Abstract
We analyzed the cause of agammaglobulinemia in a girl whose father had been diagnosed as having X-linked agammaglobulinemia (XLA). Flow cytometric analysis revealed the lack of peripheral B cells with the block of B-cell differentiation in the stages between pro-B cells and pre-B cells in the bone marrow, and the defect of the Bruton tyrosine kinase (BTK) expression on monocytes. We found a BTK gene mutation in the first single base pair of intron 11 in her father and heterozygous mutation in the patient at the site. Sequence analysis of abnormally smaller-sized polymerase chain reaction (PCR) products of cDNA confirmed splicing abnormalities due to the mutation. Maternally derived X chromosome was exclusively inactivated in peripheral blood and oral mucosal cells. This is the first report of female XLA caused by heterozygous BTK gene abnormality and extreme nonrandom inactivation of X chromosome on which normal BTK gene is located.
Introduction
A 10-month-old Japanese girl was admitted to our hospital because of frequent respiratory infections and otitis media during the last 2 months. She was the second child of nonconsanguineous parents. Her father was diagnosed as having X-linked agammaglobulinemia (XLA) at the age of 3 years by recurrent infections, extremely low levels of serum immunoglobulins, and the lack of peripheral B cells. Her mother, elder brother, and maternal and paternal grandmothers were healthy. Serum immunoglobulin G (IgG), IgA, and IgM concentrations were 0.06 g/L (6 mg/dL), 0.01 g/L (1 mg/dL), and 0.05 g/L (5 mg/dL), respectively. Informed consent was obtained, and the study was done in accordance with the ethical standards of the responsible committee on human experimentation (Regional Committee of Ethics for Human Research at the Faculty of Medicine, Kyushu University).
Study design
Three-color flow cytometric analysis was performed for identification of lymphocyte subsets and B-cell precursor cells in bone marrow.1
Each exon of Bruton tyrosine kinase (BTK) gene was amplified by polymerase chain reaction (PCR) as previously described.2 PCR primer pair for intron 11 boundary of BTK was 5′-TAAAAGCAATGAGGCTGTAG-3′ and 5′-AAGTGGGACGGGCACAGCAT-3′. Direct sequencing of PCR products was performed and analyzed by an ABI 310 DNA sequencer (Applied Biosystems, Tokyo, Japan).
Analysis of X-chromosome inactivation was performed as previously described.3 Briefly, an aliquot of DNA was digested with methylation-sensitive enzyme HpaII (Takara Shuzo, Otsu, Japan) and amplified by PCR at exon 1 of androgen receptor gene with specific primers.3 PCR products were run on a GeneGel Excel 12.5 24 Kit (Amersham Pharmacia Biotech, Uppsala, Sweden) and separated by electrophoresis on GenePhor (Amersham Pharmacia Biotech). Gels were silver-stained with Hoefer Automated Gel Stainer (Amersham Pharmacia Biotech).
We searched microsatellite markers in NT_011642 that contains XIST gene. The fluorescence-labeled PCR products were loaded into ABI 3100 DNA Analyzer equipped with Genescan software (Applied Biosystems) for genotyping. The marker alleles were visualized using Genotyper software (Applied Biosystems). Among 108 CA repeats that we found, 7 microsatellite markers showed polymorphism. PCR primers for the microsatellite marker no. 7 were GGAATAGCAAGGGCAGTAAAGC and TTCACTCCCTCCTGGTATGTAC.
Results and discussion
Results
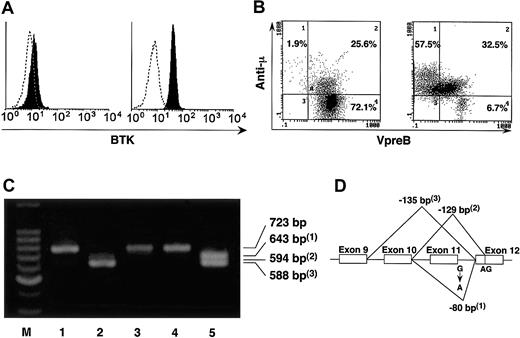
Immunologic characteristics of the patients. Flow cytometric analysis revealed the lack of B cells in peripheral blood (data not shown) and the defect of BTK expression on monocytes of the patient as well as her father (Figure 1A; data not shown). Her mother and paternal grandmother expressed normal levels of BTK without showing bimodal or mosaic pattern of BTK expression (data not shown).4 B-cell differentiation was blocked in the stages between pro- and pre-B cells in bone marrow of the patient (Figure 1B).5 We found a mutation in the first single base pair of intron 11 (G > A) of BTK gene in her father and heterozygous mutation in the patient at the same site (Figure 1D; data not shown). The cDNA amplification between exon 6 and exon 13 of BTK showed abnormally smaller-sized products in the patient and her father (Figure 1C). Sequence analysis of the PCR products revealed splicing abnormality due to the mutation of intron 11 (Figure 1D). No other genetic abnormality was observed in the patient by sequencing all the coding regions of BTK. High-resolution X-chromosome analysis of peripheral blood cells revealed no structural abnormality (data not shown).
Defect of BTK and block of B-cell differentiation. (A) Intracellular staining of BTK was performed and BTK expression was analyzed in peripheral blood CD14+ cells of the girl with agammaglobulinemia (left) and a healthy control (right). (B) The μ chain and VpreB were stained intracellularly. Analysis gate was set on CD19+ and/or VpreB-positive cells. In contrast to the normal B-cell development in the bone marrow of a healthy control (right), maturational block of B-cell precursors occurred and neither VpreB- μ + (low) nor VpreB- μ + (high) cells were observed in the patient (left). (C) The cDNA amplification was performed by PCR between exon 6 and exon 13 of BTK in the family members. Abnormally smaller-sized PCR products (1-3) were observed in the patient (lane 2) and her father (lane 5), although her paternal grandmother (lane 1), her mother (lane 3), and her brother (lane 4) expressed BTK mRNA with normal size. The primer pair was 5′-ATGCTATGGGCTGCCAAATT-3′ and 5′-GGTCCTTTGGATCAATTTCC-3′, and the expected size of the normal PCR product is 723 bp. M indicates marker. (D) The abnormally smaller-sized PCR products (1-3) in panel C were sequenced and found to be the result of splicing abnormality caused by the mutation of intron 11.
Defect of BTK and block of B-cell differentiation. (A) Intracellular staining of BTK was performed and BTK expression was analyzed in peripheral blood CD14+ cells of the girl with agammaglobulinemia (left) and a healthy control (right). (B) The μ chain and VpreB were stained intracellularly. Analysis gate was set on CD19+ and/or VpreB-positive cells. In contrast to the normal B-cell development in the bone marrow of a healthy control (right), maturational block of B-cell precursors occurred and neither VpreB- μ + (low) nor VpreB- μ + (high) cells were observed in the patient (left). (C) The cDNA amplification was performed by PCR between exon 6 and exon 13 of BTK in the family members. Abnormally smaller-sized PCR products (1-3) were observed in the patient (lane 2) and her father (lane 5), although her paternal grandmother (lane 1), her mother (lane 3), and her brother (lane 4) expressed BTK mRNA with normal size. The primer pair was 5′-ATGCTATGGGCTGCCAAATT-3′ and 5′-GGTCCTTTGGATCAATTTCC-3′, and the expected size of the normal PCR product is 723 bp. M indicates marker. (D) The abnormally smaller-sized PCR products (1-3) in panel C were sequenced and found to be the result of splicing abnormality caused by the mutation of intron 11.
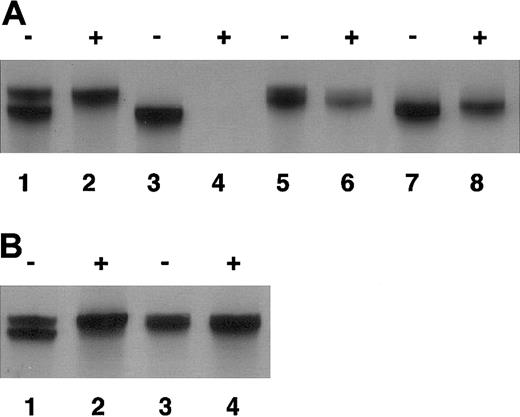
Nonrandom X-chromosome inactivation of the patient. As shown in Figure 2, maternal X chromosome was exclusively inactivated in mononuclear cells (Figure 2A) and oral mucosal cells (Figure 2B). On the other hand, random X-chromosome inactivation was confirmed in her mother and paternal grandmother by the presence of 2 types of CAG repeats, demonstrated by sequencing, after HpaII treatment in the androgen receptor exon 1 (with 2 CAG repeat difference in her mother and 1 CAG repeat difference in her paternal grandmother; data not shown). Altogether, the patient was diagnosed as having XLA associated with a defect of BTK caused by heterozygous abnormality of the BTK gene and nonrandom X inactivation of maternally derived X chromosome in which normal BTK gene is located, which was a novel alteration without evidence of inheritance.
Nonrandom X-chromosome inactivation of the patient. DNA was extracted from peripheral blood (A) and oral mucosal cells (B) of the family members. Exon 1 of the androgen receptor, which contains CAG repeat, was amplified as described in “Study design” with (+) or without (–) the digestion by methylation-sensitive HpaII before amplification. (A) Lane 1 and 2 indicate the patient; lane 3 and 4, her father; lane 5 and 6, her mother; and lane 7 and 8, her paternal grandmother. (B) Lane 1 and 2 indicate the patient; and lane 3 and 4, her mother.
Nonrandom X-chromosome inactivation of the patient. DNA was extracted from peripheral blood (A) and oral mucosal cells (B) of the family members. Exon 1 of the androgen receptor, which contains CAG repeat, was amplified as described in “Study design” with (+) or without (–) the digestion by methylation-sensitive HpaII before amplification. (A) Lane 1 and 2 indicate the patient; lane 3 and 4, her father; lane 5 and 6, her mother; and lane 7 and 8, her paternal grandmother. (B) Lane 1 and 2 indicate the patient; and lane 3 and 4, her mother.
Discussion
X chromosome is usually inactivated randomly in all somatic cells in early embryogenesis.6 Skewed preferential inactivation of nonmutated X chromosome can lead to the development of X-linked recessive disorders in females.7-11
On the other hand, Wengler et al12 reported that in female carriers of Wiskott-Aldrich syndrome (WAS), almost complete nonrandom inactivation of X chromosome with WASP gene mutation was observed in hematopoietic cells. Selective advantage of X chromosome without the mutation would inhibit the development of the disease in female heterozygotes of X-linked recessive disorders.11 On the other hand, Ariga et al13 reported that mutant WASP message was detected in peripheral blood cells in female carriers of WAS. In addition, WAS and X-linked thrombocytopenia of female heterozygotes are reported even without complete inactivation of nonmutated X chromosome.14,15 Therefore, it is likely that WAS can develop in heterozygotes because the selective advantage of X chromosome without the mutation does not appear to be completely dominant.
Although XLA is one of the most common primary immunodeficiency disorders affecting humoral immunity, this is the first report of a female case. As BTK is indispensable for B-cell development,16 only progenitor cells that have inactivation of X chromosome with BTK mutation can develop into mature B cells. Even a small population of progenitors with inactivation of X chromosome with BTK mutation would give a chance to the development of B cells and their expansion in the periphery. Therefore, it is reasonable to suggest that the development of XLA in females with heterozygous mutation of BTK is more restricted than other X-linked recessive disorders including WAS.
Possible reasons for extremely unbalanced X-chromosome inactivation observed in this female patient might include a stochastic event,11 a dysregulation of X-chromosome inactivation caused by defect of XIST gene17,18 which selects the X chromosome to inactivate, and defect of Tsix gene,19 which selects X chromosome to avoid accumulation of XIST gene mRNA in coordination with CTCF.20 In this patient, no abnormality was observed in XIST, its promoter, or mRNA expression levels. Some polymorphisms demonstrated the XIST mRNA expression of both maternally and paternally derived X chromosomes (data not shown). We further examined microsatellite markers in the NT_011642 region to analyze the possibility of deletion in a region adjacent to XIST. A microsatellite marker no. 7, a 180-bp distance from XIST gene to the telomere, showed heterozygous pattern of AC repeats, which suggests no large deletion in this region (data not shown). We therefore concluded that the nonrandom inactivation of X chromosome in this patient was caused by unknown mechanisms other than XIST.
Prepublished online as Blood First Edition Paper, September 4, 2003; DOI 10.1182/blood-2003-06-1964.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Yuka Sasaki and Saifuddin Ahrmed (Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University) for helping with the sequencing and Kunitaka Joh-o (Department of Pediatric Cardiology, Kyushu Kouseinenkin Hospital) for the encouragement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal