HEMOSTASIS, THROMBOSIS, AND VASCULAR BIOLOGY
Human C-reactive protein (huCRP) is a major acute-phase protein synthesized in the liver under interleukin-1 (IL-1) and IL-6 control. An elevated concentration of huCRP is currently considered as both a marker and an independent predictor of cardiovascular disease.1 HuCRP is indeed present in atheromatous plaques, and a recent in vivo study indicates that huCRP may have a direct proatherogenic role via the up-regulation of angiotensin type 1 receptors.2 Lowering huCRP levels could therefore be of benefit in modulating the evolution of atherosclerosis.
Statins and fibrates are cholesterol-lowering agents that also decrease plasma levels of huCRP.3 The hypolipidemic effect of fibrates is obtained via the nuclear receptor peroxisome proliferator–activated receptor-alpha (PPAR-α). In addition, PPAR-α activation by fibrates has been shown to act as a negative regulator of inflammatory processes by antagonizing the activity of the transcription factor pathways (eg, nuclear factor κ B [NFκB]).
The statins block cholesterol synthesis by interrupting the conversion of 3-hydroxymethylglutaryl coenzyme A (HMG-CoA) to mevalonate. In addition, they may also have anti-inflammatory effects; rosuvastatin specifically suppressed the expression of the inflammation parameters monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor α (TNF-α) in the vessel wall.4 It has been suggested that the pleiotropic effects of HMG-CoA reductase inhibitors and PPAR-α activators (eg, inhibition of tissue factor and plasminogen activator inhibitor 1 [PAI-1] expression and of endothelin-1 [ET-1] secretion) participate in the capacity of these molecules to reduce coronary heart disease.
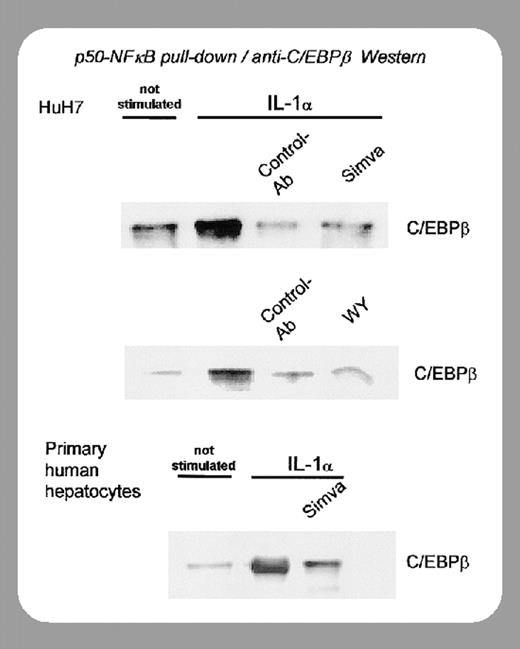
Whether the huCRP-lowering capacity of statins and fibrates is secondary to reduced atherogenesis or a direct independent effect has been clarified by recent studies from the Gaubius Laboratory (Leiden, The Netherlands) on the mechanism of action of these agents. It was initially shown that fibrates reduce CRP levels by down-regulation of IL-1–stimulated CRP gene expression via reduction of nuclear p50-NFκB–C/EBPβ (CCAAT/enhancer binding protein β) complex formation.5
In this issue of Blood, Kleemann and colleagues (page 4188) have shown that atorvastatin and fenofibrate, at doses higher than those required for cholesterol lowering, decrease basal and IL-1β–induced plasma huCRP levels. Since these experiments were performed in nonatherosclerotic huCRP mice, the authors clearly demonstrate that these compounds exert a direct anti-inflammatory action independent of cholesterol lowering and atherogenesis. Furthermore, using human liver slices it was shown that these drugs suppressed the effect of IL-1β at the transcriptional level. The suppression of IL-1–induced huCRP gene transcription appears to involve both an up-regulation of IκBα, an inhibitor of the activity of NFκB, and a reduction of nuclear p50-NFκB-C/EBPβ complexes. It is of note that this direct anti-inflammatory effect was obtained with both the statins and fenofibrate.
Since huCRP may accelerate the progression of atherosclerosis in apolipoprotein E (ApoE)–deficient mice,2 these results suggest that lowering huCRP with statins or fibrates will regulate the progression of atherosclerosis. However, the possibility that decreasing huCRP concentration may contribute to arrest plaque formation and decrease the likelihood of cardiovascular accidents, as do the cholesterol-lowering doses of statins in humans, remains to be demonstrated. A response will be obtained in this regard from the ongoing low-cholesterol/high-huCRP trial “Justification for the Use of Statins in Primary Prevention: an Interventional Trial Evaluating Rosuvastatin (JUPITER).”6

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal