Type II congenital dyserythropoietic anemia (CDA-II or HEMPAS) is an autosomal recessive disorder, representing the most frequent form of congenital dyserythropoiesis.
Recently, the molecular basis of the retsina (ret) phenotype that resulted in zebrafish from mutations in the gene that encodes the erythroid anion exchanger 1 (AE1, also called SLC4A1) was reported.1 The high number of binucleated erythroblasts, the presence of “double membranes,” and the reduction in posttranslational glycosylation of AE1, observed in the ret fish, are all reminiscent of the human CDA-II. Since the gene responsible for CDA-II in humans has not yet been identified, it is highly relevant to ask whether it could be AE1.
In first approximation, this does not seem likely for several reasons. First, as the authors themselves note, in a majority of families with CDA-II that have been subjected to linkage analysis, the disease maps to 20q11.2,2 whereas AE1 maps to 17q21-q22. Second, complete inactivation of AE1 in mice3 and in cattle4 causes severe hemolytic anemia, but not the CDA-II phenotype. Third, human erythroid AE1 absence has been found to cause severe hereditary spherocytosis (HS) but, again, no CDA-II.5
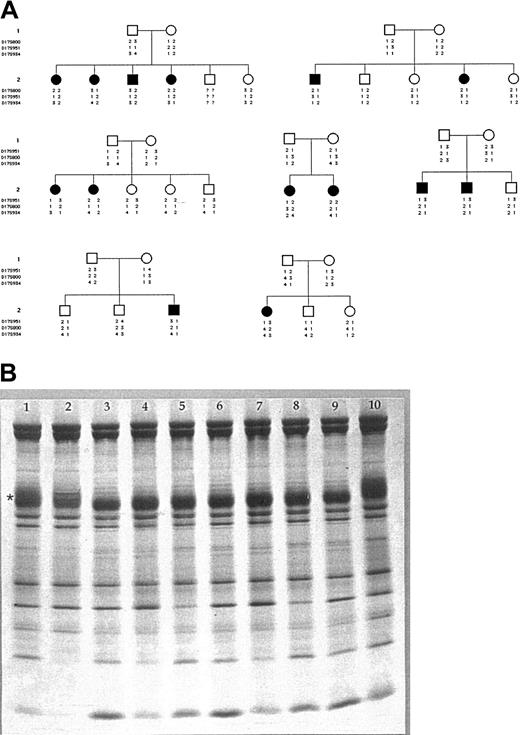
In order to test whether AE1 mutations might be responsible for a subset of patients with CDA-II we have used the resources of an international registry6 in which 108 patients are enrolled. In 14 of these patients, belonging to 7 unrelated families, the disease was not linked to 20q11.2 (Figure 1A).7 Of these 14 patients, 6 had a particularly severe form of CDA-II (they were transfusion dependent from the age of 1 year); the remaining 8 patients had “typical” CDA-II. In 5 families we were able to exclude linkage to chromosome 17. In addition, we proceeded in all 14 patients to quantitate erythrocyte AE1 by means of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).8,9 Whereas in patients with HS the amount of AE1 was significantly reduced (73.90 ± 2.42% of controls, P < .0001), in patients with CDA-II the amount of AE1 was virtually normal (96.50 ± 2.07% of controls, P = .783). In terms of the possible pathophysiologic significance of AE1 in CDA-II, while all these patients had the underglycosylation of AE1 that is characteristic of this condition, we did not find any difference in AE1 levels between patients with severe CDA-II versus “typical” CDA-II (96.83 ± 2.48% versus 96.25 ± 1.83%, respectively; P = .621) (Figure 1B).10
Exclusion of AE1 gene as cause of CDA-II. (A) Pedigrees of 7 families with CDA-II: haplotype analysis of 3 markers tightly linked to the SLC4A1 gene in 7 CDA-II families unlinked to CDAN2 locus on chromosome 20. White symbols indicate healthy subjects; black symbols, patients. (B) SDS-PAGE of red blood cell membrane proteins. SDS-PAGE was performed using the discontinuous buffer system of Laemmli with acrylamide linear gradient from 5% to 15%. The proteins were stained with Coomassie brilliant blue and gels were scanned using a laser densitometer (Ultroscan; Pharmacia, Uppsala, Sweden). The amount of the major membrane proteins was then expressed as the value of the relative surface area present under the densitometric curve, and the results were normalized to the protein 4.1. Erythrocyte membrane analysis of each patient and control was performed 3 times using different gels. The reproducibility of AE1 and protein 4.1 measurements was within a range of ± 4%. The amount of AE1 and protein 4.1 was compared with normal values obtained from controls. * indicates AE1; 1 and 10, controls; 2, HS due to AE1 deficiency; 3, 4, and 5, “typical” CDA-II; and 6, 7, 8, and 9, severe CDA-II.
Exclusion of AE1 gene as cause of CDA-II. (A) Pedigrees of 7 families with CDA-II: haplotype analysis of 3 markers tightly linked to the SLC4A1 gene in 7 CDA-II families unlinked to CDAN2 locus on chromosome 20. White symbols indicate healthy subjects; black symbols, patients. (B) SDS-PAGE of red blood cell membrane proteins. SDS-PAGE was performed using the discontinuous buffer system of Laemmli with acrylamide linear gradient from 5% to 15%. The proteins were stained with Coomassie brilliant blue and gels were scanned using a laser densitometer (Ultroscan; Pharmacia, Uppsala, Sweden). The amount of the major membrane proteins was then expressed as the value of the relative surface area present under the densitometric curve, and the results were normalized to the protein 4.1. Erythrocyte membrane analysis of each patient and control was performed 3 times using different gels. The reproducibility of AE1 and protein 4.1 measurements was within a range of ± 4%. The amount of AE1 and protein 4.1 was compared with normal values obtained from controls. * indicates AE1; 1 and 10, controls; 2, HS due to AE1 deficiency; 3, 4, and 5, “typical” CDA-II; and 6, 7, 8, and 9, severe CDA-II.
Based on genetic analysis and on biochemical findings, we suggest that in at least the vast majority of cases human CDA-II is not due to AE1 mutations. It is intriguing that, in zebrafish, AE1, through its protein 4.1R-binding domain, was found to affect the poles of the mitotic spindle specifically in erythroid cells; as a result, AE1 deficiency ultimately affects the completion of chromosome segregation in these cells, thus mimicking certain morphologic features of CDA-II. Therefore, in the rare patients in whom linkage to AE1 has not been ruled out, it will be important to look specifically for mutations within this particular domain of human AE1. At any rate, since these cases are a minority, there must be in humans at least one other gene involved in this pathway.
Supported by Telethon (prog GP0202Y02), Italian Cancer Research Association (AIRC), Italian University and Research Ministry (MURST) Projects of Relevant Interest (progetti PRIN) (Italy), Ministero della Sanità and Ministry of Instruction, University and Research (MIUR FIRB) project (A.I.), and Regione Campania.
We thank the families for their enthusiastic cooperation and hospitality, and Prof Narla Mohandas for stimulating discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal