Abstract
During early embryogenesis, blood vessels and hematopoietic cells arise from a common precursor cell, the hemangioblast. Recent studies have identified endothelial progenitor cells in the peripheral blood, and there is accumulating evidence that a subset of these cells is derived from precursors in the bone marrow. Here we show that adult bone marrow–derived, phenotypically defined hematopoietic stem cells (c-kit+, Sca-1+, lineage–) give rise to functional endothelial cells. With the exception of the brain, donor-derived cells are rapidly integrated into blood vessels. Durably engrafted endothelial cells express CD31, produce von Willebrand factor, and take up low-density lipoprotein. Analysis of DNA content indicates that donor-derived endothelial cells are not the products of cell fusion. Self-renewal of stem cells with hematopoietic and endothelial cell potential was revealed by serial transplantation studies. The clonal origin of both hematopoietic and endothelial cell outcomes was established by the transfer of a single cell. These results suggest that adult bone marrow–derived hematopoietic stem cells may serve as a reservoir for endothelial cell progenitors.
Introduction
The close temporal and physical proximity of the developing hematopoietic and vascular systems has led to the hypothesis that blood cells and vascular tissue arise from a common progenitor cell.1-3 In vitro differentiation of embryonic stem cells reveals the existence of a bipotential progenitor cell, or hemangioblast, that differentiates into endothelium and cells of the hematopoietic lineages.4,5 Hematopoietic stem cells (HSCs) appear to be critical for early embryonic blood vessel development and wild-type HSC rescue–impaired angiogenesis in acute myeloid leukemia 1 (AML1)–deficient embryos.6 In contrast to early development, the complex functional relationships between hematopoiesis and the vascular compartment in postnatal life have only recently been investigated, and we now have evidence that adult blood vessels produce stem cell survival signals.7
Traditional models of vascular repair in the adult propose that angiogenesis results from the sprouting of new vessels through the recruitment of local endothelial progenitor cells (EPCs) from neighboring blood vessel walls. Recently however, several studies have demonstrated that similar to HSCs,8 EPCs circulate in the peripheral blood.9-13 EPCs can be mobilized into the blood by signals produced by ischemic tissue or following the administration of exogenous cytokines.14,15 These mobilized cells migrate to the site of ischemic injury where they participate in the repair of vascular tissue. EPCs can differentiate into smooth muscle cells, and there is evidence that they participate in the abnormal vascular remodeling associated with atherosclerosis.16 Furthermore, the impaired recruitment of EPCs appears to significantly block tumor angiogenesis and inhibit tumor growth.17 Bone marrow (BM)–derived EPCs have also been shown to contribute to the neovasculature in the newborn18 and to repopulate the liver endothelium of transplant recipients.19 Although there is accumulating evidence that subsets of BM-derived cells have the potential to differentiate into vascular tissue, the relationship between endothelial cell (EC) precursors and the HSC compartment remains incompletely understood.
Here we show that in addition to contributing to long-term multilineage hematopoiesis, highly purified populations of bone marrow–derived HSCs give rise to functional ECs in many somatic tissues. Reconstitution of the endothelial compartment with donor-derived cells occurs at the clonal level. Serial transplantation studies produce a high frequency of functional endothelium providing direct evidence for the self-renewal of stem cells with EC potential.
Materials and methods
Mice
Male donor ROSA26 or C57BL/6-TgN(ACTBEGFP)1Osb mice (8-12 weeks old) and female C57Bl6 recipient mice (8-12 weeks old) were used. Mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained in a breeding colony in the animal care facility at the Oregon Health and Sciences University.
Isolation of KSL cells
Donor BM was prepared using a modified version of a previously described procedure.20 Single-cell suspensions of BM were labeled with antibodies to c-kit–allophycocyanin (APC), Ly6AE-B SA-PharRed (Sca-1), and a phycoerythrin-conjugated lineage mixture (B220, CD3, CD5, CD4, CD8, Mac-1, Gr-1, Ter119) (BD Pharmingen, San Diego, CA). HSCs (KSL cells) were enriched to homogeneity by double sorting c-kit+/sca-1+/lineage-negative (lin–) cells using a fluorescence-activated cell sorter (FACS) Vantage (Becton Dickinson, San Jose, CA). Dead cells were excluded by a combination of scatter gates and propidium-iodide staining. For single-cell transplantation, primary sorted green fluorescent protein (GFP+) KSL cells were resorted into 96-well plates using an automated cell deposition unit (ACDU) device (Becton Dickinson). The presence of a single GFP+ cell per well was confirmed by fluorescence microscopy.
Transplantation studies
Recipient mice were treated with 1200 cGy of lethal irradiation in 2 equal doses of 600 cGy delivered 3 hours apart. The indicated number of donor cells were injected intravenously into the retro-orbital plexus. Recipients received antibiotic water for one month after transplantation (neomycin sulfate at 1.1 g/L and polymyxin B sulfate at 167 mg/L).7 For single-cell transplantations, individual GFP+ KSL cells were combined with 300 000 Sca-1–depleted BM cells and injected intravenously.
Hematopoietic engraftment
Peripheral blood was obtained by retro-orbital bleeding. Red cells were depleted by sedimenting erythrocytes in 3% Dextran (T-500; Amersham Pharmacia Biotech, Uppsala, Sweden) followed by hypotonic lysis. Recipient blood was analyzed for multilineage engraftment of donor-derived GFP+ cells. Lineage markers were B220, CD3, and Mac-1/Gr-1 (BD Pharmingen).
EC engraftment
Recipient tissues harvested at the indicated time points after transplantation were fixed in 4% paraformaldehyde, washed, dehydrated in 30% sucrose, then cryopreserved in optimal cutting temperature (OCT). Tissue sections (5 μm) were incubated with blocking buffer, then incubated overnight at 4°C with the primary antibodies anti–von Willebrand Factor (VWF; 1:400; DAKO, Glostrup, Denmark), anti-CD31 (1:100; BD Pharmingen), or anti-CD45 (1:25, BD Pharmingen). Sections were washed and incubated with secondary antibody conjugated to Cy3 (Chemicon, Temecula, CA), and the nuclei were counterstained with DAPI (4,6 diamidino-2-phenylindole). Sections were examined and photographed with a Zeiss Axiophot microscope using a true color AxioCam camera and standard epifluorescence filters for fluorescein isothiocyanate (FITC), Cy3, and DAPI (Carl Zeiss, Jena, Germany). Images were digitally combined using Axio Vision software (Carl Zeiss). Z-stack images were obtained with a BioRad 1024 ES laser scanning microscope (BioRad, Hercules, CA) and analyzed using Delta Vision deconvolution software (BioRad). Cell counts were performed by 3 independent observers. An individual portal vein cross-section was considered engrafted if it contained 10% or more donor-derived ECs. A minimum of 200 portal veins were counted per recipient mouse, and the results from 3 to 6 mice per transplantation group is shown. The ratio of GFP+ portal veins to total portal veins was determined and the standard error of the mean (SEM) was calculated. A 2-tailed t test was used to evaluate differences between transplantation groups.
Detection of the progeny of ROSA26 donor cells
Recipient tissues were analyzed for β-galactosidase activity in frozen sections of OCT cryopreserved tissue. Sections were incubated with 1 mg/mL X-gal (5-bromo-4-chloro-3-indolyl β-D-galactopyranoside; Sigma Chemical, St Louis, MO), 2 mM magnesium chloride, 5 mM potassium ferrocyanide, and 5 mM potassium ferricyanide. Tissue sections were counterstained with Nuclear Fast Red (Biomeda, Hayward, CA).
Fluorescence in situ hybridization (FISH)
FISH analysis was performed on paraffin-embedded liver tissue obtained from female C57BL/6 mice that received transplants of male HSCs (KSL). Sections were treated with 8% sodium thiocyanate (80°C) for 10 minutes, immersed in a 37°C pepsin solution (0.2 g pepsin/50 mL 0.1 M HCl) for 15 minutes, and then denatured at 72°C with a 2 × saline-sodium citrate buffer (SSC)/70% formamide solution for 5 minutes. A Y-Chromosome Mouse PAINT probe (labeled with Cy3 fluorescent tag; Cambio, Cambridge, United Kingdom) was added and slides were incubated at 42°C for 16 hours. Slides were washed in a 2 × SSC/0.2% nonidet P-40 (NP-40) solution, rinsed in double-distilled (dd) H2O, and then counterstained with DAPI II (Cambio). Nuclear localization of Y signals was evaluated by confocal microscopy.
Uptake DiI-Ac-LDL by GFP+ ECs
Recipients of donor HSCs were injected intravenously with 40 μL DiI-Ac-LDL (1,1′-dioctadecyl-1,3,3,3′,3′-tetramethylindocarbocyanine-labeled acetylated low-density lipoprotein [LDL]; Biomedical Technologies, Stoughton, MA). After 2 to 4 hours, liver tissue was harvested, fixed in 4% paraformaldehyde, and then cryopreserved in OCT. Tissue sections were evaluated by confocal microscopy as described in “Endothelial cell engraftment.”
Analysis of DNA content
Single-cell suspensions of liver tissue from recipient mice that received transplants with GFP+ KSL cells were passed through a 35-μm filter. Liver cells were layered on a fetal bovine serum cushion and centrifuged to remove glycogen. Liver cells were incubated with the anti–CD31-APC and biotinylated anti-CD45.2 followed by streptavidin-PharRed (BD Pharmingen). Cell sorting was used to enrich populations of viable GFP+, CD31+, CD45– cells (ECs) and GFP+, CD31+, CD45+ cells (hematopoietic cells). Analysis of the DNA content of sorted cell populations was performed with a FACS Calibur flow cytometer (Becton Dickinson) after fixing sorted cells with 50% ethanol then labeling with 20 μg/mL propidium iodide. Normal spleen cells were used as a control.
Results
EC outcomes from bone marrow–derived cells
To evaluate the potential of adult bone marrow–derived cells to differentiate into vascular endothelium, BM or BM-derived hematopoietic stem cells (c-kit+, Sca-1+, lineage–, KSL cells now referred to as HSCs) were transplanted into irradiated (1200 cGy) recipients. At 5 days after transplantation, only rare donor-derived cells were observed in recipient tissues (data not shown). However by 2 weeks, donor-derived cells were readily detected in the peripheral blood and throughout the vascular compartment of the liver (Figure 1). Evaluation of cross-sections of the endothelial surface of mid- to large-sized portal veins was performed as these vessels are frequent (40-50 per liver tissue section) and generally free of passenger leukocytes. Donor-derived cells were readily detected in the intima of portal vessels using 3 independent sources of donor cells. Marked BM-derived cells from ROSA26 donors,21 enhanced GFP donors,22 and wild-type male donor cells (male → female recipients) were consistently integrated into the endothelial surface of vessels for up to 8 months following transplantation (Figure 1B-F). By contrast, nonirradiated recipients of KSL cells did not demonstrate GFP+ cells in the EC compartment indicating that radiation-induced damaged is required for the differentiation and engraftment of KSL cells into vascular endothelium (data not shown).
Bone marrow–derived cells differentiate into portal vein endothelium. Following transplantation of BM or HSCs (KSL), recipient liver was examined for donor-derived CD31+/VWF+ cells. (A) Hematoxylin and eosin stain of recipient liver tissue showing the lumen of a portal vein (L). (B) Y-chromosome–positive (red) male donor cell in the nucleus of a cell in the intima of a portal vein 8 months after KSL transplantation detected by Y-PAINT/FISH with DAPI (blue) nuclear staining. (C) X-gal detection of LacZ expression in a ROSA26 donor-derived cell (blue) in a portal vein. (D-F) A portal vein from a GFP+ KSL transplant recipient demonstrating the expression of CD31 (red, D) and GFP (green, E). (F) Merged image. (G-I) A portal vein from a GFP+ KSL recipient demonstrating the expression of VWF (red, G) and GFP (green, H). (I) Merged image. (J) Confocal microscopy images of a donor-derived GFP+, CD31+, DAPI+ cell in a portal vein; (K) a GFP+, VWF+, DAPI+ cell in a portal vein. (B,J-K, 500 KSL cells per recipient; C, 106 BM cells per recipient; D-F,G-I, 2000 KSL cells per recipient. Scale bars: A, D-I, 20 μm; B-C, 10 μm; J-K, 5 μm; L indicates portal vein lumen.)
Bone marrow–derived cells differentiate into portal vein endothelium. Following transplantation of BM or HSCs (KSL), recipient liver was examined for donor-derived CD31+/VWF+ cells. (A) Hematoxylin and eosin stain of recipient liver tissue showing the lumen of a portal vein (L). (B) Y-chromosome–positive (red) male donor cell in the nucleus of a cell in the intima of a portal vein 8 months after KSL transplantation detected by Y-PAINT/FISH with DAPI (blue) nuclear staining. (C) X-gal detection of LacZ expression in a ROSA26 donor-derived cell (blue) in a portal vein. (D-F) A portal vein from a GFP+ KSL transplant recipient demonstrating the expression of CD31 (red, D) and GFP (green, E). (F) Merged image. (G-I) A portal vein from a GFP+ KSL recipient demonstrating the expression of VWF (red, G) and GFP (green, H). (I) Merged image. (J) Confocal microscopy images of a donor-derived GFP+, CD31+, DAPI+ cell in a portal vein; (K) a GFP+, VWF+, DAPI+ cell in a portal vein. (B,J-K, 500 KSL cells per recipient; C, 106 BM cells per recipient; D-F,G-I, 2000 KSL cells per recipient. Scale bars: A, D-I, 20 μm; B-C, 10 μm; J-K, 5 μm; L indicates portal vein lumen.)
Donor-derived cells incorporated into the luminal surface were evaluated for the expression of EC markers. CD31 (platelet-EC adhesion molecule 1) was detected in the portal veins, central veins, sinusoids, and arterioles of recipient liver tissue. CD31 expression demonstrated a characteristic linear staining pattern on the surface of ECs (Figure 1D-F). By contrast, von Willebrand factor (VWF), a coagulation factor, showed a punctate expression pattern on the cell surface and within the cytoplasm of ECs of large vessels (Figure 1G-I). Colocalized expression of endothelial markers in individual donor-derived GFP+ cells was confirmed by confocal microscopy (Figure 1J-K). All GFP+ donor cells that were integrated into the intimal surface of recipient vessels expressed both CD31 and VWF. These donor-derived cells also expressed the EC marker VE-cadherin (data not shown).
Uptake of low-density lipoprotein by KSL cell–derived endothelium
In addition to producing VWF, ECs are functionally defined by their capacity to take up low-density lipoprotein (LDL) from the plasma. To further evaluate the functional activity of donor-derived ECs, mice that received transplants 8 weeks previously of GFP+ HSCs were injected intravenously with DiI-labeled acetylated-LDL (ac-LDL). Within 3 hours of injection, examination of the liver by confocal microscopy revealed high levels of LDL uptake throughout the endothelial compartment of the liver. Individual GFP+ ECs demonstrated clear evidence of ac-LDL uptake that was indistinguishable from neighboring host endothelium (Figure 2D).
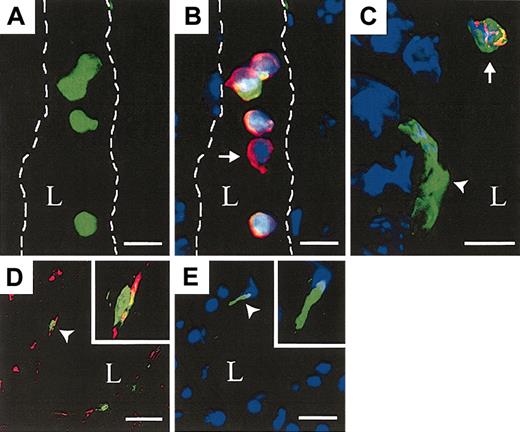
HSCs give rise to circulating hematopoietic cells and functional ECs. The liver of recipients that received transplants of 500 GFP+ KSL cells was evaluated. (A) GFP+ KSL cell–derived blood cells within a longitudinal section of a hepatic vessel (lumen (L) outlined, FITC channel only). (B) Merged image showing donor, GFP+ (green), DAPI+ (blue) nucleated blood cells coexpressing CD45 (red) and a single host-derived GFP–, CD45+ blood cell (arrow). (C) A portal vein with a donor DAPI+, GFP+, CD45– EC (arrowhead) near a donor DAPI+, GFP+, CD45+ blood cell (arrow). (D) Low-density lipoprotein (Dil-Ac-LDL, red) uptake in GFP+ ECs. Insert shows higher magnification of a single GFP+, Dil-Ac-LDL cell (arrow head). (E) A GFP+, CD45–, DAPI+ EC (arrowhead) within a portal vein from a 500 GFP+ KSL recipient 2 weeks after transplantation not injected with ac-LDL. Scale bars: A-B, 10 μm; C, 5 μm; and D-E, 20μm; original magnification, × 60 (D-E inserts).
HSCs give rise to circulating hematopoietic cells and functional ECs. The liver of recipients that received transplants of 500 GFP+ KSL cells was evaluated. (A) GFP+ KSL cell–derived blood cells within a longitudinal section of a hepatic vessel (lumen (L) outlined, FITC channel only). (B) Merged image showing donor, GFP+ (green), DAPI+ (blue) nucleated blood cells coexpressing CD45 (red) and a single host-derived GFP–, CD45+ blood cell (arrow). (C) A portal vein with a donor DAPI+, GFP+, CD45– EC (arrowhead) near a donor DAPI+, GFP+, CD45+ blood cell (arrow). (D) Low-density lipoprotein (Dil-Ac-LDL, red) uptake in GFP+ ECs. Insert shows higher magnification of a single GFP+, Dil-Ac-LDL cell (arrow head). (E) A GFP+, CD45–, DAPI+ EC (arrowhead) within a portal vein from a 500 GFP+ KSL recipient 2 weeks after transplantation not injected with ac-LDL. Scale bars: A-B, 10 μm; C, 5 μm; and D-E, 20μm; original magnification, × 60 (D-E inserts).
Donor-derived ECs are readily distinguished from hematopoietic cells
Both CD3123,24 and VWF25 are expressed by subsets of hematopoietic cells. Although donor-derived hematopoietic cells were usually distinguishable from vascular endothelium by morphologic criteria, it was important to demonstrate that the donor cells detected on endothelial surfaces were not adherent hematopoietic cells. Donor-derived cells were evaluated for coexpression of the panhematopoietic marker CD45. Both donor-derived and host-derived hematopoietic cells within the lumen of liver vessels were readily identified (Figure 2A-B). Populations of GFP+ CD45+ hematopoietic cells were also found throughout the liver in a perivascular distribution, a finding consistent with differentiation of donor-derived HSCs into hepatic macrophages or Kuppfer cells (data not shown). By contrast, all GFP+ cells integrated into the endothelial surface of blood vessel lumens were uniformly CD45– (Figure 2C).
EC outcomes are not the result of cell fusion
Recently, in vitro studies have demonstrated that the fusion of bone marrow–derived cells26 or neural progenitor cells27 with embryonic stem cells produces a tetraploid (4N) cell population that remarkably retains its potential to differentiate into multiple tissue types. These findings raise the question of whether a fusion event between a donor-derived BM cell and host-derived endothelial progenitor cell may contribute to the donor-derived endothelium observed in our study. To address this issue, the DNA content of engrafted GFP+ cells in transplant recipients was analyzed. Single-cell suspensions from the liver of GFP+ HSC recipients were evaluated for donor-derived cells by flow cytometry (Figure 3). GFP+ cells were identified and sorted into EC (CD31+, CD45–) and hematopoietic cell (CD31+, CD45+) populations. Both cell populations were fixed and assayed for DNA content using propidium iodide. Between 93% to 96% of sorted ECs and sorted hematopoietic cells demonstrated 2N DNA. These GFP+ cell populations were also evaluated by interphase FISH (male donor → female recipients). Sorted, donor-derived cells displayed a single X signal and a single Y signal, consistent with the presence of diploid ECs and hematopoietic cells (data not shown). These 2 independent approaches do not exclude the existence of a rare, 4N or greater GFP+ cell population, however they demonstrate that the vast majority of donor-derived ECs are not derived from stable cell fusion events.
Analysis of the DNA content of donor-derived hematopoietic cells and ECs in recipients of 500 KSL cells. Top left panel shows GFP+ donor-derived cells in a recipient liver by flow cytometry. Top right panel indicates cell sorting gates for GFP+, CD31+, CD45– ECs and GFP+, CD31+, CD45+ hematopoietic cells. Sorted cell populations were fixed with ethanol and analyzed for DNA content with propidium iodide. Lower panels show propidium iodide uptake by sorted GFP+, CD31+, CD45– ECs, GFP+, CD31+, CD45+ hematopoietic cells, and control normal spleen cells. The percentage of cells in S/G2/M of the cell cycle is indicated.
Analysis of the DNA content of donor-derived hematopoietic cells and ECs in recipients of 500 KSL cells. Top left panel shows GFP+ donor-derived cells in a recipient liver by flow cytometry. Top right panel indicates cell sorting gates for GFP+, CD31+, CD45– ECs and GFP+, CD31+, CD45+ hematopoietic cells. Sorted cell populations were fixed with ethanol and analyzed for DNA content with propidium iodide. Lower panels show propidium iodide uptake by sorted GFP+, CD31+, CD45– ECs, GFP+, CD31+, CD45+ hematopoietic cells, and control normal spleen cells. The percentage of cells in S/G2/M of the cell cycle is indicated.
Long-term engraftment of self-renewing stem cells with EC potential
Long-term, lineage-specific differentiation and the capacity to self-renew are the principal functional determinants of stem cell activity. To evaluate the extent and the durability of donor-derived endothelium, the portal veins of recipient mice were examined. Cross-sections of the lumen of individual vessels ranged in size from 20 to 40 nucleated cells. Individual portal veins were scored positive only if 10% or more of the cells on the luminal surface were CD45– and GFP+, VWF+ or GFP+, CD31+. Analysis of donor-derived cells 14 days after transplantation revealed that 33% to 38% of the portal veins contained GFP+ ECs in recipients of either unfractionated BM cells or an equivalent number of HSCs (Figure 4A). At 2 weeks after transplantation, a dose response was observed with recipients of 2000 KSL cells showing donor ECs in 32% ± 2.4% of vessels, while the EC progeny of 500 KSL cells was detected in 20% ± 1.8% of portal veins (P < .01). By 6 to 8 months after transplantation, recipients of unfractionated BM cells had similar numbers of GFP+ ECs (44% ± 4.0%), while the long-term recipients of 500 donor KSL cells had an increased frequency of GFP+ ECs over time (42% ± 3.9%). Every tissue section in each mouse examined demonstrated donor-derived ECs. Typical individual liver tissue sections contained between 40 to 60 portal vein cross-sections with a mean of 150 GFP+ ECs per section. Based on these results, we estimate that up to 5% of the portal vein endothelium was donor derived at 6 to 8 months after transplantation.
High levels of donor-derived ECs and hematopoietic cells following primary and secondary transplantation. (A) Liver from recipients of GFP+ BM (2-5 × 106 cells), GFP+ KSL (500-2000 cells), or from unfractionated bone marrow (WBM) (106 cells) serially transplanted from primary recipients (secondary transplantation) was examined to determine the frequency of portal veins with GFP+ ECs. Portal veins were considered positive if 10% or more of the ECs integrated within the vessel wall were GFP+, CD31+, or VWF+. 2nd Tx indicates secondary transplantation. (Error bars indicate SEM; n = 3-6 mice per group [P ≤ .01]). (B) Donor-derived, multilineage hematopoiesis in the peripheral blood of a secondary recipient 3 months after transplantation. The percentage of GFP+ donor cells expressing individual hematopoietic lineage markers is indicated.
High levels of donor-derived ECs and hematopoietic cells following primary and secondary transplantation. (A) Liver from recipients of GFP+ BM (2-5 × 106 cells), GFP+ KSL (500-2000 cells), or from unfractionated bone marrow (WBM) (106 cells) serially transplanted from primary recipients (secondary transplantation) was examined to determine the frequency of portal veins with GFP+ ECs. Portal veins were considered positive if 10% or more of the ECs integrated within the vessel wall were GFP+, CD31+, or VWF+. 2nd Tx indicates secondary transplantation. (Error bars indicate SEM; n = 3-6 mice per group [P ≤ .01]). (B) Donor-derived, multilineage hematopoiesis in the peripheral blood of a secondary recipient 3 months after transplantation. The percentage of GFP+ donor cells expressing individual hematopoietic lineage markers is indicated.
To directly measure the self-renewal capacity of cells with both endothelial and hematopoietic potential, serial transplantation studies were performed. At 8 months after primary transplantation with HSCs, BM was harvested from primary recipients and transplanted into secondary irradiated recipients. Analysis of the peripheral blood demonstrated high levels of donor-derived, multi-lineage hematopoietic reconstitution (Figure 4B). Liver vessels from secondary recipients were evaluated at 3 months after secondary transplantation, and a mean of 31% ± 7.7% of portal veins were engrafted with GFP+ ECs (Figure 4A). The frequency of EC outcomes was similar to that observed in primary recipients of BM and HSCs. Serial transplantation studies revealed that KSL cells and their progeny in the BM of primary recipients retain the capacity to undergo both endothelial and hematopoietic differentiation.
HSCs contribute to the EC compartment of multiple tissues
Studies of EC engraftment kinetics and self-renewal potential focused on the liver because of the ease of identifying and quantitating GFP+ ECs in cross-sections of mid- to large-sized portal veins. Donor-derived ECs were also frequently detected in the liver microvasculature (sinusoids) and in branches of the hepatic artery, demonstrating that the EC differentiation of BM and KSL cells was not restricted to the portal vessel system (data not shown). The presence of GFP+ ECs in the vasculature of several other tissues was assayed by evaluating the coexpression of CD31, VWF, and the absence of CD45 expression. Donor-derived ECs were detected in all tissue sections of lung, heart, skeletal muscle, and intestine examined (Figure 5). The overall frequency of GFP+ ECs in these diverse organs ranged from 30 to 150 cells per tissue section. Although many GFP+, CD45+ hematopoietic cells were detected in the brain of HSC recipients, a mean of only 1 GFP+, CD45– cell was found per 5 tissue sections. These rare cells were not usually associated with recognizable vascular structures (data not shown). Based on these results, the frequency of donor-derived GFP+ ECs in the brain is at least 100-fold lower than in other tissues examined.
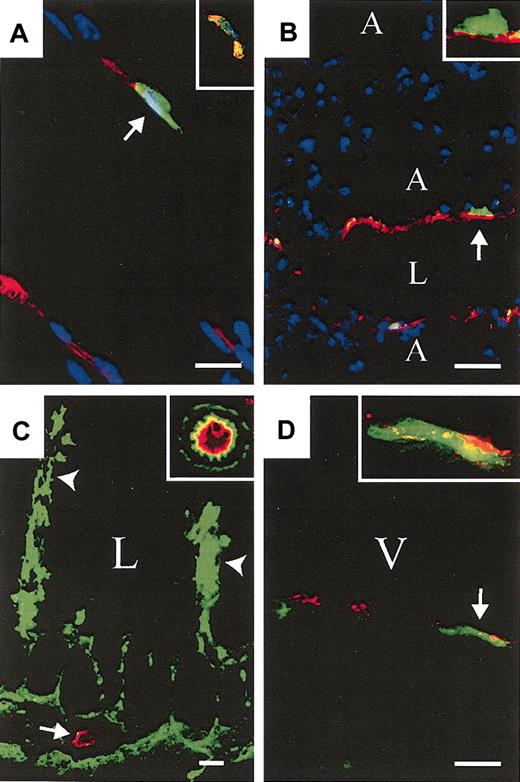
Bone marrow–and HSC-derived ECs are detected in multiple tissues. (A) GFP+ (green) KSL-derived CD31+ (red) cell (arrow) and a GFP+, VWF+ (red) cell (insert) in skeletal muscle. (B) GFP+ BM-derived CD31+ cells in lung (arrow; insert: high magnification; A indicates alveolus, L indicates vascular lumen). (C) GFP+ BM-derived CD31+ cells in the distal small intestine. Donor-derived gut-associated lymphatic tissue is indicated (arrowheads) and a cross-section of a GFP+ CD31+ vessel is shown (arrow; insert: high magnification of neighboring GFP+ vessel). (D) GFP+ KSL-derived VWF+ cells in ventricular free wall of the heart (arrow; insert: higher magnification; V indicates ventricular cavity). (A,D, 2000 KSL cells; B-C, 106 BM. A-B, DAPI nuclear stain (blue). Scale bars: A, 10 μm; B, 20 μm; C, 25 μm; and D, 10 μm.) Original magnifications: × 40 (A,C inserts); × 60 (B,D inserts).
Bone marrow–and HSC-derived ECs are detected in multiple tissues. (A) GFP+ (green) KSL-derived CD31+ (red) cell (arrow) and a GFP+, VWF+ (red) cell (insert) in skeletal muscle. (B) GFP+ BM-derived CD31+ cells in lung (arrow; insert: high magnification; A indicates alveolus, L indicates vascular lumen). (C) GFP+ BM-derived CD31+ cells in the distal small intestine. Donor-derived gut-associated lymphatic tissue is indicated (arrowheads) and a cross-section of a GFP+ CD31+ vessel is shown (arrow; insert: high magnification of neighboring GFP+ vessel). (D) GFP+ KSL-derived VWF+ cells in ventricular free wall of the heart (arrow; insert: higher magnification; V indicates ventricular cavity). (A,D, 2000 KSL cells; B-C, 106 BM. A-B, DAPI nuclear stain (blue). Scale bars: A, 10 μm; B, 20 μm; C, 25 μm; and D, 10 μm.) Original magnifications: × 40 (A,C inserts); × 60 (B,D inserts).
ECs are clonally derived from HSCs
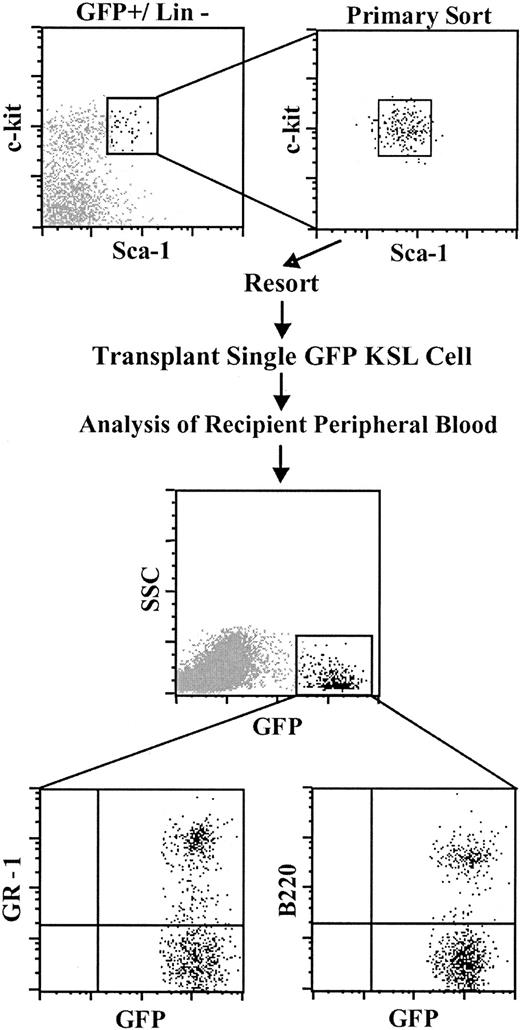
Although donor BM–derived HSCs were double sorted to phenotypic homogeneity, it remained possible that functional heterogeneity within the KSL cell population was primarily responsible for the endothelial and hematopoietic outcomes observed. To address this question, single GFP+ KSL donor cells were sorted into individual tissue-culture wells. Following visual confirmation of a single sorted cell per well, Sca-1– host type BM cells were added to each well, and this entire cell mixture was transplanted into a single irradiated recipient. The Sca-1– BM cells are depleted in long-term HSC activity but contain multilineage hematopoietic progenitors that provide protection from lethal irradiation. Analysis of the peripheral blood of single KSL cell recipients 6 to 8 weeks after transplantation showed circulating donor-derived GFP+ hematopoietic cells in the peripheral blood of 10% of recipient mice (8/79), a frequency of chimerism similar to that previously reported.28-30 The frequency of donor-derived cells ranged from 0.3% to 5.0% of the total nucleated blood cells, and GFP+ myeloid and lymphoid hematopoiesis was readily detected (Figure 6). Confocal microscopy of liver tissue from 3 of 3 chimeric mice that received transplants of a single KSL cell revealed the presence of GFP+ ECs integrated into the vessel wall of both portal veins and venous sinusoids (Figure 7). Individual GFP+ cells integrated into the endothelium were analyzed sequentially for the expression of CD45 followed by CD31 and VWF expression. These donor-derived ECs were readily distinguished from donor- and host-derived hematopoietic cells (Figure 7D,F). A low frequency of donor-derived ECs (< 1% of 500 KSL cell recipients) was observed, suggesting a correlation between the levels of donor hematopoietic and endothelial cell engraftment. However, a large-scale limit dilution analysis of single KSL cell recipients would be required to establish this relationship. Our finding of both GFP+ ECs and GFP+ hematopoiesis derived from a single KSL cell demonstrates hemangioblast activity in the KSL subset of adult bone marrow.
Transplantation of a single HSC produces multilineage hematopoiesis in the peripheral blood of recipient mice. Top panels: Isolation of single GFP+ KSL cells. (Primary and secondary cell sorting gates are shown). Middle panel: Donor-derived cells in the peripheral blood of a recipient of a single GFP+ KSL cell. Lower panels: GFP+ peripheral blood myeloid cells (GR-1) and B cells (B220) derived from a single KSL cell.
Transplantation of a single HSC produces multilineage hematopoiesis in the peripheral blood of recipient mice. Top panels: Isolation of single GFP+ KSL cells. (Primary and secondary cell sorting gates are shown). Middle panel: Donor-derived cells in the peripheral blood of a recipient of a single GFP+ KSL cell. Lower panels: GFP+ peripheral blood myeloid cells (GR-1) and B cells (B220) derived from a single KSL cell.
Transplantation of a single HSC gives rise to donor-derived ECs. (A) Portal vein from a recipient of a single GFP+ KSL cell 6 months after transplantation demonstrating the expression of CD31 (Cy3, red channel only). (B) High magnification of area in the box indicated in panel A, showing a GFP+ luminal cell (green channel only) integrated into the vessel wall. (C) Merged image, the box indicating the same donor GFP+ (green), CD31+ (red) EC with a DAPI+ nucleus (blue) (insert: high magnification of the same cell). (D) A GFP+, CD45– EC within the vessel wall (solid arrow) and a CD45+ (red) leukocyte (open arrowhead). (E) The same GFP+, CD45– EC sequentially labeled with VWF (red) (solid arrow). (F) A GFP+, CD45– donor EC in a liver venous sinusoid (solid arrow) and a donor CD45+ (red) leukocyte (open arrowhead) 6 months after transplantation. (G) The same GFP+ CD45– EC sequentially labeled with CD31 (red). (Scale bars: A, 20 μm; C: 20 μm; and D-G: 10 μm; DAPI+ nuclei (blue); L indicates lumen.) Original magnification, × 60 (B,C insert).
Transplantation of a single HSC gives rise to donor-derived ECs. (A) Portal vein from a recipient of a single GFP+ KSL cell 6 months after transplantation demonstrating the expression of CD31 (Cy3, red channel only). (B) High magnification of area in the box indicated in panel A, showing a GFP+ luminal cell (green channel only) integrated into the vessel wall. (C) Merged image, the box indicating the same donor GFP+ (green), CD31+ (red) EC with a DAPI+ nucleus (blue) (insert: high magnification of the same cell). (D) A GFP+, CD45– EC within the vessel wall (solid arrow) and a CD45+ (red) leukocyte (open arrowhead). (E) The same GFP+, CD45– EC sequentially labeled with VWF (red) (solid arrow). (F) A GFP+, CD45– donor EC in a liver venous sinusoid (solid arrow) and a donor CD45+ (red) leukocyte (open arrowhead) 6 months after transplantation. (G) The same GFP+ CD45– EC sequentially labeled with CD31 (red). (Scale bars: A, 20 μm; C: 20 μm; and D-G: 10 μm; DAPI+ nuclei (blue); L indicates lumen.) Original magnification, × 60 (B,C insert).
Discussion
Our results demonstrate that within 14 days of transplantation, bone marrow (BM) and an equivalent number of BM-derived KSL cells make equally significant contributions to the EC compartment. The differentiation of donor cells into ECs within 2 weeks is similar to the kinetics observed during the reconstitution of the hematopoietic compartment by unfractionated bone marrow or phenotypically defined HSCs.31 EC progeny of HSCs persist for at least 8 months, and serial transfer experiments reveal a self-renewal potential of endothelial stem cell activity that parallels the self-renewal of the HSC compartment. Importantly, our studies specifically exclude cell fusion as the principal mechanism responsible for the generation of functional ECs from stem cells. This finding is particularly relevant in view of the observation that bone marrow cells have the capacity to fuse with embryonic stem cells and differentiate in vitro.26 Recently, Wang et al have shown that regenerating hepatocytes in a mouse model of tyrosinemia can arise from the stable fusion of bone marrow–derived cells with host hepatocytes.32 This study provides the first evidence of a functional role for cell fusion in vivo and raises the issue of whether cell fusion is in part responsible for the reported differentiation of HSCs into divergent lineages including neurons,33 muscle,34 and epithelial cells.29 While our results did not detect a significant number of cells with 4N or greater DNA, it is important to note that we have not excluded the possibility that a rare cell fusion event occurred followed by a reduction division yielding 2 diploid cells.32
Single-cell transplantation studies show that HSCs are capable of generating both a hematopoietic and an EC outcome at the clonal level. These results support the hypothesis that hemangioblast activity, originally identified in early development,2,35 persists into the adult life.12,18,36 Studies by Pelosi et al demonstrate that single adult human CD34+ KDR+ hematopoietic stem/progenitor cells give rise to ECs following serial passage of these cells in long-term culture.12 It will be important to extend this finding of adult human hemangioblast activity using an in vivo model system. Recently, Grant et al used a retinal neovascularization model to demonstrate that the clonal progeny of phenotypically defined adult mouse HSCs participate in the process of vascular repair.15 These investigators found that donor-derived neovascularization in the retina was dependent on both ischemic injury and an exogenous source of vascular endothelial growth factor. As the retina is an extension of the central nervous system, this result is consistent with our findings of only rare nonhematopoietic donor-derived cells in the brain of transplant recipients. Our results extend these observations by showing widespread incorporation of donor-derived cells into the EC compartment without additional postirradiation vascular injury. However, it is important to note that ECs were not observed in nonirradiated recipients of KSL cells, indicating that radiation-induced tissue damage is required for generating endothelial outcomes in this model system.
Several recent studies suggest an important role for circulating endothelial progenitor cells (EPCs) in vascular tissue homeostasis. EPCs participate in processes as diverse as tumor angiogenesis,17 atherosclerosis,16 graft arterial disease,37 and repair following ischemic injury.14,38-40 Most of these studies used recipients of marked donor BM more than 8 weeks after transplantation. Therefore it remains unknown if the donor-derived EPCs are mobilized directly from the BM into the circulation and then migrate to sites of vascular injury. Alternatively, donor BM–derived EPCs could have already seeded peripheral tissues with subsequent local recruitment to sites of vascular repair. With the exception of the central nervous system, we have demonstrated rapid, widespread seeding of the EC compartment in many somatic tissues supporting this second possibility. These results underscore the importance of determining the role of BM-derived EC progenitors, circulating EPCs, and locally resident endothelial progenitor cells in the maintenance and repair of adult vascular tissue.
Supported by grants HL-69133 and HD-039251 to W.H.F.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, September 4, 2003; DOI 10.1182/blood-2003-05-1684.
The authors wish to thank Aurelie Snyder for her expertise with confocal microscopy, Greg Faulkner for operating the cell sorter, and Yumi Cox for her assistance with in situ hybridization.



![Figure 4. High levels of donor-derived ECs and hematopoietic cells following primary and secondary transplantation. (A) Liver from recipients of GFP+ BM (2-5 × 106 cells), GFP+ KSL (500-2000 cells), or from unfractionated bone marrow (WBM) (106 cells) serially transplanted from primary recipients (secondary transplantation) was examined to determine the frequency of portal veins with GFP+ ECs. Portal veins were considered positive if 10% or more of the ECs integrated within the vessel wall were GFP+, CD31+, or VWF+. 2nd Tx indicates secondary transplantation. (Error bars indicate SEM; n = 3-6 mice per group [P ≤ .01]). (B) Donor-derived, multilineage hematopoiesis in the peripheral blood of a secondary recipient 3 months after transplantation. The percentage of GFP+ donor cells expressing individual hematopoietic lineage markers is indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-05-1684/6/m_h80145471004.jpeg?Expires=1767737147&Signature=icedFZbSJ50EzNj9nDu8MHcvx0YzYZ5pc0Rr8y27I-3jSTp8Cw0todCAF2DUFbSsd7CUYscvWqDocoF8iriyEEWtH5Q2aj3t8v1aOdLvOIw9-wHTS-Y5SFqNdPAnzygck9iOZpR8vMg4Hajih9nXtqMsohtcQrV5amYOhC3lYg-pOuuK9woB2nICsLZLsTxcPeWKbt5CB3nZM0sgAK0PFDHXaNMosznuMFlBFWeiYEvWxQqT7~akh~A-Vmrck9zEgjJsjD57O7s4Z3hY9VM5dgp9vrInuG4OE8PiFYiHx08COd~yq8JFqCW68EsvKFsQZTgApIJVrB4p2KW7WC-3SA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal