Abstract
Monocytes and macrophages participate in a wide variety of host defense mechanisms. Annexin II, a fibrinolytic receptor, binds plasminogen and tissue plasminogen activator (t-PA) independently at the cell surface, thereby enhancing the catalytic efficiency of plasmin production. We demonstrated previously that annexin II on the surface of both cultured monocytoid cells and monocyte-derived macrophages promotes their ability to remodel extracellular matrix. Here, we demonstrate that human peripheral blood monocytes represent the major circulating annexin II–expressing cell. Annexin II supported t-PA–dependent generation of cell surface plasmin and the matrix-penetrating activity of human monocytes. Compared to polymorphonuclear leukocytes, monocytes supported a 12.9-fold greater rate of plasmin generation in the presence of exogenous t-PA, and this activity was largely attributable to annexin II. Likewise, anti–annexin II IgG directed against the t-PA–binding tail domain inhibited plasminogen-dependent, cytokine-directed monocyte migration through extracellular matrix. On differentiation of monocytes to macrophages, there was a 2.4-fold increase in annexin II–specific mRNA, and a 7.9-fold increase in surface annexin II. Thioglycolate-elicited peritoneal macrophages, furthermore, displayed an additional 3.8-fold increase in annexin II surface expression compared with resident cells. Thus, annexin II–mediated assembly of plasminogen and t-PA on monocyte/macrophages contributes to plasmin generation, matrix remodeling, and directed migration.
Introduction
Annexin II belongs to a family of widely distributed, phospholipid-binding, calcium-regulated, peripheral membrane proteins known as the annexins.1 Annexin II is richly expressed in the epithelial cells of intestine and lung and is also found on the cell surface of vascular endothelial cells, myelomonocytic leukemia cells,2 and cells of several monocyte-like lines.3 Through the expression of independent binding sites for both plasminogen and tissue plasminogen activator (t-PA), annexin II assembles these 2 proteins on the cell surface, thereby accelerating the production of plasmin.4 Kinetic studies indicate that annexin II enhances the catalytic efficiency (kcat/Km) of t-PA–dependent plasminogen activation by 60-fold,5 suggesting that endothelial cell surface generation of plasmin likely contributes to fibrinolytic surveillance, thereby promoting blood vessel patency.
Monocytes and macrophages are large, motile, phagocytic cells that have a wide range of biosynthetic and secretory activities depending on their exposure to local stimuli and mediators.6 They participate routinely in host defense mechanisms, including antigen presentation and clearance of debris, and contribute critically to acute and chronic inflammatory responses, pathogen clearance, and wound healing.7,8 In many pathologic processes, such as atherosclerosis or disorders of lipid metabolism, furthermore, lipid-laden macrophages accumulate in tissues.9 The monocyte/macrophage, moreover, may play a role in tumor progression, through elaboration of matrix metalloproteinase 9 (MMP-9),10 and through its participation in neovessel development.11 All of these functions may depend on the ability of the monocyte to regulate and localize proteolytic activity at the cell surface.
Assembly of plasminogen activators on cell surfaces not only localizes them to a specific microenvironment but also augments their catalytic efficiency and protects them from inhibitors.1 Much previous work, conducted mainly with cultured cell lines, has demonstrated that cells of the monocyte lineage have the capacity to bind plasminogen, urokinase, and t-PA.3,12,13 Additional studies show that monocytoid cells can not only modulate the density of their receptors for plasminogen and t-PA,14-16 but they also synthesize t-PA on stimulation by inflammatory mediators such as lipopolysaccharide, interleukin 4 (IL-4), and interferon γ (IFN-γ).17,18 Indeed, in coronary atherosclerosis, t-PA is expressed in areas infiltrated by CD68+ macrophage-like cells,19 and, macrophage-like cells found within abdominal aortic aneurysm lesions express t-PA.20 In the central nervous system, macrophage-like microglia up-regulate and release t-PA in response to excitotoxic injury.21 Thus, the t-PA–controlled arm of the fibrinolytic system may be activated in specific instances of macrophage recruitment and excitation.
We previously reported that annexin II is a major plasminogen-binding site that regulates the migratory activity of monocytoid cells in vitro.3 In the present study, we asked whether annexin II expressed on human peripheral blood monocytes and their derived macrophages supports the t-PA–dependent generation of plasmin. Additionally, we asked whether annexin II–dependent plasmin contributes to monocyte/macrophage-directed migration.
Materials and methods
Isolation of human leukocyte fractions
According to a protocol approved by the Institutional Review Board, human peripheral blood mononuclear cells (PBMCs) were isolated from healthy volunteers and from surplus buffy coat samples (New York Blood Center, New York, NY) less than 24 hours after donation. Whole blood collected in 3.2% sodium citrate and buffy coat samples were diluted in a ratio of 1:1 with monocyte buffer (phosphate buffered saline [PBS] supplemented with 0.5% bovine serum albumin and 1.5% citrate dextrose phosphate), and the PBMCs were isolated via density gradient centrifugation through Ficoll-Paque Plus (Amersham Pharmacia Biotech, Piscataway, NJ).22,23 The white cell layer was carefully pipetted from the surface of the separation medium, resuspended in 50 mL monocyte buffer to remove residual medium, and centrifuged at 250g for 10 minutes. The supernatant was removed and this process repeated 2 times.24 Monocytes were enriched to 97% to 99% purity by magnetic sorting (Miltenyi Biotec, Auburn, CA) whereby PBMCs were incubated with monoclonal CD14 IgG-conjugated super-paramagnetic microbeads (20 μL/1 × 107 cells, 15 minutes, 4°C), and then applied to a pre-equilibrated LS selection column positioned within a MidiMACS magnet (Miltenyi Biotec, Auburn, CA). The column was washed with 12 mL monocyte buffer, gently removed from the magnet, and then eluted with monocyte buffer.25,26 Trypan blue exclusion of eluted cells was routinely 99%.
Polymorphonuclear (PMN) cells were isolated from 50 mL whole blood collected from healthy donors. Whole blood anticoagulated with sodium citrate was diluted in a ratio of 1:1 with monocyte buffer and centrifuged (200g, 15 minutes). The pellet was resuspended to a volume of 70 mL in 0.9% NaCl and erythrocytes removed via sedimentation through 3.5% dextran (21°C, 45 minutes). The leukocyte layer was collected and centrifuged (300g, 4°C, 10 minutes), and the cell pellet resuspended in 7.5 mL 0.9% NaCl. Following Ficoll gradient centrifugation as described, the supernatant was aspirated, and the remaining erythrocytes removed via hypotonic lysis whereby the 300g cell pellet was resuspended in 6 mL distilled water that was supplemented with 3.5% NaCl after 30 seconds. The cells were then washed once in 40 mL monocyte buffer (300g, 4°C, 10 minutes), and the cell pellet, consisting of 99% PMN cells by Wright stain, was collected and washed twice in monocyte buffer.
Human monocyte–derived macrophage culture
Human macrophages were derived from peripheral blood monocytes via differential adherence.23,24,27,28 PBMCs were suspended in monocyte complete medium (RPMI containing 10% human AB serum, 1% l-glutamine, 1% penicillin, and 1% streptomycin), and plated onto plastic 100-mm Falcon dishes. The cells were incubated (12 hours, 37°C), and subsequently washed with monocyte complete medium to remove nonadherent cells. The adherent cells were maintained in culture for 4 to 18 days, changing the medium every 2 to 3 days.
For preparation of cell lysates, cells were harvested at specific time intervals by gentle trituration into calciumand magnesium-free HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered saline (HBS; 37°C). The cells were washed with calciumand magnesium-free HBS and centrifuged (16 000g, 10 minutes), and the cell pellet stored at –80°C. On the day of the experiment, cells were thawed on ice and cell lysates prepared as previously described.29
Fluorescence-activated cell sorting
Fluorescence-activated cell sorting (FACS) was performed using 5 × 105 monocytes and 5 × 105 neutrophils obtained from healthy donors. Cells were incubated with fluorescein isothiocyanate (FITC)–conjugated monoclonal IgG directed against annexin II (Transduction Laboratories, Lexington, KY), phycoerythrin (PE)–conjugated monoclonal IgG directed against CD14 (PharMingen, San Diego, CA), or appropriately conjugated isotype-matched controls (each 50 μg/mL PBS; 1 hour, 4°C), and then washed twice with PBS. Fluorescence data were acquired via a Becton Dickinson FACScalibur, and analyzed with Cell Quest software (BD Biosciences, Bedford, MA).
Western blotting
To estimate expression of cell surface and cytosolic annexin II in monocytes, nonmonocyte PBMCs, and macrophages, EGTA (ethylene glycol tetraacetic acid) elution of calcium-dependent cell surface proteins was carried out as previously described.3,29,30 Briefly, macrophages (1 × 106) were washed twice in 1 mL HBS and then incubated in 300 μL HBS containing 10 mM EGTA (37°C, 30 minutes). The supernatant was collected after centrifugation (500g, 5 minutes). In all eluate samples, release of lactate dehydrogenase (LDH), an index of cell lysis, was monitored and found to be undetectable.31
In addition, cell surface biotinylation was performed. Cells were incubated with the non–cell membrane-permeable reagent sulfosuccinimidyl-2-(biotinamido)-ethyl-1,3-dithioproprionate (0.5 mg/mL, 20 minutes, 4°C, sulfo-NHS-SS-biotin; Pierce, Rockford, IL), washed, and then lysed in lysis buffer (20 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, 150 mM NaCl, 2 mM EDTA [ethylenediaminetetraacetic acid], 10% glycerol, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, pH 8.0; 4°C, 30 minutes).32 Biotinylated proteins were separated from cytosolic proteins by precipitation with streptavidin-conjugated Sepharose beads (4°C, 1 hour).
After 4 washes, bead-associated proteins were released into Laemmli sample buffer, and resolved by 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) as previously described.33 Following transfer to nitrocellulose membranes, annexin II was detected using monoclonal anti–annexin II IgG (0.125 μg/mL; Zymed, South San Francisco, CA). CD71 was detected using monoclonal anti-CD71 IgG (0.25 μg/mL; PharMingen), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), with monoclonal anti-GAPDH IgG (8 μg/mL; Biodesign International, Saco, ME). Primary antibodies were followed with horseradish peroxidase (HRP)–conjugated ovine antirabbit or antimouse IgG (Amersham Pharmacia Biotech). Bound HRP was visualized using the enhanced chemiluminescence (ECL) kit (Amersham Pharmacia Biotech).
Plasmin generation assays
Cells (1 × 106/well) were suspended in 1 mL incubation buffer (IB; HBS containing 3 mM CaCl2, 1 mM MgCl2) and incubated with 170 nM N-terminal glutamic acid plasminogen (American Diagnostica, Stamford, CT; 1 hour, 4°C). Cells were washed twice with IB, incubated with either rabbit polyclonal anti–annexin II directed against the t-PA–binding “tail” peptide (MSTVHEILKLSLEGD; Covance Research Products, Princeton, NJ) or preimmune rabbit IgG (each 60 μg/mL, 45 minutes, 4°C), and then washed again in IB. Both t-PA (12 nM) and AFC-81 (d-valine-leucine-lysine-7-amino-4-trifluoromethyl coumarin; Enzyme Systems Products, Livermore, CA; 166 μM), a fluorogenic plasmin substrate, were added, and substrate hydrolysis measured at 5-minute intervals as relative fluorescent units (RFUs) at 400 nm excitation and 505 nm emission in a SpectroMax Gemini XS fluorescence spectrophotometer (Molecular Devices, Sunnyvale, CA), as previously described.34 Initial rates of plasmin generation were calculated using linear regression analysis of plots of RFU versus time squared as described.35
Matrix invasion assays
Human peripheral blood monocytes (5 × 104) were suspended in monocyte serum-free medium (MSFM; Gibco, Carlsbad, CA), and added to porous (8-μm) tissue culture inserts coated with Matrigel (BD Biosciences) as previously described.3,34 Cells were preincubated with or without rabbit polyclonal anti–annexin II IgG or preimmune rabbit IgG (each 40 μg/mL; 4°C, 45 minutes) prior to plating in tissue culture inserts. Matrix-coated inserts were placed in a 12-well plate containing MSFM supplemented with 50 ng/mL monocyte chemoattractant protein-1 (MCP-1; R&D Systems, Minneapolis, MN). Plasminogen (1 μg/mL) was added to appropriate inserts and cells incubated (18 hour, 37°C). Cells that migrated to the lower well were enumerated by 2 independent observers by counting cells within 5 fields at × 40 magnification. Monocyte migration was confirmed by the DNA Fluororeporter system (Molecular Probes, Eugene, OR).
RNA preparation and Northern blotting
Total RNA was extracted from freshly isolated human peripheral blood monocytes (day 0) and human monocyte-derived macrophages (HMDMs; day 15) using Trizol reagent (Life Technologies, Bethesda, MD). Equal amounts of RNA were resolved on 1% agarose gels, transferred to ζ-probe membranes (Bio-Rad, Hercules, CA),2 and hybridized to a 32P-labeled human annexin II probe in QuickHyb solution (Stratagene, La Jolla, CA). The probe was generated by random-prime labeling (Roche Molecular Biochemicals, Indianapolis, IN), using a 300–base pair (bp) human annexin II tail-specific cDNA as template as previously described.34 Autoradiograms were scanned and digitized using Adobe Photoshop and analyzed by densitometry using the Scion Image (Beta-3 version) software package (Scion, Frederick, MD).
Peritoneal macrophage isolation and Western blot analysis
Thioglycolate-elicited peritoneal macrophages were obtained from Swiss Webster mice as described previously.36 Mice were given intraperitoneal injections of 3 mL 3% Brewer thioglycolate medium containing 0.3 mM thioglycolate (Difco, Detroit, MI). Four days later, cells were harvested by lavage with cold Dulbecco PBS (DPBS). Peritoneal cells were recovered by centrifugation, resuspended in RPMI containing 10% fetal bovine serum (FBS), and plated in appropriate wells. After 2 hours of cell adherence, nonadherent cells were washed free.
Resident and thioglycolate-elicited macrophages were lysed in Tris-buffered saline containing 1% Triton X-100 (TTBS), aprotonin (1 μg/mL), EDTA (0.5 mg/mL), and leupeptin (0.5 μg/mL) for 10 minutes at 4°C. The lysates were centrifuged (14 000g, 10 minutes, 4°C), and the supernatant mixed with 6 × SDS sample buffer and boiled for 5 minutes under nonreducing conditions. For Western blotting, equal amounts of lysate proteins were electrophoresed in 4% to 15% polyacrylamide gradient gels and transferred to polyvinylidene difluoride (PVDF) membranes.3 The membrane was blocked in TTBS containing 5% dry defatted milk (1 hour), washed twice (TTBS), and incubated 1 hour with 0.5 μg/mL monoclonal anti–human annexin II IgG (clone 5, IgG1; Transduction Laboratories) in TTBS containing 3% dry defatted milk. The membrane was washed twice (TTBS), incubated 1 hour in TTBS containing 3% dry defatted milk and biotinylated rabbit anti–mouse IgG (1:30 000; Pierce), washed again twice (TTBS), and incubated with preformed avidin-biotin–HRP complexes (Pierce) in DPBS/0.1% Tween-20 for 1 hour. Bound HRP was visualized using the ECL kit (Amersham Pharmacia Biotech).
Results
Human monocytes express cell surface annexin II
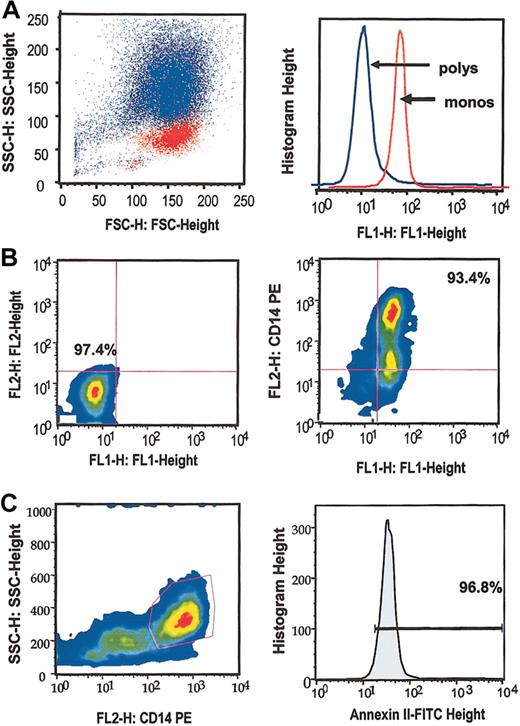
In addition to conventional Wright staining, flow cytometric analyses verified that isolated monocyte and PMN leukocyte cell fractions were at least 96% pure (Figure 1A left panel). In addition, we noted that 92.3% ± 1.3% (SEM, n = 4) of cells in the monocyte fraction, but only 6.2% ± 2.3% (SEM, n = 4) of cells in the PMN fraction were positive for cell surface annexin II (Figure 1A right panel). Whereas the monocyte fraction did not react with isotype-matched control antibodies (Figure 1B left panel), nearly all (96.3% ± 2.2%, SEM, n = 4) cells positive for the monocyte marker CD14 were also positive for annexin II (Figure 1B right panel). Finally, 96.3% ± 1.1% (SEM, n = 4) of CD14+ cells (Figure 1C left panel) were also positive for cell surface annexin II (Figure 1C right panel). These data indicate that essentially all isolated peripheral blood monocytes expressed cell surface annexin II.
Flow cytometric analysis. (A) Superimposed scatter plots (left panel) demonstrate discrete populations of isolated monocytes (red) and PMN neutrophils (blue) by analysis of side scatter (SSC) versus forward scatter (FSC). Histogram depicts cell surface annexin II expression by monocytes (red) and neutrophils (blue) as FL1 fluorescence (right panel). (B) Scatter plot of FL1 (FITC) versus FL2 (PE) fluorescence on human monocytes. Both isotype controls (left panel) and annexin II-FITC and CD14-PE signals (right panel) are shown. (C) Plot of CD14 positivity versus SSC (left panel). Histogram shows annexin II expression (FL1) among CD14+ cells (right panel).
Flow cytometric analysis. (A) Superimposed scatter plots (left panel) demonstrate discrete populations of isolated monocytes (red) and PMN neutrophils (blue) by analysis of side scatter (SSC) versus forward scatter (FSC). Histogram depicts cell surface annexin II expression by monocytes (red) and neutrophils (blue) as FL1 fluorescence (right panel). (B) Scatter plot of FL1 (FITC) versus FL2 (PE) fluorescence on human monocytes. Both isotype controls (left panel) and annexin II-FITC and CD14-PE signals (right panel) are shown. (C) Plot of CD14 positivity versus SSC (left panel). Histogram shows annexin II expression (FL1) among CD14+ cells (right panel).
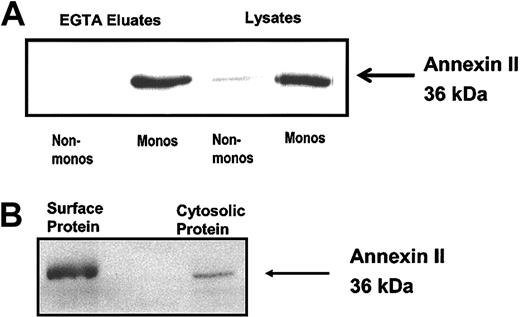
Western blot analysis of cell surface EGTA eluates and wholecell lysates confirmed monocyte expression of annexin II on the cell surface and in the cytosol, whereas equivalent numbers of monocyte-depleted PBMCs showed minimal cytosolic, and no cell surface, annexin II (Figure 2A). Similarly, when monocyte cell surface proteins were biotinylated using a membrane-impermeable reagent, a protein comigrating with authentic annexin II and reacting specifically with anti–annexin II IgG was recovered in the pool of streptavidin-precipitated proteins (Figure 2B). Together these experiments revealed that the monocyte, rather than the PMN neutrophil or a nonmonocyte mononuclear cell, is the major annexin II–expressing leukocyte in human peripheral blood.
Peripheral blood monocyte expression of annexin II. (A) Western blot analysis of cell surface expression of annexin II on human monocyte-depleted PBMCs (Non-monos) and monocytes (Monos). EGTA eluates or whole cell lysates (30 μg) were resolved on a 12.5% SDS-polyacrylamide gel and immunoblotted with anti–annexin II IgG. (B) Cell surface biotinylation of surface proteins on human monocytes. Surface biotinylated proteins were isolated by streptavidin precipitation, resolved on a 12.5% SDS-polyacrylamide gel, and immunoblotted with anti–annexin II IgG. Whole cell lysate protein was used as a positive control.
Peripheral blood monocyte expression of annexin II. (A) Western blot analysis of cell surface expression of annexin II on human monocyte-depleted PBMCs (Non-monos) and monocytes (Monos). EGTA eluates or whole cell lysates (30 μg) were resolved on a 12.5% SDS-polyacrylamide gel and immunoblotted with anti–annexin II IgG. (B) Cell surface biotinylation of surface proteins on human monocytes. Surface biotinylated proteins were isolated by streptavidin precipitation, resolved on a 12.5% SDS-polyacrylamide gel, and immunoblotted with anti–annexin II IgG. Whole cell lysate protein was used as a positive control.
Annexin II promotes plasminogen activation on human monocytes
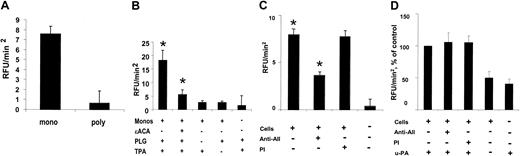
To examine the role of annexin II in monocyte-related fibrinolytic activity in the presence of t-PA, a fluorogenic assay of plasminogen activation was used. In these experiments, human monocytes supported a 12.7-fold greater rate of t-PA–dependent plasmin generation than human PMN cells (7.6 ± 0.8 versus 0.6 ± 1.2 RFU/min2, SE, n = 5, P < .003; Figure 3A). Plasmin generation, furthermore, was cell, plasminogen, and t-PA dependent, and was inhibited by 70.6% in the presence of the lysine analog, ϵ-aminocaproic acid (17.0 ± 3.0 versus 5.0 ± 1.0 RFU/min2, SE, n = 3, *P < .04; Figure 3B). Polyclonal IgG directed against the annexin II tail peptide, the putative t-PA–binding site,37 specifically inhibited t-PA–dependent plasmin generation at the monocyte cell surface by 55.0% (8.0 ± 0.5 versus 3.6 ± 0.3 RFU/min2, SE, n = 3, *P < .003), whereas no inhibition was seen in the presence of preimmune rabbit IgG (Figure 3C). In contrast, anti–annexin II–tail peptide IgG had no effect on urokinase plasminogen activator (u-PA)–dependent plasmin generation by human monocytes (Figure 3D). Thus, although nonannexin-binding sites may exist, cell surface annexin II contributes significantly to t-PA–dependent, cell-specific plasmin generation at the human monocyte cell surface.
Monocyte plasmin generation. (A) Comparison of t-PA–dependent plasmin generated in the presence of suspended human peripheral blood monocytes (mono) and polymorphonuclear neutrophils (poly; 1 × 106 cells/sample). Data collected over 90 minutes are shown as mean relative fluorescence units per minute squared (RFU/min2) ± SE, n = 5, P < .003. (B) Effect of ϵ-aminocaproic (ϵACA) on t-PA–dependent plasmin generation in the presence of human monocytes (Monos). Monocytes (1 × 106) were incubated with or without ϵ-ACA (10 mM) in the presence of plasminogen (PLG; 170 nM) and t-PA (TPA; 12 nM). Shown are mean values ± SE, n = 3; *P < .04. (C) Effect of anti–annexin II IgG (60 μg/mL) on the t-PA–dependent generation of plasmin by monocytes. Cells were incubated in the presence of anti–annexin II polyclonal IgG directed against the t-PA–binding site (Anti-AII) or preimmune rabbit IgG (PI), as described in “Materials and methods.” Shown are mean values ± SE, n = 3; *P < .003. (D) Effect of anti–annexin II IgG (Anti-AII) versus preimmune IgG (PI) on u-PA–dependent plasmin generation by human monocytes. Shown are mean RFU/min2 values expressed as percent of control (± SE, n = 3).
Monocyte plasmin generation. (A) Comparison of t-PA–dependent plasmin generated in the presence of suspended human peripheral blood monocytes (mono) and polymorphonuclear neutrophils (poly; 1 × 106 cells/sample). Data collected over 90 minutes are shown as mean relative fluorescence units per minute squared (RFU/min2) ± SE, n = 5, P < .003. (B) Effect of ϵ-aminocaproic (ϵACA) on t-PA–dependent plasmin generation in the presence of human monocytes (Monos). Monocytes (1 × 106) were incubated with or without ϵ-ACA (10 mM) in the presence of plasminogen (PLG; 170 nM) and t-PA (TPA; 12 nM). Shown are mean values ± SE, n = 3; *P < .04. (C) Effect of anti–annexin II IgG (60 μg/mL) on the t-PA–dependent generation of plasmin by monocytes. Cells were incubated in the presence of anti–annexin II polyclonal IgG directed against the t-PA–binding site (Anti-AII) or preimmune rabbit IgG (PI), as described in “Materials and methods.” Shown are mean values ± SE, n = 3; *P < .003. (D) Effect of anti–annexin II IgG (Anti-AII) versus preimmune IgG (PI) on u-PA–dependent plasmin generation by human monocytes. Shown are mean RFU/min2 values expressed as percent of control (± SE, n = 3).
Anti–annexin II IgG inhibits human monocyte invasion through extracellular matrix
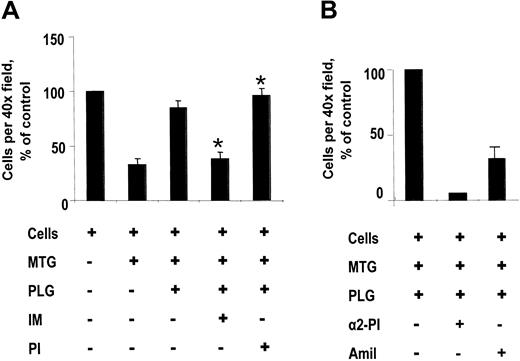
We used a modified Boyden chamber system to assess the role of annexin II in human monocyte migration through a matrix barrier (Matrigel) in response to MCP-1 (Figure 4). Because Matrigel is a source of t-PA, we did not add any additional exogenous plasminogen activator in these experiments. In the absence of any chemoattractant agent, there was minimal migration of monocytes to the lower well.38 In response to MCP-1, 75.1% ± 12.1% (SEM, n = 5) of monocytes plated migrated through uncoated wells into the lower chamber. Furthermore, although MCP-1–directed monocyte migration was significantly inhibited in the presence of Matrigel, an extract of murine tumor basement membrane composed primarily of laminin,39 this blockade was reversed on addition of plasminogen to the upper well (Figure 4A). Preincubation of human monocytes with IgG directed against the t-PA–binding “tail” peptide, but not preimmune IgG, furthermore, also inhibited monocyte migration by 60% ± 5% (mean ± SE, n = 4, *P < .01). Addition of α2-plasmin inhibitor (50 μg/mL), furthermore, completely blocked plasminogen-dependent monocyte migration to the lower chamber, whereas addition of amiloride (10 μM), a specific urokinase inhibitor,40 blocked 62% to 80% of migration to the lower chamber (Figure 4B). These data indicate that plasminogen-dependent, MCP-1–directed monocyte migration through Matrigel was, in part, dependent on annexin II, required plasmin proteolytic activity, and may be driven by u-PA in the absence of sufficient exogenous t-PA.
MCP-1–directed human monocyte migration. (A) Effect of anti–annexin II IgG. Monocytes (5 × 105) were added to porous (8-μm) polycarbonate tissue culture inserts coated with Matrigel (MTG), after being incubated (45 minutes, 4°C) in the presence or absence of anti–annexin II tail peptide IgG (IM; 40 μg/mL; ± SE, n = 4), preimmune rabbit IgG (PI; 40 μg/mL; ± SE, n = 4). After 18 hours, plasminogen (PLG)–dependent migration in response to MCP-1 was expressed as percent of cells migrated under control conditions per × 40 field (*P < .01). (B) Effect of protease inhibitors. Monocytes (1 × 105) were added to Matrigel-coated porous inserts in the presence or absence of amiloride (10 μM; Amil; ± range, n = 2) or α2-plasmin inhibitor (50 μg/mL; α2-PI; ± range, n = 2). After 18 hours, cell migration in response to MCP-1 was expressed as percent of control.
MCP-1–directed human monocyte migration. (A) Effect of anti–annexin II IgG. Monocytes (5 × 105) were added to porous (8-μm) polycarbonate tissue culture inserts coated with Matrigel (MTG), after being incubated (45 minutes, 4°C) in the presence or absence of anti–annexin II tail peptide IgG (IM; 40 μg/mL; ± SE, n = 4), preimmune rabbit IgG (PI; 40 μg/mL; ± SE, n = 4). After 18 hours, plasminogen (PLG)–dependent migration in response to MCP-1 was expressed as percent of cells migrated under control conditions per × 40 field (*P < .01). (B) Effect of protease inhibitors. Monocytes (1 × 105) were added to Matrigel-coated porous inserts in the presence or absence of amiloride (10 μM; Amil; ± range, n = 2) or α2-plasmin inhibitor (50 μg/mL; α2-PI; ± range, n = 2). After 18 hours, cell migration in response to MCP-1 was expressed as percent of control.
Annexin II expression is enhanced on monocyte differentiation
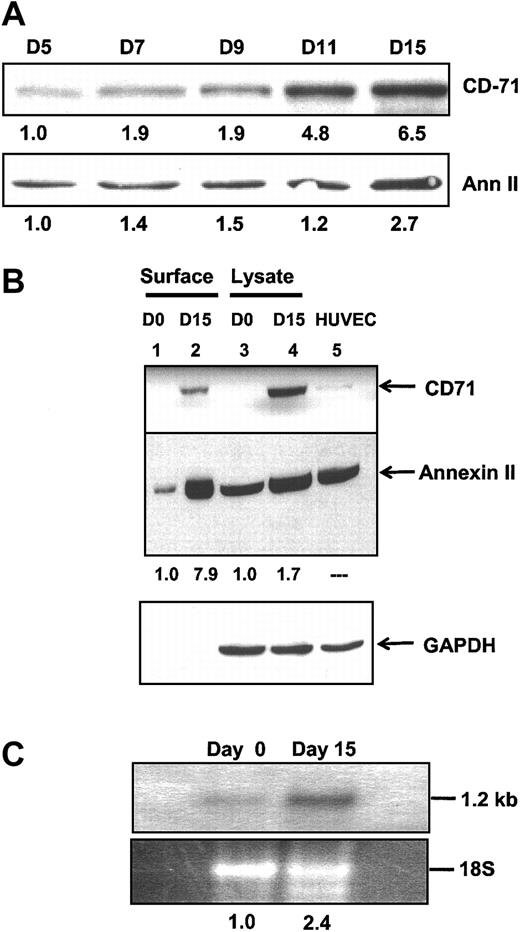
To further clarify the potential role of annexin II in monocyte/macrophage function, HMDMs were maintained in culture and harvested at selected time points. Whole HMDM lysates were subjected to SDS-PAGE under reducing conditions and probed for expression of annexin II and CD71, a macrophage marker not expressed on monocytes (Figure 5A).41,42 CD71 was expressed by monocyte/macrophages in only trace amounts on day 5, but increased 6.5-fold in intensity by day 15. At the same time, there was a 2.7-fold increase in total cellular annexin II. When cell surface proteins were examined by Western blot of surface biotinylated proteins (Figure 5B), we noted a 7.9-fold increase in annexin II cell surface expression between day 0 and day 15 (Figure 5B lanes 1 and 2) and a 1.7-fold increase in cytosolic annexin II (Figure 5B lanes 3 and 4). Finally, Northern blot analysis of total monocyte/macrophage mRNA on day 0 and day 15 revealed a 2.4-fold increase in steady-state levels of annexin II mRNA compared to 18S rRNA used as a loading control (Figure 5C). These data indicated that expression of both annexin II mRNA and annexin II protein was amplified on monocyte differentiation; in addition, cell surface expression of annexin II was preferentially increased over total cell expression.
Annexin II expression by HMDMs. (A) Western blot analysis of whole cell lysates of HMDMs maintained in culture and harvested at the time intervals shown. Blots were probed with anti-CD71 IgG, a macrophage-specific marker and monoclonal anti–annexin II IgG. The level of expression relative to day 5 is shown beneath each band. (B) Western blot analysis of annexin II expression by monocytes and HMDMs. Either biotinylated cell surface proteins (lanes 1 and 2) or whole cell lysates (25 μg, lanes3 and 4) from peripheral blood monocytes on day 0 (lanes 1 and 3) or from HMDMs on day 15 (lanes 2 and 4) were examined by immunoblotting with anti-CD71, anti–annexin II, and anti-GAPDH IgG. Human umbilical vein endothelial cell (HUVEC) lysate (lane 5) served as a positive control. Cell surface biotinylated proteins and whole cell lysates were prepared as described in “Materials and methods.” The level of monocyte/macrophage expression of annexin II relative to day 0 is shown for each lane. (C) Northern blot. Total RNA from freshly harvested monocytes (day 0) or from same-donor HMDMs allowed to differentiate in culture for 2 weeks (day 15) was extracted, resolved by agarose gel electrophoresis, transferred to ζ-probe membranes, and incubated with a 32P-random-primed annexin II cDNA probe. The relative intensities of the radiographic annexin II–specific band and the corresponding ethidium bromide–stained 18S rRNA band are shown below each lane.
Annexin II expression by HMDMs. (A) Western blot analysis of whole cell lysates of HMDMs maintained in culture and harvested at the time intervals shown. Blots were probed with anti-CD71 IgG, a macrophage-specific marker and monoclonal anti–annexin II IgG. The level of expression relative to day 5 is shown beneath each band. (B) Western blot analysis of annexin II expression by monocytes and HMDMs. Either biotinylated cell surface proteins (lanes 1 and 2) or whole cell lysates (25 μg, lanes3 and 4) from peripheral blood monocytes on day 0 (lanes 1 and 3) or from HMDMs on day 15 (lanes 2 and 4) were examined by immunoblotting with anti-CD71, anti–annexin II, and anti-GAPDH IgG. Human umbilical vein endothelial cell (HUVEC) lysate (lane 5) served as a positive control. Cell surface biotinylated proteins and whole cell lysates were prepared as described in “Materials and methods.” The level of monocyte/macrophage expression of annexin II relative to day 0 is shown for each lane. (C) Northern blot. Total RNA from freshly harvested monocytes (day 0) or from same-donor HMDMs allowed to differentiate in culture for 2 weeks (day 15) was extracted, resolved by agarose gel electrophoresis, transferred to ζ-probe membranes, and incubated with a 32P-random-primed annexin II cDNA probe. The relative intensities of the radiographic annexin II–specific band and the corresponding ethidium bromide–stained 18S rRNA band are shown below each lane.
Thioglycolate-elicited macrophages up-regulate annexin II expression
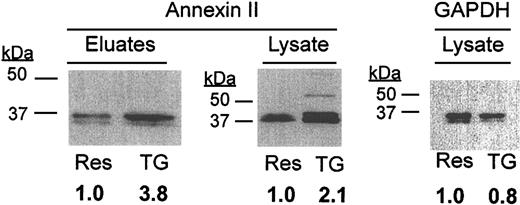
To determine whether the t-PA/annexin II system might be modulated in macrophages in vivo, we studied expression of annexin II by mouse peritoneal cells isolated from Swiss Webster mice (Figure 6). Western blot analysis revealed that whereas resident macrophages expressed annexin II in both cell surface eluates and whole cell lysates, there was a 3.8-and 2.1-fold increase, respectively, in each compartment on stimulation with thioglycolate. This increase was not reflective of increased protein loading, as judged by anti-GAPDH staining of the lysate blot, and by protein staining of the surface eluate blot (not shown). These data suggest that murine peritoneal macrophages up-regulate annexin II on activation by inflammatory mediators.
Analysis of annexin II expression in murine peritoneal macrophages. Cell surface eluates (45 μg/lane) or whole cell lysates (30 μg/lane) of resident (Res) and thioglycolate-elicited (TG) macrophages were analyzed by Western blot for annexin II and GAPDH (monoclonal anti-GAPDH; Biodesign International). TG cell expression relative to Res cells is shown below each lane.
Analysis of annexin II expression in murine peritoneal macrophages. Cell surface eluates (45 μg/lane) or whole cell lysates (30 μg/lane) of resident (Res) and thioglycolate-elicited (TG) macrophages were analyzed by Western blot for annexin II and GAPDH (monoclonal anti-GAPDH; Biodesign International). TG cell expression relative to Res cells is shown below each lane.
Discussion
In this study, we show for the first time that the human monocyte represents the major annexin II–expressing cell in flowing blood (Figures 1-2). We show, further, that annexin II mediates t-PA–dependent plasmin generation in the presence of human peripheral blood monocytes (Figure 3). If we assume that cell surface annexin II is kinetically equivalent to soluble annexin II, and that t-PA cofactor activity relates only to annexin II, an “effective concentration” of a 1 × 106/mL monocyte suspension can be estimated at about 1.3 nM soluble annexin II, whereas the same concentration of endothelial cells or acute promyelocytic leukemia (APL) cells represents about 1.6 nM and about 65 nM annexin II, respectively. These data suggest that monocyte cell surface annexin II is highly active, and could, indeed, promote peripheral blood monocyte migration through a reconstituted basement membrane barrier as indicated in Figure 4.
In addition, we demonstrate that expression of annexin II is increased when monocytes differentiate into human monocyte-derived macrophages (Figure 5). In addition to a doubling of total cellular annexin II, densitometric analysis of anti–annexin II immunoreactive bands indicates that monocyte cell surface annexin II was about 1.3% of total cellular annexin II, whereas macrophage cell surface annexin II was about 6.3% of the total. This value is similar to that estimated for endothelial cells and APL cells in previous studies (∼4% and ∼5%, respectively).2,29 Therefore, of the human cell types analyzed, HMDMs express relatively high levels of cell surface annexin II.
Finally, our data indicate that macrophages recruited to the peritoneal cavity following administration of thioglycolate expressed higher levels of annexin II than unstimulated macrophages, especially at the cell surface (Figure 6). These data suggest that monocyte-differentiating agents such as inflammatory cytokines may further enhance either overall expression of annexin II or its transport to the cell surface, or both. Our unpublished data (April 2000), however, indicate no effect of macrophage colony-stimulating factor, transforming growth factor β (TGF-β), or IFN-γ on either plasmin binding to HMDMs or the level of annexin II expression by a murine macrophage cell line. Together, these data suggest that annexin II plays an important role in human macrophage/monocyte-directed migration and recruitment and that these functions may be regulated by one or more, as yet unidentified, inflammatory mediators.
There is clear evidence that the fibrinolytic proenzyme, plasminogen, plays a central role in inflammatory macrophage recruitment. In plasminogen-deficient mice subjected to electrical injury of the femoral artery, for example, infiltration of CD45+ leukocytes into the blood vessel wall and removal of necrotic debris were drastically impaired.43,44 In addition, 1 week following ligation of the main left coronary artery, influx of macrophages into the resulting myocardial infarct was nearly absent in plasminogen-deficient mice, whereas the response in wild-type mice was exuberant.45 In a model of carotid artery transplant arteriosclerosis, furthermore, macrophages failed to infiltrate the vascular media in plasminogen-deficient, but not wild-type, mice.46 On induction of peritoneal inflammation with thioglycolate, recruitment of monocytes was severely compromised in plasminogen-null mice compared to wild-type animals, whereas influx of PMN neutrophils was essentially normal.47
In many settings, urokinase appears to act as the dominant, constitutive plasminogen activator in plasminogen-dependent macrophage recruitment. In models of mechanical or electrical injury to the femoral artery, for example, macrophage infiltration and clearance of necrotic material were specifically impaired in the urokinase-null mouse.48 When apolipoprotein E (ApoE) deficiency was superimposed on u-PA deficiency, moreover, macrophages failed to infiltrate atherosclerotic lesions and aneurysmal dilatation of the aorta was attenuated.49 Similarly, in experimental myocardial infarction, both infiltration of leukocytes, including macrophages, and subsequent ventricular rupture, were markedly and specifically reduced in u-PA–null mice.50 Deficiency of u-PA reduced glomerular macrophage infiltration in experimental crescentic glomerulonephritis.51 Finally, recruitment of macrophages to lung parenchyma during experimental infection with either Cryptococcus neoformans or Pneumocystis carinii was significantly reduced in u-PA–deficient mice.52,53
Although comparatively less is known about the potential role of t-PA during macrophage recruitment to sites of inflammation, several studies point to a significant role. In experimental monoarticular arthritis in the mouse, for example, t-PA deficiency was associated with reduced joint infiltration by macrophages.54 t-PA is produced by macrophages in the arterial wall in both severe atherosclerosis19 and in abdominal aortic aneurysm.20 In the central nervous system, macrophage-like glial cells appear to express t-PA and use it for activation, migration, and phagocytic activity,55,56 possibly through binding to annexin II.57 t-PA is also required for fibrin clearance by Schwann cells as a prelude to nerve regeneration and remyelination following experimental sciatic nerve crush.58 In addition, strong evidence suggests that t-PA is deposited in extracellular matrices in ovarian, vascular, and periodontal tissues, especially under conditions of inflammation.59-61 Matrix-associated t-PA may be available for capture by invading macrophages.
We have shown previously that immature myelomonocytic cells bind and activate plasminogen in a t-PA– and annexin II–dependent manner.2 In addition, we have demonstrated that differentiation of APL blast cells to more mature neutrophil-like cells under all-trans-retinoic acid, is associated with a loss of annexin II expression. In the present study, we demonstrate that freshly isolated human peripheral blood monocytes and HMDMs, but not mature PMN neutrophils, express cell surface annexin II and support annexin II–dependent, plasmin-mediated matrix invasion in the presence of t-PA. Although human peripheral blood monocytes produce urokinase as a constitutive plasminogen activator,62,63 there is excellent evidence that they can also secrete t-PA when stimulated with a combination of IFN-γ and lipopolysaccharide, or with IL-4 alone.17,18 In addition, human peripheral blood monocytes have been shown to bind t-PA at their surface with a Kd of about 900 nM and a Bmax of about 1.7 × 105 sites/cell,12 and in a manner that preserves the active site of t-PA. Our results show that t-PA–induced plasmin generation and monocyte migration are, in part, annexin II dependent, and indicate that macrophage secretion of t-PA and its annexin II–mediated activation of plasminogen at the cell surface could play an important role in matrix remodeling.
Interestingly, the present studies also show a significant increase in annexin II expression on progression from monocyte to macrophage. Differentiation of macrophages in vitro was associated with a 2to 3-fold increase in both total annexin II protein and steady-state mRNA levels, suggesting that the macrophage differentiation pathway either enhances the rate of annexin II mRNA transcription or prolongs the half-life of the annexin II message. Our data show, further, that expression of annexin II in macrophages is enhanced by inflammatory mediators because thioglycolate-stimulated murine macrophages showed a doubling of whole cell expression over resident macrophages. Although further studies will be necessary to reveal how these processes are regulated, it seems clear that amplification of the annexin II–plasminogen system belongs to the macrophage's recruitment repertoire.
It is intriguing to note that, in the case of the monocyte-to-macrophage transition and in the progression of resting to activated macrophage, the increase in annexin II expression is most striking at the cell surface. Whereas total cell expression increased 2to 3-fold, enhancement at the cell surface was significantly (4to 8-fold) greater. These data suggest not only an increase in synthesis of annexin II, but also the possibility of enhanced mechanisms for transport to, or retention at, the cell surface.
The potential mechanisms by which annexin II–mediated plasmin generation might promote macrophage recruitment and directed migration are manifold. First, plasmin might eliminate physical barriers to macrophage migration by promoting macrophage remodeling of extracellular matrix proteins. These include fibrin, the provisional matrix that forms after injury in most tissues, as well as laminin64,65 and fibronectin.66 Second, plasmin appears to activate MMP-1, MMP-3, and MMP-13 directly, and to activate MMP-2 and MMP-9 indirectly.67 Third, plasmin may release sequestered cytokines, such as fibroblast growth factor-2,68 or activate differentiation factors, such as TGF-β,69,70 which could amplify the inflammatory response. Fourth, plasmin71,72 as well as t-PA,56,73 may provide crucial cellular signals that could modify the macrophage's locomotive, phagocytic, or degradative capabilities. Thus, stimulus-induced synthesis and release of annexin II to the macrophage cell surface could initiate a plasmin-mediated cascade of effects directed at augmenting its recruitment to sites of injury. Such a mechanism could represent an important regulatory step in the macrophage's rapid and adaptive response to tissue injury.
Supported by grants HL 42493, HL 46403, and HL 67839 from the National Heart, Lung and Blood Institute (K.A.H.) and 0150884T from the American Heart Association (D.J.F.). C.B. was supported by a Glorney-Raisbeck Fellowship from the New York Academy of Medicine. A.T.J. was supported by training grant HL 07423 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-04-1304.
We thank Dr Andrew Nicholson for his careful review of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal