributes to the evolution of the murine MPD. We also found that bone marrow cells from Mx1-Cre, Nf1flox/flox mice are resistant to apoptosis, which is consistent with data from Myb-transformed myeloid cell lines.26
Previous studies of mice repopulated with Nf1-/- cells have emphasized the effects of Nf1 inactivation on stem cells and myeloid lineage progenitors.16,17,19 However, limited attention has been paid to lymphopoiesis. In addition to uniformly developing MPD, Mx1-Cre, Nf1flox/flox mice demonstrate lymphocytosis with increased numbers of differentiated T and B cells. These data are consistent with competitive repopulation studies, which revealed that loss of Nf1 is associated with a proliferative advantage in both myeloid and lymphoid compartments.18 The risk of lymphoid malignancies has been examined in large population-based studies of pediatric cancer registries in Japan and the United Kingdom. Whereas one group identified no significant increase,32 the other report found a 5- to 10-fold higher incidence of acute lymphoblastic leukemia and non-Hodgkin lymphoma in children with NF1.6 A child with NF1 has been described who presented with JMML and subsequently developed a T-cell lymphoma, both of which showed loss of the normal NF1 allele.33 Ingram et al34 recently reported greater thymic cellularity and a modest increase in Ras-GTP levels in RAG2-deficient mice that received transplants with Nf1-/- fetal liver cells. Paradoxically, Nf1-/- T cells showed impaired proliferation in response to T-cell mitogens. Mx1-Cre, Nf1flox/flox mice provide a model that can be used to dissect the functional consequences of ablating Nf1 in lymphoid cells.
Consistent with previous studies of primary fetal livers and of irradiated recipients reconstituted with these cells,11,16,17 we found that Mx1-Cre, Nf1flox/flox bone marrow cells model the hypersensitive pattern of CFU-GM colony growth that is a hallmark of JMML. These data establish a link between Nf1 inactivation and this distinctive cellular phenotype. On the basis of this precedent, we exposed Mx1-Cre, Nf1flox/flox bone marrow cells to retinoids, but we did not observe selective effects on the growth of mutant CFU-GM colonies. These data imply that the therapeutic effects of 13cRA in JMML are not mediated though the GM-CSF/Ras/neurofibromin pathway.
Our adoptive transfer data are consistent with a previous report in which bone marrow from recipient mice repopulated with Nf1-/- fetal liver cells induced MPD in secondary hosts that received a myeloablative dose of radiation.19 In addition, serial studies of sublethally irradiated mice demonstrated engraftment of mutant cells in most of the recipients without overt evidence of MPD after 8 months of observation. Furthermore, we did not detect splenomegaly or myeloid infiltration even in mice in which 100% of the peripheral blood leukocytes were derived from mutant cells. These data are remarkably consistent with the results of competitive repopulation experiments in which Nf1-/- fetal liver cells out-competed wild-type cells in the stem-cell compartment and in the lymphoid and myeloid lineages but failed to induce MPD in the absence of a high degree of chimerism.18 In another study in which heterozygous Nf1 inactivation cooperated with exposure to cyclophosphamide in leukemogenesis, some Nf1+/- mice showed loss of the wild-type allele without evidence of MPD when the experiment was terminated.20 Together with these data, our results in sublethally irradiated recipients suggest that the presence of wild-type bone marrow cells can retard the development of MPD in vivo. These observations also support a model in which loss of Nf1 in a susceptible bone marrow precursor confers a subtle growth advantage that progresses to Nf1-deficient hematopoiesis, followed by splenic invasion by myeloid precursors and, finally, by overt MPD. An unsettled question involves whether the MPD induced by Nf1 inactivation is caused by progressive overgrowth of a stable population of mutant cells or involves clonal selection of a subpopulation that has acquired one or more cooperating mutations. Although the absence of acquired cytogenetic aberrations in the spleens of diseased mice is consistent with the former model, additional studies are required to resolve this issue.
We and others have detected hyperactive Ras signaling in JMML bone marrows,11 in cell lines from Nf1 mutant mice,16,26 in c-kit-positive bone marrow cells isolated from the recipients of Nf1-/- fetal liver cells,17 and in heterozygous Nf1 mutant mast cells.35 We were, therefore, surprised that bone marrow mononuclear cells from Mx1-Cre, Nf1flox/flox mice demonstrated a small increase in the percentage of Ras-GTP in unstimulated cells but similar levels of phosphorylated Akt and MEK compared with control cells. However, bone marrow-derived Mac1-positive cells from Mx1-Cre, Nf1flox/flox mice that were stimulated with GM-CSF showed sustained phosphorylation of MEK. Similarly, prolonged activation of extracellular-regulated kinase (ERK) was recently reported in Nf1-deficient mouse embryonic fibroblasts in response to 1% serum.36 Our data support a mechanism whereby Nf1 inactivation deregulates Ras signaling in a subset of hematopoietic cells, which may be difficult to detect in whole bone marrow. The CFU-GM fraction comprises approximately 0.1% of the nucleated cells in Mx1-Cre, Nf1flox/flox marrow, and we speculate that these cells are especially sensitive to loss of Nf1. Measuring the activation status of signaling cascades in rare subsets of primary cells is a challenging problem that will likely require adapting new methodologies such as the flow cytometry-based approach recently described by Perez and Nolan.37 Our data also raise the possibility that some types of hematopoietic cells compensate for loss of Nf1 by attenuating signaling networks, which may further complicate ascertaining the biochemical consequences of leukemia-associated mutations in vivo.
In addition to facilitating studies that directly address the role of Nf1 in signal transduction and myeloid growth control, Mx1-Cre, Nf1flox/flox mice are a tractable experimental system for identifying molecular events that contribute to acute myeloid leukemia (AML). The classic studies of Land et al38 demonstrated the principle of oncogene cooperativity in cellular transformation. Kelly et al39 have applied this concept to AML and proposed that transcription factor fusion proteins cooperate with mutations that constitutively activate growth-promoting signal transduction pathways in leukemogenesis. Indeed, data from mice generally support the idea that neither type of leukemia-associated genetic lesion is sufficient to induce AML. For example, transgenic or knock-in models of transcription factor fusions such as AML1-ETO, PML-RARA, and MLL-AF9 either do not spontaneously develop AML or show incomplete penetrance and prolonged latency.29,40,41 By contrast, although inactivating Nf1 or expressing either BCR-ABL or a mutant FLT3 allele induces MPD,16,39,42,43 additional events are required for AML. We envision 2 general strategies for harnessing Mx1-Cre, Nf1flox/flox mice to discover genes that cooperate with Nf1 inactivation in leukemogenesis. The first strategy involves either performing genetic crosses with transgenic or knock-in strains that express fusion oncoproteins such as PML-RARA, AML1-ETO, or MLL-AF9, or transducing Nf1-deficient bone marrow with retroviruses that encode these or other leukemia-associated fusions followed by adoptive transfer into irradiated recipients. The second approach is to harness retroviral insertional mutagenesis to identify novel genes that cooperate with loss of Nf1 in leukemogenesis.44,45 In an important “proof of principle” experiment, Largaespada et al16 found that Nf1+/- mice that were infected with the BXH-2 retrovirus developed AML with increased penetrance and reduced latency compared with wild-type littermates. These investigators identified inactivation of the normal Nf1 allele in approximately 85% of BXH-2/Nf1+/- AMLs. Because both Nf1 alleles are already mutated in Mx1-Cre, Nf1flox/flox animals that have been treated with pI-pC, this strain is theoretically superior to Nf1+/- mice for performing forward genetic screens. The predictable onset of MPD and the prolonged survival of Mx1-Cre, Nf1flox/flox mice may prove advantageous for uncovering genes that cooperate in the development of AML.
Finally, Mx1-Cre, Nf1flox/flox mice and cells from these animals can be used to test molecularly targeted agents with pharmacodynamic monitoring of relevant biochemical end points in primary target cells. Potential therapeutic strategies for treating JMML include developing GM-CSF receptor antagonists (reviewed in Iversen et al46,47 and Frankel et al48 ), inhibitors of Ras processing enzymes (reviewed in Le and Shannon49 ), and agents that interfere with the activation of downstream effectors such as MEK.50 Given the important role of hyperactive Ras in myeloid malignancies (reviewed in Lee and Shannon49 and Sawyers and Denny51 ) and other cancers (reviewed in Bos52 and Hingorani and Tuveson53 ), studies in this tractable disease model might identify agents that have therapeutic potential that extend beyond hematopoietic malignancies.
Prepublished online as Blood First Edition Paper, February 24, 2004; DOI 10.1182/blood-2003-08-2650.
Supported by the U.S. Army Neurofibromatosis Research Program (Project DAMD 17-02-1-0638), the National Institutes of Health (grants CA84221and CA72614), the Jeffrey and Karen Peterson Family Foundation, and the Frank A. Campini Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Bhumi Patel and Elizabeth Davis for assistance in spectral karyotyping analysis, Sara Jew-Lim for assistance in genotyping, Jacob Rozmus for assistance with the Ras-GTP assay, Andrew Kim for assisting with adoptive transfer experiments, and Philip Chan for preparing pathologic specimens and downloading images to the MMHCC website.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal