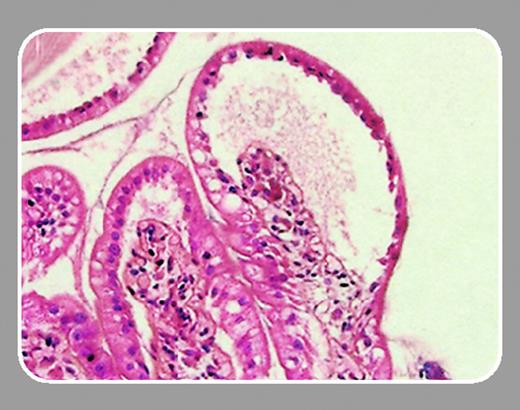
In 2 papers in this issue, Socié and colleagues (page 50) and Meignin and colleagues (page 360) address the role of both cytokine-mediated inflammation and early epithelial chimerism in human gastrointestinal GvHD. In a careful and unique analysis of gastrointestinal biopsies obtained early after allogeneic transplantation, the authors searched for a prognostic role of tumor necrosis factor α (TNFα) and Fas expression as well as a variety of parameters characterizing the cellular infiltrate. The same series of biopsies was then used to evaluate early epithelial chimerism. With these studies, they extend their first report on the high incidence of intestinal GvHD, which revealed activated eosinophils as a hallmark of GvHD.1
In their new study, Socié et al address further partners of the inflammatory cascade: while TNF expression in the lamina propria was highly characteristic of GvHD, the number of infiltrating neutrophils and a high percentage of apoptotic bodies predicted early and 1-year transplantation-related mortality even more precisely than a set of clinical parameters. These data point not only to relevance of early diagnostic biopsies obtained prior to any immunosuppressive intervention but confirm experimental data demonstrating a central role of an orchestrated inflammatory response in the gut for the pathophysiology of human GvHD.2 The data support the concept that cells of the innate immune system rather than T lymphocytes play a major role in the effector phase of GvHD. Although Socié and colleagues could not confirm earlier reports3 on a significant percentage of donor cells replacing damaged epithelia, a larger series of biopsies with a longer follow-up is needed to answer the questions on stem cell plasticity, which would allow repair of epithelia by pluripotent donor stem cells.
Should we continue to focus on these time-consuming and labor-intensive translational studies in patients? The answer is clearly positive as new approaches such as nonmyeloablative conditioning will postpone but not abrogate the problem of gastrointestinal GvHD.4 Furthermore, there is at least experimental evidence that gastrointestinal GvHD is the key to alloreaction: knock-out of chemokines or adhesion molecules attracting T cells to Peyer patches almost completely abrogated overall GvHD mortality.5 Understanding intestinal inflammation as a major player in GvHD might even open new avenues in the future to separate detrimental GvHD from graft-versus-leukemia reactions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal