Acute myeloid leukemia (AML) is characterized by abnormalities frequently affecting transcriptional control elements, leading to differentiation block.1 C/EBPα is one of the most frequently involved transcription factors, being disrupted by point mutations in 7% to 11% of cases.2,3 In the setting of the t(8;21) AML, C/EBPα function has been shown to be down-regulated by the AML-ETO fusion protein.4 The latter mechanism was reported as a peculiarity of t(8;21)-positive AML, apparently not shared by other subtypes.4
Using a quantitative real-time polymerase chain reaction (RQPCR) method,5,6 we analyzed the C/EBPα expression levels in 144 bone marrows (BMs) from AML patients at diagnosis (French-American-British [FAB] distribution: M0, 15; M1, 19; M2, 38; M3, 22; M4, 37; M5, 9; and M6, 4) and in 32 samples (14 BM and 18 peripheral blood [PB]) from healthy controls. Following karyotypic and molecular analysis, 19 of 38 FAB M2 patients were classified as t(8;21) positive and 16 of 37 FAB M4 cases as inv(16) positive. C/EBPα expression was also tested in serial samples from 18 patients during follow-up (7 characterized by t(8;21), 5 by inv(16), and 6 by a normal karyotype).
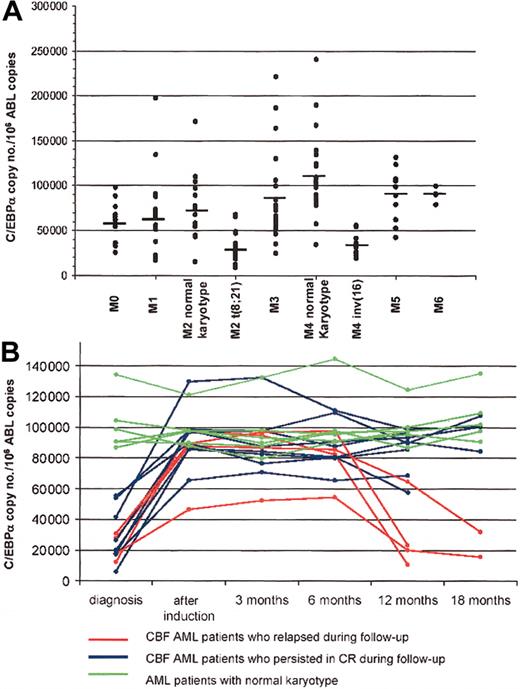
As shown in Figure 1A, we detected a significant down-modulation of C/EBPα transcript in the samples positive for the presence of the t(8,21) and inv(16) cytogenetic abnormalities with respect to the other types of AMLs (mean value of C/EBPα copies/104 ABL copies: 33 638 vs 77 813, P = .0001), which showed values similar to those found in healthy controls (77 865 and 56 845 C/EBPα copies/104 ABL copies in normal BM and PB, respectively). Moreover, the comparison between the C/EBPα values obtained in BM samples from patients with AML1/ETO-positive FAB M2 AML compared with AML1/ETO-negative FAB M2 cases demonstrated a highly significant difference (32 523 vs 71 985 C/EBPα copies/104 ABL copies, P = .0001). Similar differences were detected by analyzing FAB M4 cases with and without the CBFb/MYH11 fusion (35 644 vs 105 173 C/EBPα copies/104 ABL copies, P = .0002). The Western blot assays further confirmed these data by showing a reduction of the total amount of C/EBPα protein in AML cases with t(8;21) or inv(16) with respect to those lacking these abnormalities. A further demonstration that the down-regulation of C/EBPα is a characteristic feature of the CBF-disrupted AMLs derives from data obtained on follow-up samples (Figure 1B). In patients characterized by t(8;21) and inv(16) (7 and 5, respectively) who achieved a complete remission (CR), C/EBPα transcript amounts increased to values not significantly different from those obtained in normal samples (P = .4) or in the other subgroups of AML at onset (P = .2). Subsequently, these values remained stable in all of the samples collected during CR, and showed a significant decrease in 3 cases of t(8;21) and in 1 case of inv(16) who subsequently relapsed. By contrast, the 6 patients with normal karyotype did not show significant differences between the CEBPα expression level at onset, in CR, and at relapse.
C/EBPα expression in the different FAB subtypes of AML and during follow-up. (A) C/EBPα expression is significantly downmodulated in FAB M2 AML patients characterized by the t(8;21) and in FAB M4 characterized by inv(16) with respect to those with normal karyotype. The mean values are defined by horizontal bars. (B) C/EBPα expression is significantly downmodulated in CBF AML patients at diagnosis, and it is up-regulated during chemotherapy-induced complete remission (CR). In the 8 CBF AML patients who persisted in CR (blue lines), C/EBPα expression did not change during follow-up; by contrast in the 4 CBF AML patients who relapsed (red lines), C/EBPα expression was downmodulated again. By contrast, no differences in C/EBPα expression were noted during follow-up in the 6 patients characterized by normal karyotype (green lines). Of these 6 patients, 4 persisted in CR and 2 relapsed.
C/EBPα expression in the different FAB subtypes of AML and during follow-up. (A) C/EBPα expression is significantly downmodulated in FAB M2 AML patients characterized by the t(8;21) and in FAB M4 characterized by inv(16) with respect to those with normal karyotype. The mean values are defined by horizontal bars. (B) C/EBPα expression is significantly downmodulated in CBF AML patients at diagnosis, and it is up-regulated during chemotherapy-induced complete remission (CR). In the 8 CBF AML patients who persisted in CR (blue lines), C/EBPα expression did not change during follow-up; by contrast in the 4 CBF AML patients who relapsed (red lines), C/EBPα expression was downmodulated again. By contrast, no differences in C/EBPα expression were noted during follow-up in the 6 patients characterized by normal karyotype (green lines). Of these 6 patients, 4 persisted in CR and 2 relapsed.
Our data clearly show that decreased levels of C/EBPα expression are present also in the AML cases with inv(16) and, therefore, that down-modulation of C/EBPα is a common feature of both major subtypes of CBF AMLs (ie, t(8;21)-positive and inv(16)-positive AML).
Supported by grants from AIRC (Associazione Italiana per la Ricerca sul Cancro), MURST-COFIN (Ministero Universitá Ricerca Scientifica Tecnologica-Cofinanziamento) 2002, and AIL (Associazione Italiana contro le Leucemie).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal