Abstract
Inflammatory processes, aside from cholesterol, play a central role in atherogenesis. Human C-reactive protein (huCRP) signals systemic inflammation and independently predicts future cardiovascular risk. Cholesterol-lowering statins reduce atherosclerosis and plasma huCRP levels. Evidence is sought for a direct anti-inflammatory statin effect in vivo, independent of effects on plasma cholesterol and atherogenesis. The effect of atorvastatin and simvastatin on huCRP expression was studied in nonatherosclerotic huCRP transgenic mice and compared with another class of hypolipidemic drugs, peroxisome proliferator-activated receptor-alpha (PPARα) activators, notably fenofibrate and Wy14643. Like statins, PPARα activators combine antiatherosclerotic properties with huCRP-lowering effects. Dietary treatment with statins or PPARα activators decreased basal and interleukin-1β (IL-1β)-induced plasma huCRP levels independently of cholesterol lowering. These direct anti-inflammatory in vivo effects occurred at the transcriptional level and could be confirmed in cultured human liver slices and in human hepatoma cells transiently transfected with a huCRP promoter-driven luciferase reporter. A molecular rationale for the suppression of IL-1-induced huCRP transcription is provided by showing that statins and PPARα activators up-regulate IκBα protein expression. This results in a reduced nuclear translocation of p50-nuclear factor κ B (NFκB) and thereby decreased amounts of nuclear p50-NFκB∼CCAAT/enhancer binding protein beta (C/EBPβ) complexes, which determine the huCRP transcription rate. Our results provide conclusive evidence for a direct suppressive effect of statins and PPARα activators on huCRP expression independent of cholesterol lowering and atherogenesis. (Blood. 2004;103:4188-4194)
Introduction
It is now apparent that chronic inflammatory processes involving p50-nuclear factor κ B (NFκB) are among the dominant factors, aside from cholesterol, that modulate atherosclerosis.1-4 Clinical studies demonstrate that human C-reactive protein (huCRP), a liver-derived, p50-NFκB-regulated inflammation marker, independently predicts future cardiovascular events (summarized in Libby et al1 and Ridker5 ). Recent observations indicate that huCRP may also be directly involved in atherogenesis.6-10
Statins are cholesterol-lowering agents that reduce atherogenesis and concomitantly the inflammatory state as reflected by the decreased plasma levels of huCRP.1,11,12 The mechanism by which statins lower huCRP levels is still unknown. Since the atheromatous material itself also is a potent initiator of systemic inflammatory processes,2,13 the question arises whether the huCRP-lowering effect of statins is a primary effect or a secondary effect; that is, a consequence of the reduced atherogenesis. Because this issue is difficult to untangle in humans and CRP is not an acute-phase protein in mice, huCRP transgenic (huCRPtg) mice have been used to address this question. HuCRPtg mice carry the entire huCRP gene, including the coding region and 17 kb of the 5′ flanking promoter region, and the expression of huCRP in these mice resembles its regulation in humans.14,15 This enabled us to test whether statins, notably atorvastatin and simvastatin, exert a direct suppressive effect on huCRP expression under basal and inflammatory (interleukin-1 [IL-1]-induced) conditions, independent of lipid-lowering and/or the atherogenic process. We have compared the effects of statins with another class of hypolipidemic drugs, notably fenofibrate and the specific peroxisome proliferator-activated receptor-alpha (PPARα) activator Wy14643, which also combine antiatherosclerotic properties with anti-inflammatory effects, including huCRP-lowering in hyperlipidemic patients.16-19 We previously reported that fenofibrate, Wy 14643, and other activators of PPARα directly suppress huCRP expression in primary human hepatocytes.20
In this study, we demonstrate that statins and PPARα activators reduce basal and IL-1β-induced huCRP expression in vivo in huCRPtg mice independently of cholesterol lowering. A suppression of basal and IL-1α-induced huCRP gene expression was also observed in ex vivo-cultured human liver slices and in human HuH7 hepatoma cells using statins and PPARα activators. In previous studies we showed that huCRP gene transcription increases with increasing amounts of nuclear p50-NFκB∼CCAAT/enhancer binding protein beta (C/EBPβ) complexes.20 Here, evidence is provided that statins, similar to PPARα activators, suppress IL-1-induced huCRP expression by reducing p50-NFκB translocation into the nucleus via up-regulation of IκBα resulting in a diminished amount of nuclear p50-NFκB∼C/EBPβ complexes which are rate-limiting for huCRP transcription.
Materials and methods
Animals
huCRPtg mice14,15 were characterized by polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA)21 for huCRP expression. Experiments were approved by the Institutional Animal Care and Use Committee of TNO and were in compliance with European Community specifications regarding the use of laboratory animals. Mice had free access to water and food. Food intake and body weight were monitored throughout the study period.
Animal experiments
Male huCRPtg mice were fed either standard mouse chow or one containing either 0.01% (wt/wt) or 0.1% (wt/wt) atorvastatin (Lipitor; Pfizer, Capelle a/d Ijssel, The Netherlands), 0.1% (wt/wt) simvastatin (Zocor; MSD, Haarlem, The Netherlands), 0.03% (wt/wt) Wy 14643 (Chemsyn, Lenexa, KS), or 0.1% (wt/wt) fenofibrate (Sigma-Aldrich Chemicals, Zwijndrecht, The Netherlands). Murine and human recombinant IL-1α/-1β were purchased from Sanvertech (Heerhugowaard, The Netherlands) and used for in vivo and in vitro experiments, respectively. Comparable huCRP-inducing effects were obtained for both isoforms. For the in vivo experiments, IL-1β was injected intraperitoneally at a low (83 000 U/mouse) or a high (250 000 U/mouse) dose. HuCRP and total plasma cholesterol concentrations were determined in tail blood plasma samples by ELISA21 and kit no. 1 489 437 (Roche Diagnostics, Almere, The Netherlands), respectively. Livers of animals killed 10 hours after IL-1β treatment were used to prepare mRNA22 and cDNA (kit no. A3500; Promega, Leiden, The Netherlands). Real time (RT)-PCR analysis was performed using RT-PCR mastermix (Eurogentec, Seraing, Belgium) and the ABI7700 RT-PCR system (PerkinElmer Biosystems, Nieuwekerk a/d Ijssel, The Netherlands), following the guidelines of the manufacturer and using cyclophilin (PerkinElmer Biosystems) as a reference.
Experiments with human hepatocytes
Experiments with human liver were approved by the institutional committees of the Leiden University Medical Center and TNO-Prevention and Health. Human liver slices and primary human hepatocytes were prepared and cultured as reported.20 Briefly, liver slices and primary cells were pretreated for 16 hours with 3 μM simvastatin (Merck, Amsterdam, The Netherlands), 250 μM Wy 14643 (Chemsyn, Lenexa, KS), or vehicle (dimethyl sulfoxide [DMSO]), and subsequently stimulated with 25 ng/mL IL-1α for an additional 24 hours in the continuous presence of simvastatin, Wy 14643, or vehicle. In vitro experiments evaluating PPARα activation have been performed with Wy 14643 since fenofibrate is not applicable in tissue culture because it requires in vivo conversion into fenofibric acid. HuCRP was determined in the supernatant of the treated cells by ELISA.21
Transfection experiments
Human HuH7 hepatoma cells were cultured as specified.20 Reporter gene activity was determined using the dual-luciferase reporter assay system (Promega, Leiden, The Netherlands) essentially as described.20 A renilla luciferase construct was cotransfected to correct for differences in transfection efficiency. Briefly, cells were preincubated with simvastatin (1 μM, 3 μM, 10 μM), atorvastatin (1 μM, 3 μM, 10 μM), or vehicle for 24 hours as established previously,23 or with Wy 14643 (10 μM, 30 μM, 100 μM) for 5 hours.20 Then, 100 ng of a luciferase reporter plasmid carrying a 300-bp fragment of the huCRP promoter (phuCRP-luc)20 or 3 consecutive NFκB binding sites (p3xNFκB-luc)23 were transiently transfected into 1.2 × 105 HuH7 cells using the FUGENE6 transfection reagent (Roche Diognostics). Subsequently, cells were stimulated with IL-1α (5 ng/mL) and harvested after an additional 18 hours in the presence of the above-indicated concentrations of simvastatin, atorvastatin, Wy 14643, or vehicle.
Western blotting and coimmunoprecipitation
Cellular and nuclear extracts were prepared in the presence of proteinase inhibitors (PIs; Roche Diagnostics) as reported.20 Western blotting and coimmunoprecipitation experiments were performed following detailed protocols20 using the antibodies anti-p50-NFκB (sc-1190), anti-IκBα (sc-371), anti-C/EBP-β (sc-150), and the control antibodies anti-MMP8 (sc-8848), anti-β-actin (sc-1615), and anti-histone H1 (sc-8616). All primary and secondary antibodies were obtained from Santa Cruz Biotechnology (Heerhugowaard, The Netherlands). Immunoblots were visualized using the Super Signal West Dura Extended Duration Substrate (Pierce, St Augustin, Germany) and the luminescent image workstation (Roche Diagnostics).
Statistical methods
Significant differences were established by an analysis of variance (ANOVA) test (SPSS version 11.5 for Windows; SPSS, Chicago, IL) followed by a least significant difference (LSD) post hoc analysis. The level of statistical significance was set at a P value of less than .05. Data are presented as the mean plus or minus the standard error of the mean (SEM) unless stated otherwise.
Results
Effect of statins and PPARα activators on plasma cholesterol and basal huCRP levels
HuCRPtg mice were fed a chow diet containing either vehicle (control) or a low dose (LD) of atorvastatin (0.05% [wt/wt]) for 4 weeks. Plasma was collected weekly for cholesterol and huCRP measurements. As shown in Figure 1A, LD atorvastatin treatment resulted in a significant 21% (P < .05) decrease in cholesterol levels after 2 weeks. Plasma cholesterol levels did not further decrease after prolonged treatment. After 4 weeks, cholesterol levels of the LD group were reduced by 16% (P < .05) as compared with controls. Plasma huCRP levels in the LD group and the control group remained constant and did not significantly change over the 4-week treatment period (Figure 1C, left panel).
Effect of atorvastatin, simvastatin, fenofibrate, and Wy 14643 on plasma cholesterol and basal huCRP expression. (A) Groups of huCRPtg mice (n ≥ 5) received a chow diet containing a low dose (LD; 0.01% [wt/wt]) of atorvastatin (At) or vehicle control (Con) for 4 weeks. Plasma cholesterol was determined in tail blood and data are expressed as means ± standard deviation (SD). (B) As in panel A but mice received a high dose (HD; 0.1% [wt/wt]) of atorvastatin. (C) Plasma huCRP levels of mice were determined in tail blood at t = 0 and t = 4 weeks and data are expressed as means ± SEM. (D) As in panel A but mice received a dose of 0.1% (wt/wt) simvastatin. (E) As in panel C. (F) As in panel A but mice received 0.1% (wt/wt) fenofibrate (FF) or 0.03% (wt/wt) Wy 14643. (G) As in panel C. Statistical significant difference compared with control animals is indicated by *P < .05. ns = not significant.
Effect of atorvastatin, simvastatin, fenofibrate, and Wy 14643 on plasma cholesterol and basal huCRP expression. (A) Groups of huCRPtg mice (n ≥ 5) received a chow diet containing a low dose (LD; 0.01% [wt/wt]) of atorvastatin (At) or vehicle control (Con) for 4 weeks. Plasma cholesterol was determined in tail blood and data are expressed as means ± standard deviation (SD). (B) As in panel A but mice received a high dose (HD; 0.1% [wt/wt]) of atorvastatin. (C) Plasma huCRP levels of mice were determined in tail blood at t = 0 and t = 4 weeks and data are expressed as means ± SEM. (D) As in panel A but mice received a dose of 0.1% (wt/wt) simvastatin. (E) As in panel C. (F) As in panel A but mice received 0.1% (wt/wt) fenofibrate (FF) or 0.03% (wt/wt) Wy 14643. (G) As in panel C. Statistical significant difference compared with control animals is indicated by *P < .05. ns = not significant.
In a second experiment, the mice were treated with a higher dose (HD) of atorvastatin (0.1% [wt/wt]). As compared with LD, HD atorvastatin treatment more rapidly lowered plasma cholesterol levels, reaching maximal reduction (27%; P < .05) after 1 week (Figure 1B). The reduction in cholesterol levels in the HD group at the end of the treatment period (22%; P < .05) was comparable to that in the LD group. In contrast to LD, however, HD atorvastatin treatment very strongly reduced huCRP levels, resulting in a 93% (P < .05) decrease after 4 weeks (Figure 1C, right panel). These results indicate that the anti-inflammatory potential of atorvastatin is demonstrable at concentrations that are higher than required for cholesterol lowering. For comparison, mice were also treated with another statin, simvastatin. Treatment with 0.1% (wt/wt) simvastatin resulted in a comparable cholesterol reduction (on average 21%; P < .05) as observed with atorvastatin, but a less pronounced reduction (75%; P < .05) of plasma huCRP (Figure 1D-E). This suggests that atorvastatin has a stronger anti-inflammatory potential than simvastatin when compared at the same dose.
In another series of experiments, huCRPtg mice received a chow diet containing either 0.1% (wt/wt) fenofibrate, 0.03% (wt/wt) Wy 14643, or vehicle. Four weeks of fenofibrate treatment resulted in a 66% (P < .05) reduction of plasma huCRP levels in the absence of an effect on plasma cholesterol (Figure 1F-G). An even stronger huCRP-lowering effect (99%; P < .05) was observed with the specificPPARα activator Wy 14643; Wy 14643 did not affect plasma cholesterol levels (Figure 1F-G). In all, the data demonstrate that statins and PPARα activators reduce basal huCRP expression in vivo independently of lowering plasma cholesterol.
Effect of statins and PPARα activators on IL-1β-induced huCRP expression
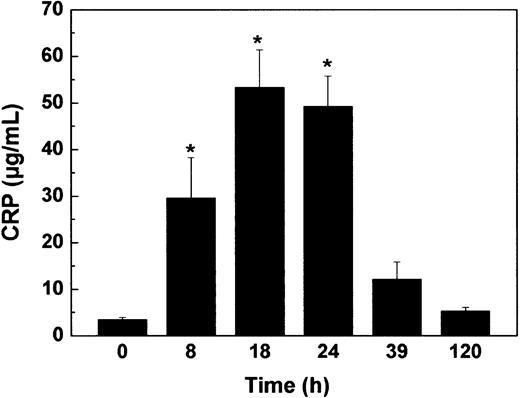
To test whether statins and PPARα activators can also suppress the in vivo induction of huCRP expression by IL-1 (ie, an acute inflammatory stimulation), we first determined the time profile of the effect of a single intraperitoneal injection of IL-1β. As shown in Figure 2, an intraperitoneal injection of 2.5 × 105 U IL-1β/mouse resulted in a time-dependent increase in plasma huCRP levels. A maximally 15-fold (P < .05) induction was reached between 18 hours and 24 hours. Plasma huCRP levels then gradually declined reaching near control values after 120 hours.
Induction of huCRP expression in huCRPtg mice. HuCRPtg mice (n = 17) received an intraperitoneal injection of 2.5 × 105 U IL-1β/mouse. Plasma huCRPs were determined in tail blood samples collected at the indicated time points. Data represent means ± SEM. *P < .05 compared with t = 0.
Induction of huCRP expression in huCRPtg mice. HuCRPtg mice (n = 17) received an intraperitoneal injection of 2.5 × 105 U IL-1β/mouse. Plasma huCRPs were determined in tail blood samples collected at the indicated time points. Data represent means ± SEM. *P < .05 compared with t = 0.
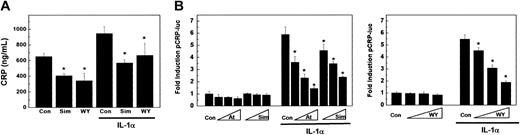
Subsequently, the effect of atorvastatin and simvastatin on IL-1β-induced huCRP expression was investigated. HuCRPtg mice were treated for 3 weeks with LD or HD atorvastatin or vehicle. Then mice received an intraperitoneal injection of either a low dose (8.3 × 104 U/mouse) or a high dose (2.5 × 105 U/mouse) of IL-1β. Plasma huCRP levels were determined 18 hours after stimulation with IL-1β. In control animals, injection of a low or a high dose of IL-1β resulted in a 5.4-fold (P < .05) and a 25.0-fold (P < .05) increase in huCRP, respectively (Figure 3A). LD atorvastatin treatment did not significantly affect basal huCRP levels or low dose or high dose IL-1β-induced huCRP expression (Figure 3A). However, HD atorvastatin treatment significantly reduced basal huCRP expression levels by 93% (P < .05), and suppressed low dose and high dose IL-1β-induced huCRP expression by 66% (P < .05) and 86% (P < .05), respectively (Figure 3A). These data indicate that atorvastatin can also suppress IL-1β-induced huCRP levels and reiterate that these suppressive effects are only observed at atorvastatin concentrations that are beyond the doses required for cholesterol lowering. When compared with HD atorvastatin, treatment with the same dose of simvastatin suppressed high dose IL-1β-induced huCRP expression by only 50% (P < .05), thus underscoring the more potent anti-inflammatory potential of atorvastatin (Figure 3B).
Effect of atorvastatin, simvastatin, fenofibrate, and Wy 14643 on IL-1β-induced huCRP expression. (A) Groups of huCRPtg mice (n ≥ 5) received a chow diet containing atorvastatin (At) at a low dose (LD; 0.01% [wt/wt]) or at a high dose (HD; 0.1% [wt/wt]), or vehicle (Con) for 3 weeks. Mice intraperitoneally received IL-1β either at a low dose (8.3 × 104 U/mouse) or at a high dose (2.5 × 105 U/mouse). (B) As in panel A but mice were fed 0.1% (wt/wt) simvastatin (Sim). (C) As in panel A but mice were fed 0.1% (wt/wt) fenofibrate (FF). (D) As in panel A but mice were fed 0.03% (wt/wt) Wy 14643 (WY). HuCRP levels were determined in tail blood which was collected 18 hours after IL-1β stimulation. Data represent means ± SEM. *P < .05 compared with control.
Effect of atorvastatin, simvastatin, fenofibrate, and Wy 14643 on IL-1β-induced huCRP expression. (A) Groups of huCRPtg mice (n ≥ 5) received a chow diet containing atorvastatin (At) at a low dose (LD; 0.01% [wt/wt]) or at a high dose (HD; 0.1% [wt/wt]), or vehicle (Con) for 3 weeks. Mice intraperitoneally received IL-1β either at a low dose (8.3 × 104 U/mouse) or at a high dose (2.5 × 105 U/mouse). (B) As in panel A but mice were fed 0.1% (wt/wt) simvastatin (Sim). (C) As in panel A but mice were fed 0.1% (wt/wt) fenofibrate (FF). (D) As in panel A but mice were fed 0.03% (wt/wt) Wy 14643 (WY). HuCRP levels were determined in tail blood which was collected 18 hours after IL-1β stimulation. Data represent means ± SEM. *P < .05 compared with control.
HuCRPtg mice were also treated with 0.1% (wt/wt) fenofibrate, 0.03% (wt/wt) Wy 14643, or vehicle for 3 weeks, and subsequently stimulated with the above 2 doses of IL-1β to induce huCRP expression. Fenofibrate treatment reduced basal huCRP expression by 63% (P < .05) and strongly suppressed induction of huCRP expression with the 2 doses of IL-1β by 88% (P < .05) and 63% (P < .05), respectively (Figure 3C). For comparison, mice treated for 3 weeks with 0.03% (wt/wt) Wy 14643 suppressed the high dose IL-1β-induced huCRP expression by 98% (P < .05), demonstrating that of the compounds tested, Wy 14643 is the most potent anti-inflammatory drug (Figure 3D). Together these data indicate that PPARα activators, like statins, also can reduce IL-1β-induced huCRP expression in vivo.
Next, the effect of atorvastatin and fenofibrate on huCRP gene expression was analyzed by RT-PCR in livers of mice killed 10 hours after IL-1β injection. HD atorvastatin and 0.1% (wt/wt) fenofibrate suppressed IL-1β-induced huCRP gene expression by 67% (P < .05) and 91% (P < .05), respectively, indicating that the suppressive effect is at the level of transcription (data not shown). The effectiveness of the atorvastatin treatment was confirmed by a 13-fold (P < .05) up-regulated hepatic expression of HMG-CoA (3-hydroxy-3-methyl-glutaryl coenzyme A) reductase in HD atorvastatin-treated animals (data not shown).In these animals, the mRNA expression level of the specific PPARα target gene acyl-CoA oxidase (ACO) was unchanged, indicating that atorvastatin did not exert its action via activation of PPARα (compare fenofibrate-treated animals which displayed a 27-fold [P < .05] up-regulation of ACO gene expression [data not shown]).
A molecular mechanism for the down-regulation of IL-1α-induced huCRP expression by statins in human hepatocytes
To elucidate the molecular mechanism by which statins reduce huCRP expression in human liver cells, freshly prepared human liver slices were incubated with simvastatin and, for comparison, the specific PPARα activator Wy 14643. After 24 hours of treatment, huCRP levels were determined in the culture medium by ELISA. Simvastatin reduced basal huCRP expression by 38% (P < .05), and a 48% (P < .05) reduction was observed with Wy 14643 (Figure 4A). Incubation of control liver slices with IL-1α significantly increased huCRP expression 1.5-fold (P < .05). Simvastatin and Wy 14643 suppressed this induction by 40% (P < .05) and 30% (P < .05), respectively (Figure 4A). Luciferase reporter gene experiments using a 300-bp fragment of the huCRP promoter, which harbors known IL-1-sensitive responsive elements for C/EBPβ and p50-NFκB,20 demonstrated that statins suppressed the effect of IL-1α at the transcriptional level. IL-1α induced huCRP promoter activity 5.9-fold in control cells; treatment with simvastatin dose-dependently suppressed this induction by maximally 60% (P < .05), which is less pronounced than the suppression obtained with atorvastatin at the same dose (76%; P < .05) or Wy 14643 (65%; P < .05) (Figure 4B).
Statin treatment suppresses IL-1-induced huCRP gene expression in human liver cells. (A) Human liver slices were incubated with 5 μM simvastatin (simva), 250 μM Wy 14643 (WY), or vehicle (DMSO; Con) for 24 hours and then stimulated with 25 ng/mL IL-1α for an additional 24 hours. HuCRP was determined in culture medium by ELISA. Data represent means ± SD of 3 experiments. (B) HuH7 cells were treated with Wy 14643 (10 μM, 30 μM, 100 μM), atorvastatin (1 μM, 3 μM, 10 μM), or simvastatin (1 μM, 3 μM, 10 μM), transiently transfected with phuCRP-luc, and subsequently were stimulated with 10 ng/mL IL-1α for 18 hours. Shown data represent means ± SD. *P < .05 compared with control.
Statin treatment suppresses IL-1-induced huCRP gene expression in human liver cells. (A) Human liver slices were incubated with 5 μM simvastatin (simva), 250 μM Wy 14643 (WY), or vehicle (DMSO; Con) for 24 hours and then stimulated with 25 ng/mL IL-1α for an additional 24 hours. HuCRP was determined in culture medium by ELISA. Data represent means ± SD of 3 experiments. (B) HuH7 cells were treated with Wy 14643 (10 μM, 30 μM, 100 μM), atorvastatin (1 μM, 3 μM, 10 μM), or simvastatin (1 μM, 3 μM, 10 μM), transiently transfected with phuCRP-luc, and subsequently were stimulated with 10 ng/mL IL-1α for 18 hours. Shown data represent means ± SD. *P < .05 compared with control.
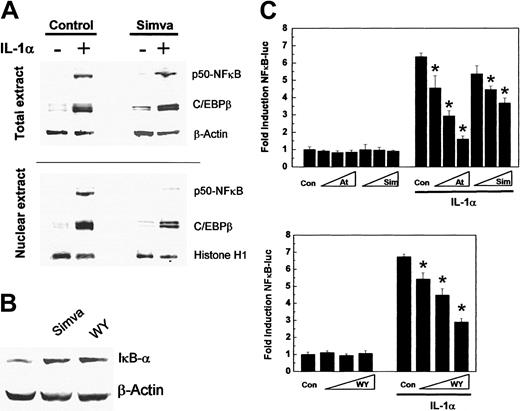
Because IL-1α-induced huCRP gene expression is regulated by the expression and nuclear translocation of the transcription factors p50-NFκB and C/EBPβ,20 the effect of simvastatin on nuclear accumulation of p50-NFκB and C/EBPβ under basal and IL-1α-stimulated conditions was analyzed next. Western blot experiments using total extracts and nuclear extracts of vehicle-treated and simvastatin-treated human HuH7 hepatoma cells stimulated or not with IL-1α showed that simvastatin did not inhibit the IL-1α-induced intracellular generation of p50-NFκB and C/EBPβ but reduced the translocation of p50-NFκB into the nucleus (Figure 5A). This can be explained by an increased expression of the intracellular inhibitor of NFκB, IκBα, by simvastatin (Figure 5B). The induction of IκBα by simvastatin was comparable to the effect of Wy 14643, an established inducer of IκBα,20 and suggests a more general suppression of NFκB activity. Indeed, simvastatin, atorvastatin, and Wy 14643 also dose-dependently inhibited IL-1α-induced NFκB-driven reporter gene activity by maximally 42% (P < .05), 75% (P < .05), and 57% (P < .05), respectively (Figure 5C).
Statin treatment inhibits NFκB activity. (A) HuH7 cells were preincubated with 3 μM simvastatin (Simva) for 24 hours and subsequently were stimulated with 10 ng/mL IL-1α for 18 hours. Western blot analysis of total cellular and nuclear extracts for p50-NFκB and C/EBPβ expression. (B) HuH7 cells were incubated with simvastatin or Wy 14643 (WY) for 24 hours and analyzed for IκBα expression in total cell extracts. Control β-actin and histone H1 expression levels confirm equal loading. (C) HuH7 cells were incubated with Wy 14643 (10 μM, 30 μM, 100 μM), atorvastatin (1 μM, 3 μM, 10 μM), simvastatin (1 μM, 3 μM, 10 μM), transiently transfected with p3xNFκB-luc, and subsequently were stimulated with 10 ng/mL IL-1α for 18 hours. Data represent means ± SD. *P < .05 compared with control.
Statin treatment inhibits NFκB activity. (A) HuH7 cells were preincubated with 3 μM simvastatin (Simva) for 24 hours and subsequently were stimulated with 10 ng/mL IL-1α for 18 hours. Western blot analysis of total cellular and nuclear extracts for p50-NFκB and C/EBPβ expression. (B) HuH7 cells were incubated with simvastatin or Wy 14643 (WY) for 24 hours and analyzed for IκBα expression in total cell extracts. Control β-actin and histone H1 expression levels confirm equal loading. (C) HuH7 cells were incubated with Wy 14643 (10 μM, 30 μM, 100 μM), atorvastatin (1 μM, 3 μM, 10 μM), simvastatin (1 μM, 3 μM, 10 μM), transiently transfected with p3xNFκB-luc, and subsequently were stimulated with 10 ng/mL IL-1α for 18 hours. Data represent means ± SD. *P < .05 compared with control.
We next questioned whether simvastatin, as a consequence of its inhibitory effect on p50-NFκB translocation, would reduce the amount of nuclear p50-NFκB∼C/EBPβ complexes that drive huCRP gene transcription. Coimmunoprecipitation experiments demonstrated that simvastatin reduced the amount of p50-NFκB∼C/EBPβ complexes induced by IL-1α in nuclei of HuH7 cells and human primary hepatocytes (Figure 6). Together these data indicate that simvastatin suppresses IL-1α-induced huCRP gene expression by up-regulating IκBα and reducing p50-NFκB translocation, resulting in a diminished amount of nuclear p50-NFκB∼C/EBPβ complexes and thereby reduced huCRP gene transcription.
Statin treatment reduces the nuclear amount of p50-NFκB∼C/EBPβ complexes. Coimmunoprecipitation was performed on nuclear extracts prepared from HuH7 cells and freshly isolated primary human hepatocytes preincubated for 17 hours with 3 μM (HuH7 cells) and 10 μM (primary hepatocytes) simvastatin (Simva), 50 μM Wy 14643 (WY), or vehicle and stimulated with 10 ng/mL IL-1α (HuH7 cells) or 25 ng/mL IL-1α (primary hepatocytes) for 18 hours. Anti-p50-NFκB or anti-MMP-8 control antibody (Control-Ab) was used for immunoprecipitation and C/EBPβ bound to precipitated p50-NFκB was detected by Western blot.
Statin treatment reduces the nuclear amount of p50-NFκB∼C/EBPβ complexes. Coimmunoprecipitation was performed on nuclear extracts prepared from HuH7 cells and freshly isolated primary human hepatocytes preincubated for 17 hours with 3 μM (HuH7 cells) and 10 μM (primary hepatocytes) simvastatin (Simva), 50 μM Wy 14643 (WY), or vehicle and stimulated with 10 ng/mL IL-1α (HuH7 cells) or 25 ng/mL IL-1α (primary hepatocytes) for 18 hours. Anti-p50-NFκB or anti-MMP-8 control antibody (Control-Ab) was used for immunoprecipitation and C/EBPβ bound to precipitated p50-NFκB was detected by Western blot.
Discussion
It is now well established that atherosclerosis is not solely a lipid disorder but also an inflammatory disease.2,3 Statins and fibrates are hypolipidemic drugs that reduce atherosclerosis development and concomitantly lower plasma levels of huCRP, a sensitive inflammation marker and independent predictor of future cardiovascular events.1,17,18,24 An important question is whether the huCRP levels are lowered as a result of a direct anti-inflammatory action of these drugs, or whether they are lowered as a consequence of reduced atherogenesis. We here provide evidence that both statins (ie, atorvastatin and simvastatin) and PPARα activators (ie, fenofibrate and Wy14643) reduce basal and IL-1β-induced huCRP gene expression in nonatherosclerotic huCRPtg mice. These anti-inflammatory effects are demonstrable at statin concentrations that are higher than required for cholesterol lowering per se or even occur without altering plasma cholesterol levels in the case of fenofibrate and Wy14643. The data indicate that statins and PPARα activators exert direct anti-inflammatory effects unrelated to atherosclerosis and lowering plasma cholesterol levels. This notion is strengthened by experiments with cultured human hepatocytes in which simvastatin and Wy14643 also reduce huCRP gene expression, and by providing a mechanistic explanation for this action. The anti-inflammatory action of statins and PPARα activators involves up-regulation of an inhibitor of NFκB, IκBα, which traps p50-NFκB in the cytosol. As a consequence, the nuclear translocation of p50-NFκB is reduced and the amount of nuclear p50-NFκB∼C/EBPβ complexes, which are crucial for huCRP gene transcription,20,25 is decreased. The results provide the first conclusive evidence for a direct down-regulating effect of statins and PPARα activators on huCRP expression in vivo.
Although it is widely accepted that the clinical benefit obtained with statins is a direct result of their lipid-lowering properties, there is still debate as to whether the additional, so-called pleiotropic effects26 of statins contribute to the clinical outcome in vascular disease, or whether all beneficial effects of statins are due to lowering plasma lipids. Recent studies in mice and cynomolgus monkeys demonstrated that statins reduce the development of atherosclerosis beyond the effect achieved by their plasma cholesterol-lowering effect alone.27-29 It was suggested that these cholesterol-independent effects of statins were the result of their anti-inflammatory action as reflected by the reduced expression of tumor necrosis factor α (TNFα), monocyte chemoattractant protein-1 (MCP-1), and vascular cell adhesion molecule 1 (VCAM-1) in atherosclerotic lesions and the down-regulation of liver-derived plasma inflammation markers; however, decisive evidence for this conclusion was lacking. In the present study we unequivocally demonstrate that statins possess anti-inflammatory potential by themselves and directly (ie, unrelated to cholesterol lowering and in absence of atherosclerosis) suppress basal and IL-1β-induced huCRP gene expression. This additional effect may also contribute to the reduced risk of cardiovascular events in normocholesterolemic patients treated with statins, an observation that cannot be fully explained by the cholesterol-lowering properties of the drugs.27,30,31 In that respect it may also be significant that other anti-inflammatory, plasma lipid-modulating drugs such as activators of PPARα and liver X receptors (LXRs) show antiatherogenic activity unrelated to their plasma cholesterol-altering activities.16,32 For example, fenofibrate was recently found to reduce atherosclerosis in ApoE-deficient mice.17 Similarly, the LXR agonist GW3965 was shown to inhibit atherogenesis in ApoE-deficient mice and low density lipoprotein receptor-deficient mice despite unfavorable effects on plasma lipids.33
Recent observations indicate that huCRP may also be directly involved in the process of atherosclerosis.6-9 A study using huCRP transgenic or nontransgenic ApoE-deficient mice demonstrates that elevated huCRP plasma levels lead to accelerated atherosclerosis, documenting a proatherogenic role for huCRP in vivo.10 Our data show that treatment with statins and PPARα activators reduces huCRP plasma levels, suggesting that these drugs may retard the process of atherosclerosis beyond the effect achieved by their lipid-lowering properties alone.
The anti-inflammatory action of statins and fibrates on huCRP expression in hepatocytes is based on up-regulation of the cytosolic inhibitor of NFκB, IκBα, resulting in reduced NFκB activity. This effect is not limited to hepatocytes since several statins have recently been shown to up-regulate the expression of IκBα in cultured human aortic endothelial cells.34 Furthermore, statins have also been reported to inhibit NFκB activation in human mesothelial cells and human aortic smooth muscle cells (haSMCs).23,34 Similarly, fibrates have been found to induce IκBα expression in huSMCs and hepatocytes.20,35 This more general effect of statins and fibrates is in accordance with the finding that atorvastatin reduces NFκB activation in atherosclerotic lesions of rabbits36 and may explain the decreased expression of the NFκB-regulated genes MCP-1 and VCAM-1 in atherosclerotic lesions.28,29 Since NFκB coordinates the expression of a wide variety of genes that control immune responses and is involved in many inflammatory diseases,4,37,38 statins and fibrates may also have beneficial effects in diseases with an inflammatory component other than atherosclerosis.39
Together, our results provide convincing evidence that statins and PPARα activators reduce huCRP gene expression by direct anti-inflammatory activities unrelated to cholesterol lowering and atherosclerosis. It remains to be investigated whether these activities also suppress other mediators of inflammatory processes, which may lead to extended therapeutic indications based on the anti-inflammatory properties of statins and fibrates.
Prepublished online as Blood First Edition Paper, February 19, 2004; DOI 10.1182/blood-2003-11-3791.
Supported by The Netherlands Organization for Scientific Research (grant 016.036.061 to R.K.) and the Netherlands Heart Foundation (grant 99.110 to R.K., grant 97.100 to J.L., and grant 99.104 to L.V.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. A. McCrory, E.Offermans, M. C. E. Maas, and M. van Erck for technical assistance. We thank Dr U. Rüther and Dr A. Zantema for helpful discussions.

![Figure 1. Effect of atorvastatin, simvastatin, fenofibrate, and Wy 14643 on plasma cholesterol and basal huCRP expression. (A) Groups of huCRPtg mice (n ≥ 5) received a chow diet containing a low dose (LD; 0.01% [wt/wt]) of atorvastatin (At) or vehicle control (Con) for 4 weeks. Plasma cholesterol was determined in tail blood and data are expressed as means ± standard deviation (SD). (B) As in panel A but mice received a high dose (HD; 0.1% [wt/wt]) of atorvastatin. (C) Plasma huCRP levels of mice were determined in tail blood at t = 0 and t = 4 weeks and data are expressed as means ± SEM. (D) As in panel A but mice received a dose of 0.1% (wt/wt) simvastatin. (E) As in panel C. (F) As in panel A but mice received 0.1% (wt/wt) fenofibrate (FF) or 0.03% (wt/wt) Wy 14643. (G) As in panel C. Statistical significant difference compared with control animals is indicated by *P < .05. ns = not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/11/10.1182_blood-2003-11-3791/6/m_zh80110462320001.jpeg?Expires=1767705940&Signature=Dqg1M23SWy~A5ihbniq4~LfHjvsUpuxyXXdkkU6-kjLck-gWFI3g1ar9kHAym-g44EO3jJJaQzO9Xs8qIAWo5xl~3DclkB6bTV6kPGo0MRTRxgctpslUXR3HKCDkcHTdfKoo1mwnShVTK8XKkkIGkXjvMG2Tiq~XojpWqGFUlQpTK~SqlftAP9u-z-URdFsMVkqayQVbVung4lHm6pK3B70NjD6M0P5ybiqkd-Wp2Y~TSbEYwU7rUvuQ8QRsRtGtx-0auiwEmz22sOvyvGP1sckCwXHQH-nceP7qOlqR~ZsH7ilSXgoZkWX4aePsf0-rIkklGUtePsiwnLAQz6xq7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Effect of atorvastatin, simvastatin, fenofibrate, and Wy 14643 on IL-1β-induced huCRP expression. (A) Groups of huCRPtg mice (n ≥ 5) received a chow diet containing atorvastatin (At) at a low dose (LD; 0.01% [wt/wt]) or at a high dose (HD; 0.1% [wt/wt]), or vehicle (Con) for 3 weeks. Mice intraperitoneally received IL-1β either at a low dose (8.3 × 104 U/mouse) or at a high dose (2.5 × 105 U/mouse). (B) As in panel A but mice were fed 0.1% (wt/wt) simvastatin (Sim). (C) As in panel A but mice were fed 0.1% (wt/wt) fenofibrate (FF). (D) As in panel A but mice were fed 0.03% (wt/wt) Wy 14643 (WY). HuCRP levels were determined in tail blood which was collected 18 hours after IL-1β stimulation. Data represent means ± SEM. *P < .05 compared with control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/11/10.1182_blood-2003-11-3791/6/m_zh80110462320003.jpeg?Expires=1767705940&Signature=wpmeFXIM-tKbzPOeAxYk1ZhmTAueakF5Hwx8vX6sd2PlEh2IjLpbMAStruibEyUPPA6EOGPc2FyZ4nB3Hhaj-8uJi0jZ-nE6~3~wNeHOAWPuuY52fAuqM5JvfmjWGPzQdqXN~e0p-1pclthAq~wK5ZW3o7dCd-j00a2iE~ZsUWrrLQbE-pxA~Jk~CKIpOWMDMonzGS2G~lUUM-uu80YFy6tB-zdg~rVru1Yc6ru41eq1HefqU~RhiwLgamUJwVxuRxu5vH9JLvGjktv9SsBAKdxOGK7514sjtB9yhEw7wzuVY-Non8juZOilDUMNhR9NBqH8Tb6eUW7VYz5Hpi8pfg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal