Abstract
Erythroid Krüppel-like factor (EKLF) plays an essential role in enabling β-globin expression during erythroid ontogeny. It is first expressed in the extraembryonic mesoderm of the yolk sac within the morphologically unique cells that give rise to the blood islands, and then later within the hepatic primordia. The BMP4/Smad pathway plays a critical role in the induction of EKLF, and transient transfection analyses demonstrate that sequences located within less than 1 kb of its transcription initiation site are sufficient for high-level erythroid-specific transcription. We have used transgenic analyses to verify that 950 bp located adjacent to the EKLF start site of transcription is sufficient to generate lacZ expression within the blood islands as well as the fetal liver during embryonic development. Of particular importance are 3 regions, 2 of which overlap endogenous erythroid-specific DNase hypersensitive sites, and 1 of which includes the proximal promoter region. The onset of transgene expression mimics that of endogenous EKLF as it begins by day 7.5 (d7.5) to d8.0. In addition, it exhibits a strict hematopoietic specificity, localized only to these cells and not to the adjacent vasculature at all stages examined. Finally, expression is heterocellular, implying that although these elements are sufficient for tissue-specific expression, they do not shield against the position effects of adjacent chromatin. These analyses demonstrate that a surprisingly small DNA segment contains all the information needed to target a linked gene to the hematopoietic compartment at both early and later stages of development, and may be a useful cassette for this purpose.
Introduction
Hemoglobin production during mammalian development is characterized by a series of switches in sites of expression of the genes coding for the α- and β-globin chains.1-3 The initial wave of transient primitive red cells is produced in the blood islands of the embryonic yolk sac. This is followed by generation of morphologically distinct definitive red cells produced in the fetal liver. After birth, the final switch to the bone marrow (and spleen in the mouse) completes the process. Concomitant with these changes, the β-like globin gene family is reprogrammed in a specific temporal sequence. The human β-like gene locus consists of embryonic (ϵ), fetal (γ), and adult (β) variants, while the murine β-like locus contains embryonic (y, bh0, bh1) and adult (β) types. Together with a far upstream locus control region, the complete cluster is located within an open erythroid-specific chromatin domain that accommodates both long- and short-range interactions that yield developmentally correct gene expression.4,5
Erythroid Krüppel-like factor (EKLF/KLF16 ) is an erythroid cell-specific transcription factor that plays a crucial role in regulation of β-like globin expression during erythroid development.7,8 This is achieved by the sequence-specific interaction of its 3 zinc fingers with the CACCC element located within the adult β-globin promoter,9 and by its association with a limited set of proteins that play critical functions in both chromatin assembly and transcriptional activation.10-12 Genetic ablation of EKLF results in a profound deficiency of β-globin expression, leading to embryonic lethality at the time when adult β-globin transcription would normally be induced in the mouse.13,14 These and other molecular and genetic studies strongly suggest that EKLF plays a critical role in consolidating the developmental switch from fetal γ- to adult β-globin expression.15-17
EKLF directs this specific effect in part by exhibiting a high level of erythroid specificity in its own expression.9,18 During murine development, EKLF expression is first detected at the neural plate stage within the blood islands of the extraembryonic mesoderm at embryonic day 7.5 (E7.5). At a later stage, EKLF is expressed within the erythroid cells of the hepatic primordia beginning at E9.5. This level of red cell specificity continues in the adult, as EKLF is expressed only in the bone marrow and within the red pulp of the spleen.18 During hematopoietic differentiation, EKLF is expressed surprisingly early, detected in multipotential primary cells19 and cell lines.20,21 As a result of its early hematopoietic expression and its later erythroid cell specificity, uncovering the mechanisms controlling EKLF genetic expression is of wide interest.
There are 2 complimentary approaches that have delineated EKLF regulation. One used embryonic stem cells, differentiating to embryoid bodies in serum-free conditions, to identify extracellular inducers that are required for EKLF expression. These studies established that BMP4 plays a necessary role in directing EKLF expression, and that the BMPR/Smad pathway is critical for its success.22 However, the competence of cells present in the embryoid bodies to respond to BMP4 is transient, and its induction of EKLF is not immediate. As a result, transcriptional activation of another factor may be required for induction.
The second approach used mapping of endogenous DNAse hypersensitive sites along with transient transfection assays to localize the transcriptional control elements of the EKLF gene. These studies found that the proximal promoter region, containing GATA and CCAAT elements, is critical for minimal activity.23 More global analyses24 found that the EKLF transcription unit is in an open chromatin domain, and that 2 erythroid cell-specific DNase hypersensitive sites (EHS1 and EHS2) are located within 1 kb of its transcriptional initiation site. The distal erythroid-specific hypersensitive site (EHS1), located within a region that is conserved in sequence with the human EKLF genomic locus, imparts a strong enhancing activity to the minimal proximal promoter. In vitro analyses demonstrated that distinct DNA binding activities in erythroid cells interact with the core region of this enhancer. Together, these distal and proximal elements, which reside with 950 bp of the start site of EKLF transcription, are responsible for its high-level erythroid cell-specific expression and may provide a platform for its control via extracellular induction pathways.24
However, a critical issue that remains is to establish that these elements are sufficient for tissue-specific expression within the developing murine system, as transient assays cannot address issues of developmental timing, localization, and variegation. Previous analyses have shown that a larger promoter construct generated erythroid expression of a linked gene in transgenic mice.25,26 However, the importance of all the mapped upstream and proximal elements for tissue-specific expression and whether they are sufficient to establish the correct temporal and topographic onset of EKLF expression have not been investigated. As a result, we approached this problem by analyzing transgenic mice using many of the same constructs that had been previously tested in transient assays.24 These studies enable us to conclude that all the stage- and cell-specific elements that control EKLF expression in vivo reside within its 950-bp promoter, and that expression is directed precisely to the blood cells. This element also recapitulates the correct onset of endogenous EKLF expression in the developing embryo. As a result, this small domain is now a well-characterized DNA cassette that can be used to direct erythroid-specific expression of any linked gene during development.
Materials and methods
EKLF promoter regions were subcloned as HindIII/BglI fragments from the previously described CAT reporter clones24 into the SmaI site of the p460 lacZ reporter.27 Mutation of the proximal GATA site was performed with the Quick-Change kit (Stratagene, La Jolla, CA) as recommended by the manufacturer using 5′CCTGGGTCTTATTAGGGAAGACAGC3′ as the mutagenic primer. This alters the antisense TGATAA sequence (at -60 base pairs [bp]) to TAATAA, a change that disrupts its interaction with the GATA1 protein.23 The -950 construct24 was the parent clone (considered wild type) from which all deletion and mutagenic versions were derived.
Transgenic mice were obtained by standard procedures28 after injection of the NotI-excised promoter/lacZ fragment from each construct into pronucleus of one-cell stage fertilized eggs (Mount Sinai Mouse Genetics Shared Research Facility, New York, NY). Genotyping was performed by Southern blotting or by polymerase chain reaction with lacZ-specific primers.29
Detection of β-galactosidase activity in staged embryos was performed as described after fixation with formaldehyde/gluteraldehyde.27 Paraffin sections were generated (7-10 μm) after postfixation in formaldehyde and visualized on a Zeiss Axiophot (Zeiss, Thornwood, NY). Circulating blood cells from day 10.5 (d10.5) yolk sacs were isolated in phosphate-buffered saline and stained as described.30,31 In all experiments, multiple embryos (> 10) were analyzed at each stage for each transgenic line. Analysis of wild type and each mutant line with Fisher exact test gives values between 0.061 and 0.098.
Results
Both red cell-specific DNAse hypersensitive sites are required, along with the proximal EKLF promoter, for proper erythroid expression of the transgene
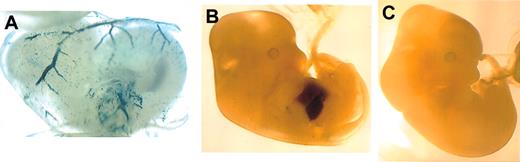
We first wished to determine if the minimal 950-bp region of the proximal EKLF promoter, shown to be sufficient for red cell-specific transcription by deletion analysis in transient assays,24 could drive cell-specific expression in mice. For this we fused this region of the promoter to a lacZ reporter, established transgenic lines, and monitored expression by whole-mount staining of timed embryos. Positive embryos showed specific transgene expression both in the primitive erythroid cells of the d10.5 yolk sac and in the definitive population within the d12.5 fetal liver (Figure 1). These results demonstrate that the -950 EKLF promoter is sufficient to recapitulate erythroid-specific reporter expression in vivo.
Whole-mount analysis of transgene expression driven by the -950 EKLF promoter during embryogenesis. Expression is observed in the circulating primitive erythroid cells in the yolk sac of a transgenic d10.5 embryo (A) and in the definitive erythroid cells in the fetal liver of a transgenic d12.5 embryo (B), but not in a nontransgenic littermate (C). Original magnification, × 50 (A); and × 20 (B-C).
Whole-mount analysis of transgene expression driven by the -950 EKLF promoter during embryogenesis. Expression is observed in the circulating primitive erythroid cells in the yolk sac of a transgenic d10.5 embryo (A) and in the definitive erythroid cells in the fetal liver of a transgenic d12.5 embryo (B), but not in a nontransgenic littermate (C). Original magnification, × 50 (A); and × 20 (B-C).
The -950 promoter element contains 2 erythroid cell-specific hypersensitive sites, a distal one at approximately -700 bp and a proximal one at approximately -300 bp.24 Our previous studies had shown that the distal EKLF hypersensitive site (EHS1) was required for generating high-level transcription of a reporter in transient assays.24 In contrast, deletion of the proximal EKLF hypersensitive site (EHS2) had no effect. We constructed the same deletions within the context of the -950 EKLF promoter upstream of the lacZ reporter and established multiple transgenic lines with each construct (Figure 2). As expected from the previous transgenic analyses, deletion of EHS1, either alone or in combination with EHS2, disabled the promoter's ability to establish lacZ expression in the blood in a total of 11 lines. However in this case, deletion of EHS2 also incapacitated the promoter's ability to establish expression (7 lines). We conclude that, unlike what is seen in the transient assays, both of the open chromatin domains in the EKLF promoter, located within 950 bp of transcription initiation site, are necessary to establish any detectable transgenic expression (3 of 5 lines). It is of note that we did not uncouple primitive from definitive expression by using any of these variant constructs.
Deletion analysis of the -950 EKLF promoter. The images at the top of the figure show the typical appearances of positive and negative d10.5 and d13.5 embryos. At the bottom of the figure are schematic diagrams of the deletions/point mutations analyzed in the study. The results from analyzing embryos derived from multiple lines (positive/total lines examined) are shown for each construct after analysis at either d10.5 or d13.5. EHS1 and EHS2 are the locations of erythroid-specific DNase hypersensitive sites as described in “Results,” and the GATA site point mutation in the minimal promoter is also shown. Original magnification, × 50 (left); and × 20 (right).
Deletion analysis of the -950 EKLF promoter. The images at the top of the figure show the typical appearances of positive and negative d10.5 and d13.5 embryos. At the bottom of the figure are schematic diagrams of the deletions/point mutations analyzed in the study. The results from analyzing embryos derived from multiple lines (positive/total lines examined) are shown for each construct after analysis at either d10.5 or d13.5. EHS1 and EHS2 are the locations of erythroid-specific DNase hypersensitive sites as described in “Results,” and the GATA site point mutation in the minimal promoter is also shown. Original magnification, × 50 (left); and × 20 (right).
The earlier studies had uncovered an important role for the GATA site located within the proximal promoter at -60 bp, both for minimal23 and EHS1-enhanced24 transcriptional activity. We tested its requirement in mice by generating transgenic lines whose reporter contains a point mutation within the context of the full promoter (-950 construct) that disrupts GATA1 binding. We found that none of the lines established with this construct generated any red cell-specific lacZ activity (Figure 2). We conclude that the proximal promoter region, particularly the GATA sequence, also plays a critical role in enabling expression of the EKLF promoter.
Transgene expression begins at the expected time in development and is strictly limited to the hematopoietic compartment
In situ experiments had shown that the onset of EKLF expression begins at the neural plate stage at d7.5.18 We monitored lacZ expression in our -950 EKLF promoter lines to determine whether the onset of transgene expression also begins at approximately the same time window. Embryos at d7.0 to d7.5 were negative for lacZ expression (Figure 3A), but those at d7.5 to d8.0 were positive in a ring pattern in the yolk sac (Figure 3B-D) resulting from successful onset of lacZ expression within the blood islands. As expected from changes in the pattern of endogenous embryonic globin expression during this time,32 the lacZ signal is more dispersed throughout the yolk sac in a punctate pattern in d8.0 to d8.5 embryos (Figure 3E-F). We conclude that, within the resolution of our time frame, the -950 EKLF promoter can direct transgene expression at a stage coincident with the onset of the endogenous promoter.
Transgene expression by the -950 promoter at initial stages of yolk sac blood island development. LacZ expression is not apparent in d7.0 to d7.5 embryos (A), but becomes detectable as a ring of expression (indicated by the arrows) at the blood islands by d7.5 to d8.0 (B-D). The expression profile becomes more diffuse by d8.0 to d8.5, leading to a punctate pattern throughout the yolk sac (E-F). In panel B, P indicates posterior, and A indicates anterior. Original magnification, × 75. Three embryos are shown in panels B-D; 2 embryos are shown in panels E-F.
Transgene expression by the -950 promoter at initial stages of yolk sac blood island development. LacZ expression is not apparent in d7.0 to d7.5 embryos (A), but becomes detectable as a ring of expression (indicated by the arrows) at the blood islands by d7.5 to d8.0 (B-D). The expression profile becomes more diffuse by d8.0 to d8.5, leading to a punctate pattern throughout the yolk sac (E-F). In panel B, P indicates posterior, and A indicates anterior. Original magnification, × 75. Three embryos are shown in panels B-D; 2 embryos are shown in panels E-F.
Although expression within the yolk sac was consistent with reporter expression in the circulating primitive cell, we wished to analyze this more carefully to see if lacZ was also expressed within the vascular system.33 For this we sectioned d7.5 to d10.5 yolk sacs and examined these under high power (Figure 4). The analysis of multiple sections demonstrate that transgene expression is strictly limited to the hematopoietic, and not the vascular (ie, endoderm or endothelial), component of the yolk sac at any of the stages examined.
Transgene expression in sections of stained embryos. LacZ expression is specifically localized to the round hematopoietic cells in the yolk sacs of d7.5 to d8.0 (A-B), d8.0 to d8.5 (C), and d10.5 (D-F) embryos and is not observed in either the endodermal or endothelial layers. Arrowheads direct attention to the lacZ-positive hematopoietic cells between the endothelial and endodermal layers of the yolk sac. emb indicates embryo; ys, yolk sac; en, endothelial layer; and ve, visceral endoderm. Original magnification, × 100. Two samples are shown in panels A-B; 3 samples are shown in panels D-F.
Transgene expression in sections of stained embryos. LacZ expression is specifically localized to the round hematopoietic cells in the yolk sacs of d7.5 to d8.0 (A-B), d8.0 to d8.5 (C), and d10.5 (D-F) embryos and is not observed in either the endodermal or endothelial layers. Arrowheads direct attention to the lacZ-positive hematopoietic cells between the endothelial and endodermal layers of the yolk sac. emb indicates embryo; ys, yolk sac; en, endothelial layer; and ve, visceral endoderm. Original magnification, × 100. Two samples are shown in panels A-B; 3 samples are shown in panels D-F.
Transgene expression is heterocellular
Inspection of stained embryos made it clear that not all positive embryos expressed lacZ to the same extent. This was particularly evident upon examination of d10.5 embryos, some of which contained only a punctate distribution of signal and others that contained a robust signal that filled the vascular system of the yolk sac (data not shown). To address whether this was due to low transgene expression in many cells versus high expression in a low number of cells, we isolated circulating d10.5 erythroblasts from single yolk sacs and stained these individually. These results (Figure 5A-B) show that lacZ expression is heterocellular, with some embryos containing a range of positive cells from 1% to 50%. LacZ expression in transverse sections from a d13.5 embryo, although specifically localized only to the fetal liver, again is not homogeneously distributed (Figure 5C). This property is not line dependent, as littermates exhibit this wide range of positivity. These results are most consistent with a binary model of gene expression, whereby a promoter/enhancer element increases the probability, and not the rate, of adjacent gene expression (see “Discussion”). Although EHS1 and EHS2 are both required for establishment of tissue-specific lacZ expression, they do not confer this property to all cells that contain the transgene.
Heterocellular transgene expression. Circulating blood from d10.5 yolk sacs (A-B) or a transverse section through a d13.5 embryo (C) demonstrates a mosaic pattern of transgene expression in positive transgenics (B-C). Also shown is a negative control littermate (A). (A-B) Large diffracting cells are primitive erythroid cells from the embryo, and small concave cells are adult definitive cells from the mother. liv indicates liver. Original magnification, × 100 (A-B); and × 75 (C).
Heterocellular transgene expression. Circulating blood from d10.5 yolk sacs (A-B) or a transverse section through a d13.5 embryo (C) demonstrates a mosaic pattern of transgene expression in positive transgenics (B-C). Also shown is a negative control littermate (A). (A-B) Large diffracting cells are primitive erythroid cells from the embryo, and small concave cells are adult definitive cells from the mother. liv indicates liver. Original magnification, × 100 (A-B); and × 75 (C).
Discussion
EKLF plays a critical role in establishing the switch in expression to the adult β-globin gene in the differentiating erythroid cell. At the same time, it, itself, exhibits a remarkably restricted pattern of expression during mammalian development. We have demonstrated that all the information required for this resides within a 950-bp region adjacent to the start of transcription. This region contains 2 erythroid-specific hypersensitive sites along with the minimal promoter. In contrast to that seen with some other erythroid (eg, GATA134,35 ) or hematopoietic (eg, SCL36 and GATA237 ) promoters where both downstream and/or upstream regions are required, the EKLF promoter is relatively small and localized to the 5′ side of the transcription unit. This should provide a useful cassette to guide expression of a linked gene to the erythroid compartment.
The -950 promoter overlaps 2 erythroid hypersensitive sites (EHS1 and EHS2) and contains the minimal promoter (that extends to -77 bp) required to direct transcription. The murine EHS1 sequence is conserved with the human promoter, and transient expression data demonstrate that this element is required to generate high-level expression of the EKLF gene.24 EHS1 behaves as a classical enhancer element in these assays, and our present studies verify its importance in vivo for transgene expression. EHS1 overlaps both the core enhancer located at -715 to -666 bp24 and the GATA/E box site26 implicated in earlier studies.
Although EHS2 was dispensable for promoter activity in transient assays, it was required for transgene expression. This was surprising, as this region of the promoter has little sequence homology to the human promoter (unlike EHS1 and the proximal region24 ). However, the fact that it is marked by a strong endogenous hypersensitive site would suggest either that it forms a sequence-independent open chromatin structure or that its removal creates a deleterious situation in the context of chromatin (after integration of the transgene) that has little negative effect upon a transiently transfected structure.
On the other hand, consistent with the transient expression tests,23,24 the present studies show that the proximal GATA site is absolutely required for transgene expression. Although GATA1 protein can bind to this site in vitro23 and in vivo,38 EKLF is still expressed in its absence39 and also in the absence of FOG1.40 One alternative is that GATA2 binds this site early in vivo, as GATA2 recognizes the same consensus sequence41 and its onset in expression precedes that of EKLF (unlike GATA1, which parallels that of EKLF).18,42 Given that GATA2 levels drop during differentiation, it is possible that GATA2 interacts with the EKLF promoter early in hematopoiesis, but that it is replaced by GATA1 later on, analogous to a GATA2/GATA1 transition model recently shown for the regulation of the GATA2 gene.43
The temporal and tissue-restricted control in expression by the -950 promoter is striking, as its onset cleanly parallels that of endogenous EKLF, and its ability to generate lacZ expression specifically within the hematopoietic (ie, future erythroid) cells of the developing yolk sac distinguishes it from other promoters that have been similarly analyzed. For example, SCL contains a more complex promoter, part of which directs expression to both the vascular and hematopoietic system.36,44 Our inability to find lacZ expressed in any of the vascular components of the yolk sac suggests that the EKLF promoter is not expressed in the hemangioblast, the postulated common precursor for hematopoietic and vascular cells.45-47 This is consistent with the embryonic stem cell experiments, where differentiation to embryoid bodies in the absence of serum did not result in EKLF induction, yet hemangioblast markers such as flk1 and PECAM were still expressed.22 In addition, genetic ablation of EKLF does not lead to any vascular defects.13,14
Although the -950 promoter is sufficient for its tissue-specific onset, lacZ expression was heterocellular and highly variegated. Random integration of a transgene into chromosomes can give rise to position effects, leading either to activation or silencing (discussed in Alami et al48 ). Variegating position effects that lead to mosaic expression patterns result from differences in the number of cells that express the transgene within the same tissue and are most consistent with a binary model for transcription.31,49,50 This model postulates that promoter/enhancer elements increase the probability that a promoter will be on or off, rather than acting as a rheostat and gradually controlling the rate of transcription. Not all erythroid promoters exhibit variegation when tested in transgenic mice. For example, the GATA1 promoter also directs a mosaic, heterocellular pattern of transgene expression,35,38 but the minimal erythroid ankyrin promoter directs expression in all erythroid cells of all transgenics.51
Current models suggest that insulator elements are the critical constituents that serve to protect against position effects both by blocking the activity of an external enhancer and by acting as a barrier to silencing.52 These also coincide with DNase hypersensitive sites in vivo, and the best-characterized vertebrate enhancer blocking elements contain CTCF binding sites.53 The EKLF genomic region contains 2 constitutive DNase hypersensitive sites, one at approximately -8.0 kb and the other at approximately +5.5 kb.24 However, these sites are not conserved in sequence with the human EKLF genomic region,54,55 and a search with the vertebrate CTCF element56 revealed that this sequence is not present in either location (data not shown). The -950 EKLF region also does not exhibit the properties of a barrier element,57 as the transgene was subject to position effect variegation. Although we have not investigated the histone acetylation/methylation status at the EKLF genomic locus directly (as has been performed with the chicken β-globin HS4 element58 ), this enables us to infer that the -950 element is missing the type of insulator activity that would prevent ectopic expression and ensure that all transgenics express the transgene only in the erythroid cell. One prediction from these studies is that fusion of the -950 EKLF promoter to a proper insulator (such as the chicken β-globin HS4 element) will repress variegation and lead to homogeneous red cell expression in all transgenics (as has been observed for the murine AE1 [Band 3] promoter59 ). Nonetheless, such a property is not a prerequisite for using the EKLF promoter as an erythroid-specific cassette (analogous to rescue studies that have used the GATA1 promoter60,61 ).
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-09-3231.
Supported by Public Health Service grant R01 DK48721 (J.J.B.) and Shared Facility grant R24 CA88302.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Kevin Kelley and Tom Lufkin for reagents and advice, and Dr Emma Whitelaw for the single cell staining protocol. We also thank the Mount Sinai Mouse Genetics Shared Research Facility directed by Dr Kelley for help in generation of transgenic embryos and mice, and members of the Bieker lab for comments.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal