Abstract
The clinical use of doxorubicin (DOX), an anthracycline chemotherapeutic agent, is limited by cardiotoxicity. The possible involvement of iron in DOX-induced cardiotoxicity became evident from studies in which iron chelators were shown to be cardioprotective. Iron overload is found in hereditary hemochromatosis, a genetic disorder prevalent in individuals of European descent. We hypothesized that Hfe deficiency may increase susceptibility to DOX-induced toxicity. Acute cardiotoxicity and iron changes were studied after treatment with DOX in Hfe knock-out (Hfe-/-) mice and wild-type mice. DOX-induced iron metabolism changes were intensified in Hfe-/- mice, which accumulated significantly more iron in the heart, liver, and pancreas, but less in the spleen compared with wild-type mice. In addition, Hfe-deficient mice exhibited significantly greater sensitivity to DOX-induced elevations in serum creatine kinase and aspartate aminotransferase. Increased mortality after chronic DOX treatment was observed in Hfe-/- mice and Hfe+/-mice compared with wild-type mice. DOX-treated Hfe-/- mice had a higher degree of mitochondrial damage and iron deposits in the heart than did wild-type mice. These data demonstrate that Hfe deficiency in mice increases susceptibility to DOX-induced cardiotoxicity and suggest that genetic mutations related to defects in iron metabolism may contribute to its cardiotoxicity in humans. (Blood. 2003;102:2574-2580)
Introduction
Doxorubicin (DOX) is an anthracycline antibiotic and one of the most active anticancer agents. It is effective in the treatment of acute leukemias and malignant lymphomas as well as a number of solid tumors. Cardiotoxicity, however, is a major problem in the clinical use of DOX. Its cardiac toxicity has been well established in both physiologic and ultrastructural studies and has been demonstrated after both acute and chronic treatments.1 Chronic cardiotoxicity is cumulative, dose dependent, and may occur in up to 20% of patients treated with DOX.2
The proposed mechanism for the cytotoxic effect of DOX is the production of reactive oxygen species (ROS) during its intracellular metabolism.3 DOX can form a stable complex with ferric iron,4 and the formed complex undergoes self-reduction to ferrous iron, resulting in the generation of a semiquinone free radical of DOX.5 The semiquinone reacts with oxygen to form a superoxide anion radical that, in the presence of iron, catalyzes conversion to a hydroxyl radical.6 In turn, this potent radical causes lipid peroxidation and DNA damage.4,7
The involvement of iron in anthracycline-induced cardiotoxicity is further suggested by studies in which iron chelators were shown to be cardioprotective.8 Conversely, iron loading was found to potentiate anthracycline toxicity.9,10 In this context, pre-existing genetic iron overload may well amplify the iron-based toxicity of anthracyclines. The identification of patients at a high risk of developing DOX-related cardiotoxicity may help decide their inclusion for treatment with cardioprotective agents.11
Genetic iron overload is found in hereditary hemochromatosis (HH), a genetic disorder that affects up to 1 in 250 individuals of European descent and has a carrier rate of between 10% and 15%.12 It is characterized by excessive dietary iron absorption in the small intestine.13 The excess iron is stored in the parenchymal cells of major tissues, primarily the liver, heart, pancreas, pituitary, and joints, where its accumulation eventually leads to severe tissue damage.14,15
The gene defective in HH, HFE, encodes a major histocompatibility complex (MHC) class I-like protein,16 consistent with the earlier finding that β2-microglobulin (β2m)-deficient mice develop progressive iron overload similar to that seen in HH.17,18 Confirmation that mutations of HFE cause HH was provided by the demonstration of iron overload in mice with targeted disruption of the Hfe gene.19-21 A major advance into how the HFE-β2m complex influences iron metabolism was the observation that it associates with the transferrin receptor,22,23 an essential component of the transferrin-dependent cellular iron uptake pathway.
Two mutations of the HFE gene are linked to the development of HH: the first, Cys282Tyr, causes HH in homozygotes; the second, His63Asp, may cause HH when associated with Cys282Tyr (compound heterozygotes).24
In this study, we tested the hypothesis that Hfe alters susceptibility to anthracycline toxicity, namely to DOX. The results demonstrate that both homozygous and heterozygous Hfe-deficient mice are more sensitive to DOX toxicity than are wild-type mice and that the alterations in iron storage and iron metabolism induced by DOX are exacerbated in these animals.
Materials and methods
Animals
All procedures were performed in accordance with Canadian Council on Animal Care guidelines and were approved by the Institutional Animal Care Committee of the Centre Hospitalier de l'Université de Montréal (CHUM). Hfe-/- mice have been described previously,20 and those used in these experiments were of the 129/SvEvTac strain.
Acute treatment
Female Hfe-/- mice and wild-type controls (8 weeks old) were injected once intraperitoneally with saline or DOX at 20 mg/kg (Sigma Chemical, St Louis, MO). On days 4, 6, and 12 after DOX treatment, the animals were anesthetized; blood samples and saline-perfused organs were collected for analysis.
Chronic treatment
The 8-week-old wild-type, Hfe+/-, and Hfe-/- female mice were injected intraperitoneally with saline or DOX (0.5 mg/mL) at a dose level of 5 mg/kg body weight per injection, for a total cumulative dose of 30 mg/kg. They were injected once a week with a 2-week interval. In agreement with Canadian Council on Animal Care guidelines concerning end points, moribund animals were killed to avoid suffering. Pilot experiments were performed to determine the conjunction of symptoms predictive of death in less than 24 hours. Symptoms included hunched posture, dehydration, pinched face, starry coat, more than 10% body weight loss, and inactivity. At this point, the animals were unable to feed themselves. Blood samples and saline-perfused organs were collected from the survivors 3 weeks after the last injection, as well as from moribund mice.
Hematologic measurements and transferrin saturation
EDTA (ethylenediaminetetraacetic acid)-treated blood samples were obtained by orbital puncture under anesthesia. Hemoglobin, hematocrit, and mean corpuscular volume were measured with an ABC vet counter (ABX hematologie, Montpellier, France). Serum iron and total iron-binding capacity (TIBC) were determined as described previously,17 by colorimetric assay, using a Kodak Ektachem DT60 instrument (Johnson & Johnson, Ortho Clinical Diagnostics, Mississauga, ON, Canada). Transferrin saturation (TS) was calculated from TIBC and serum iron values.
Measurement of organ iron concentration
Liver, heart, pancreas, and spleen iron content was assessed by acid digestion of tissue samples, followed by iron quantification with atomic absorption spectroscopy.17
Biochemical analysis
Serum creatine kinase (CK), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) activities were measured with the Kodak Ektachem DT60 instrument.
Histopathology
Samples of liver, spleen, heart, and pancreas were fixed in buffered 4% formaldehyde and stained with hematoxylin/eosin or Masson trichrome blue for demonstration of fibrosis. Ferric iron (Fe(III)) was detected by Prussian blue staining.
Electron microscopy and X-ray microanalysis
Small pieces of heart were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer. The tissue was washed twice with the same buffer and divided into 2 groups: one group was osmicated with 1% OsO4, reduced with 1.5% potassium ferrocyanide, and stained with 4% aqueous uranyl acetate and Reynold lead citrate. The other group was processed without OsO4 or staining. Ultrathin sections of a thickness of 70 to 100 nm were prepared using an ultramicrotome (Reichert-Jung Ultracut-E, Vienna, Austria) equipped with a diamond knife. The sections were transferred to 300-mesh copper TEM grids and investigated with a JEOL JEM-2000FX transmission electron microscope (TEM) at 80 kV (JEOL EM 2000FX; Joel LTD, Tokyo, Japan). Qualitative X-ray microanalysis was performed on the nonosmicated/unstained specimens using PGT-Prism energy-dispersive spectrometer (EDS; Princeton Gamma-Tech, Princeton, NJ). Elemental analysis was carried out at 80 kV with a beam diameter of 50 nm for 100 seconds.
Statistical analysis of the data
The results are presented as means ± SD. The Student t test was used for comparison between the control and knock-out mouse groups. Differences with P values less than .05 were considered significant.
Results
Iron metabolism changes after acute DOX treatment
Recently, anthracyclines were shown to modify cellular iron metabolism in vitro by inactivating iron regulatory proteins (IRPs)25 via the formation of anthracycline-iron complexes.26,27 To evaluate changes in iron metabolism after acute DOX treatment in vivo, wild-type and Hfe-/- mice were given a single injection of DOX (20 mg/kg, intraperitoneally) or saline (n = 7-8 per group). Serum was collected on day 4 and analyzed for iron levels and TS. As expected,20 saline-treated Hfe-/- mice showed elevated serum iron and TS compared with saline-treated wild-type controls (Table 1). After DOX treatment, similar values were observed in both strains, with serum iron and TS increasing in wild-type mice to levels close to those observed in saline-treated Hfe-/- controls. Elevated serum iron was also observed in Hfe-/- mice after DOX treatment (Table 1).
Serum iron (SI), total iron binding capacity (TIBC), and transferrin saturation (TS) by strain on day 4 after a single DOX injection (20 mg/kg)
Treatment . | SI, μM . | TIBC, μM . | TS, % . |
|---|---|---|---|
| Wild-type | |||
| Saline | 24.8 ± 7.1 | 43.2 ± 4.1 | 58.1 ± 18.7 |
| DOX | 42.4 ± 8.1* | 45.9 ± 3.7 | 92.8 ± 18.2* |
| Hfe-/- | |||
| Saline | 41.7 ± 5.8† | 42.1 ± 5.9 | 100.4 ± 13.3† |
| DOX | 50.1 ± 8.1* | 51.0 ± 7.4* | 106.0 ± 6.0 |
Treatment . | SI, μM . | TIBC, μM . | TS, % . |
|---|---|---|---|
| Wild-type | |||
| Saline | 24.8 ± 7.1 | 43.2 ± 4.1 | 58.1 ± 18.7 |
| DOX | 42.4 ± 8.1* | 45.9 ± 3.7 | 92.8 ± 18.2* |
| Hfe-/- | |||
| Saline | 41.7 ± 5.8† | 42.1 ± 5.9 | 100.4 ± 13.3† |
| DOX | 50.1 ± 8.1* | 51.0 ± 7.4* | 106.0 ± 6.0 |
Data are presented as means ± SD.
P < .05, comparison between saline-treated and DOX-treated mice (n = 7-8 per group).
P < .05, comparison between wild-type and mutant mice with the same treatment.
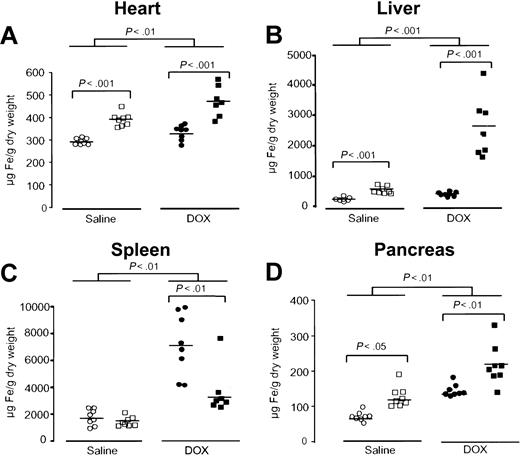
Next, the effect of acute DOX treatment on tissue iron concentrations was evaluated in the heart, liver, spleen, and pancreas. Iron levels in the heart of saline-treated Hfe-/- mice were higher than in saline-treated wild-type mice (P < .001, Figure 1A). After DOX treatment, both wild-type and Hfe-/- mice showed a significant increase in iron levels in the heart (P < .01, Figure 1A). However, DOX led to significantly higher iron accumulation in the heart of Hfe-/- mice compared with wild-type mice receiving the same treatment (P < .001, Figure 1A). As expected, higher iron levels were found in the liver of saline-treated Hfe-/- mice than in wild-type controls (P < .001, Figure 1B), evidencing the preexisting iron burden in this mouse strain.20 After DOX treatment, liver iron levels rose dramatically in Hfe-/- mice (P < .001), whereas a much smaller, but yet significant, increase was noted in wild-type mice (P < .001, Figure 1B). Conversely, in the spleen, iron concentration was remarkably augmented in DOX-treated wild-type controls (P < .01), whereas the same treatment induced a significantly lower elevation in Hfe-/- mice (P < .01, Figure 1C). To exclude the possibility that this increase was a reflection of massive cell death with the corresponding loss of spleen weight,28 therefore masking the real capacity of the spleen to store iron, we calculated the amount of total iron in the spleen. Total iron was significantly augmented in the spleen of DOX-treated wild-type mice (49 ± 24 μg Fe to 82 ± 24 μg Fe, P < .02), and it remained unchanged in treated Hfe-/- mice (50 ± 22 μg Fe to 39 ± 11 μg Fe). Finally, in the pancreas, iron levels were also increased in both mouse groups after treatment (P < .01), with higher concentrations being found in Hfe-/- mice (P < .01, Figure 1D).
Acute DOX treatment induces alterations in iron metabolism that are exacerbated in Hfe-deficient mice. Iron concentration in the heart (A), liver (B), spleen (C), and pancreas (D) of saline-treated (white symbols) and DOX-treated, day 4, (black symbols), wild-type (○, •) and Hfe-/- (□, ▪) mice. Horizontal bars represent means. Differences between strains and between treatments were determined by Student t test.
Acute DOX treatment induces alterations in iron metabolism that are exacerbated in Hfe-deficient mice. Iron concentration in the heart (A), liver (B), spleen (C), and pancreas (D) of saline-treated (white symbols) and DOX-treated, day 4, (black symbols), wild-type (○, •) and Hfe-/- (□, ▪) mice. Horizontal bars represent means. Differences between strains and between treatments were determined by Student t test.
Tissue iron concentrations in wild-type mice after a single injection of DOX tended to return to pre-injection levels by day 12, whereas in Hfe-/- mice they remained elevated, especially in the liver, suggesting that these mice recover at a slower rate (data not shown).
These results indicate that acute DOX treatment induces alterations in iron metabolism in vivo, as is evidenced by an increase in tissue and serum iron levels. These alterations are intensified in Hfe-/- mice.
Cellular distribution of excess iron in the heart and liver
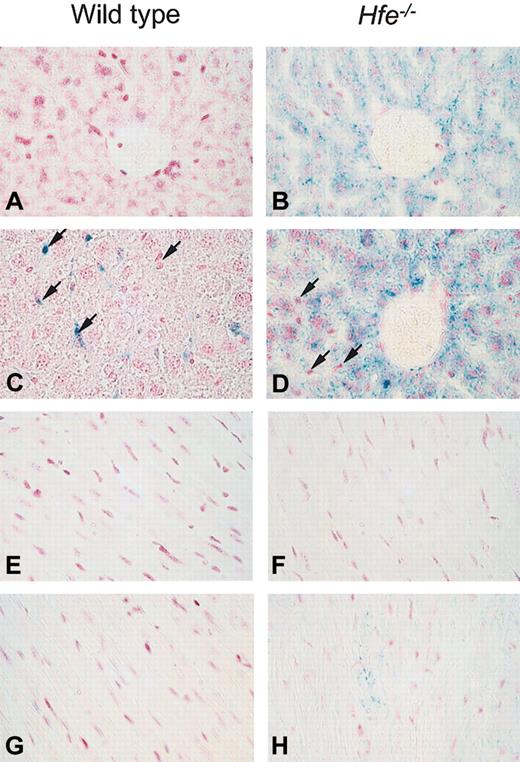
A pivotal factor in iron-related toxicity is the presence of iron in the parenchymal cells of affected tissues, as opposed to reticuloendothelial macrophages, which are believed to be more resistant to iron toxicity.29 The cellular distribution of ferric iron was determined by Prussian blue staining on days 4 (n = 7-8) and 12 (n = 7) after DOX treatment. No iron was detected in the liver and heart of saline-treated wild-type mice (Figure 2A,E). As reported previously,20 in saline-treated Hfe-/- mice iron was present predominantly in parenchymal cells of the liver (Figure 2B) but not the heart (Figure 2F). After DOX treatment, iron deposition in Hfe-/- mice was found mainly in parenchymal cells (Figure 2D) and cardiomyocytes at day 4 (Figure 2H) and day 12 (data not shown). In contrast, DOX-treated wild-type mice either did not show any iron staining in the heart and liver, or iron was detected only in liver Kupffer cells in 2 mice of 8 at day 4 (Figure 2C) and in 2 mice of 7 at day 12 (data not shown). Additional histologic examination of the liver showed no pathology other than distinct iron storage in both mouse strains.
Iron storage in hepatic cells and heart after acute DOX treatment in wild-type and Hfe-deficient mice (Prussian blue staining). Light micrographs of liver (A-D) and heart (E-F) sections from saline-treated (A-B and E-F) or DOX-treated, day 4, (C-D and G-H) wild-type and Hfe-/- mice. In the livers of Hfe-/- mice, parenchymal cells with increased iron content after DOX treatment are visible around the portal spaces. In DOX-treated wild-type mice, iron could be detected only in 2 of 8 mice at day 4 (C) and 2 of 7 mice at day 12 and was found in Kupffer cells (arrows, C-D). In the heart, iron was detected in cardiomyocytes of DOX-treated Hfe-/- mice (H). Original magnification, × 400.
Iron storage in hepatic cells and heart after acute DOX treatment in wild-type and Hfe-deficient mice (Prussian blue staining). Light micrographs of liver (A-D) and heart (E-F) sections from saline-treated (A-B and E-F) or DOX-treated, day 4, (C-D and G-H) wild-type and Hfe-/- mice. In the livers of Hfe-/- mice, parenchymal cells with increased iron content after DOX treatment are visible around the portal spaces. In DOX-treated wild-type mice, iron could be detected only in 2 of 8 mice at day 4 (C) and 2 of 7 mice at day 12 and was found in Kupffer cells (arrows, C-D). In the heart, iron was detected in cardiomyocytes of DOX-treated Hfe-/- mice (H). Original magnification, × 400.
Thus, DOX induces iron accumulation in different cells of the liver in wild-type compared with Hfe-/- mice. In the heart, iron is detected in cardiomyocytes of Hfe-/- mice after DOX treatment.
Effect of acute DOX treatment on serum CK, AST, and ALT
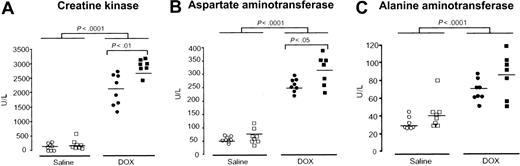
The increase in tissue iron levels observed in Hfe-/- mice after DOX treatment may potentiate its toxic effect by free radical generation.30 To evaluate the sensitivity of cardiac and liver tissues to DOX-induced damage, serum levels of CK, AST, and ALT were analyzed in wild-type and Hfe-/- mice 4 days after a single injection of DOX (20 mg/kg, intraperitoneally). Hfe-deficient mice displayed a significant increase of CK and AST compared with wild-type mice (P < .01 and P < .05, Figure 3A-B), suggesting that these animals are more sensitive to heart and muscle damage. A significant increase in ALT levels was also observed in DOX-treated animals, but no significant differences were observed between wild-type and Hfe-/- mice (Figure 3C).
Effect of Hfe deficiency on DOX-induced increase in serum creatine kinase (CK), aspartate aminotransferase (AST), and alanine aminotransferase (ALT). Serum CK (A), AST (B), and ALT (C) were measured in saline-treated (white symbols) and DOX-treated, day 4, (black symbols) wild-type (○, •) and Hfe-/- (□, ▪) mice. Horizontal bars represent means. Differences between strains and between treatments were determined by Student t test.
Effect of Hfe deficiency on DOX-induced increase in serum creatine kinase (CK), aspartate aminotransferase (AST), and alanine aminotransferase (ALT). Serum CK (A), AST (B), and ALT (C) were measured in saline-treated (white symbols) and DOX-treated, day 4, (black symbols) wild-type (○, •) and Hfe-/- (□, ▪) mice. Horizontal bars represent means. Differences between strains and between treatments were determined by Student t test.
Survival of mice chronically treated with DOX
In view of the frequency of heterozygous individuals harboring HFE mutations in populations of European descent,31 we included heterozygous Hfe-deficient (Hfe+/-) mice in studies of chronic DOX toxicity, a clinically relevant feature.32
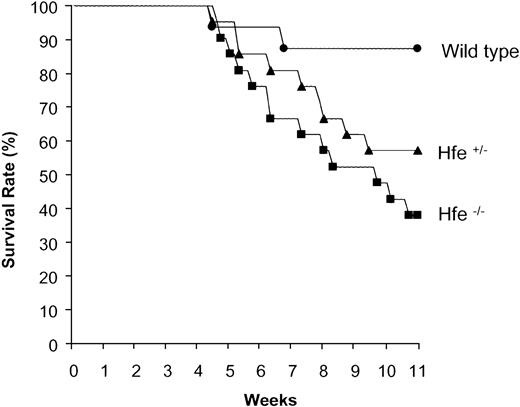
Wild-type, Hfe+/- and Hfe-/- mice were chronically treated with DOX as described in “Materials and methods.” Mortality after DOX treatment was observed to start at a cumulative dose of 25 mg/kg body weight. As shown in Figure 4, chronically administered DOX induced 62% lethality in Hfe-/- mice, 43% in Hfe+/- mice, and 12% in wild-type mice.
Increased mortality of heterozygous (Hfe+/-) and homozygous (Hfe-/-) mice chronically treated with DOX. Representative experiment showing the survival rates of wild-type (•), Hfe+/- (▴), and Hfe-/- (▪) mice (n = 16 per group) chronically treated with DOX.
Increased mortality of heterozygous (Hfe+/-) and homozygous (Hfe-/-) mice chronically treated with DOX. Representative experiment showing the survival rates of wild-type (•), Hfe+/- (▴), and Hfe-/- (▪) mice (n = 16 per group) chronically treated with DOX.
Body weight changes are presented in Table 2. DOX treatment caused a significant reduction in the body weight of dying mice (P < .05), irrespective of the genotype. Conversely, mice that did survive until the end of the experiment responded differently to chronic DOX treatment, depending on their genotype. DOX-treated wild-type mice had similar body weights as their saline-treated controls, whereas the body weight of DOX-treated Hfe+/- and Hfe-/- mice was significantly lower than that of their matched controls (P < .05, Table 2). A significant reduction of heart and liver weight was observed in all nonsurviving DOX-treated mice (P < .05), whereas in the survivors the decrease in heart and liver mass was intermediate (data not shown).
Body weight changes by strain in chronically DOX-treated mice (total cumulative dose, 30 mg/kg)
. | Body weight, g . | . | |
|---|---|---|---|
| Treatment . | Initial . | Terminal . | |
| Wild-type | |||
| Saline | 20.8 ± 2.3 | 26.3 ± 4.0 | |
| DOX | |||
| Survivors | 21.3 ± 0.8 | 25.2 ± 4.5 | |
| Nonsurvivors | 20.4 ± 2.2 | 18.4 ± 0.6* | |
| Hfe+/− | |||
| Saline | 20.3 ± 2.2 | 24.8 ± 2.0 | |
| DOX | |||
| Survivors | 21.8 ± 2.4 | 22.2 ± 1.5*† | |
| Nonsurvivors | 21.3 ± 2.3 | 17.0 ± 1.3* | |
| Hfe−/− | |||
| Saline | 19.6 ± 1.9 | 24.3 ± 3.2 | |
| DOX | |||
| Survivors | 19.9 ± 1.1 | 21.2 ± 1.9*† | |
| Nonsurvivors | 20.0 ± 1.4 | 17.8 ± 2.0* | |
. | Body weight, g . | . | |
|---|---|---|---|
| Treatment . | Initial . | Terminal . | |
| Wild-type | |||
| Saline | 20.8 ± 2.3 | 26.3 ± 4.0 | |
| DOX | |||
| Survivors | 21.3 ± 0.8 | 25.2 ± 4.5 | |
| Nonsurvivors | 20.4 ± 2.2 | 18.4 ± 0.6* | |
| Hfe+/− | |||
| Saline | 20.3 ± 2.2 | 24.8 ± 2.0 | |
| DOX | |||
| Survivors | 21.8 ± 2.4 | 22.2 ± 1.5*† | |
| Nonsurvivors | 21.3 ± 2.3 | 17.0 ± 1.3* | |
| Hfe−/− | |||
| Saline | 19.6 ± 1.9 | 24.3 ± 3.2 | |
| DOX | |||
| Survivors | 19.9 ± 1.1 | 21.2 ± 1.9*† | |
| Nonsurvivors | 20.0 ± 1.4 | 17.8 ± 2.0* | |
Data are presented as means ± SD.
P < .05, comparison between saline-treated and DOX-treated mice.
P < .05, comparison between wild-type and mutant mice with the same treatment.
Taken together, the capacity of Hfe-/- and, importantly, Hfe+/- mice to resist chronic administration of DOX was strikingly affected.
Iron metabolism changes in chronically DOX-treated mice
To evaluate iron metabolism changes induced by chronic DOX administration, serum iron, TIBC, TS, and organ iron concentrations were measured in saline-treated and DOX-treated survivors and, when possible, in moribund animals. As presented in Table 3, serum iron and TS were significantly higher in both Hfe homozygous and heterozygous mice treated with saline than in wild-type controls (P < .0001), revealing that Hfe+/- mice also display an intermediate iron burden compared with wild-type and Hfe-/-mice. Moribund DOX-treated wild-type and Hfe+/- mice had significantly increased TS and decreased TIBC compared with their respective saline-treated controls (P < .001), whereas in Hfe-/- mice TS remained close to 100%, and TIBC was slightly reduced but did not reach statistical significance. In marked contrast, all DOX-treated surviving mice, irrespective of their genotype, had similar serum iron, TS, and TIBC as their matched saline-treated controls.
Serum iron (SI), total iron binding capacity (TIBC), and transferrin saturation (TS) by treatment in mice chronically treated with DOX
Treatment . | SI, μM . | TIBC, μM . | TS, % . |
|---|---|---|---|
| Wild-type | |||
| Saline | 21.4 ± 2.9 | 47.6 ± 4.4 | 45.0 ± 6.2 |
| DOX | |||
| Survivors | 26.2 ± 4.9 | 47.2 ± 2.8 | 55.1 ± 9.7 |
| Nonsurvivors | 30.5 ± 6.8* | 33.3 ± 10.6* | 93.0 ± 9.3* |
| Hfe+/− | |||
| Saline | 31.9 ± 4.3† | 46.1 ± 1.8 | 67.0 ± 9.0† |
| DOX | |||
| Survivors | 25.8 ± 2.9 | 47.8 ± 3.7 | 54.0 ± 8.0 |
| Nonsurvivors | 30.6 ± 6.8 | 33.4 ± 5.8* | 94.5 ± 18.0* |
| Hfe−/− | |||
| Saline | 41.5 ± 3.7† | 42.4 ± 2.1‡ | 98.0 ± 7.4† |
| DOX | |||
| Survivors | 39.3 ± 8.3 | 41.3 ± 7.4‡ | 95.0 ± 7.7 |
| Nonsurvivors | 36.3 ± 10.2 | 36.6 ± 10.0 | 99.5 ± 8.1 |
Treatment . | SI, μM . | TIBC, μM . | TS, % . |
|---|---|---|---|
| Wild-type | |||
| Saline | 21.4 ± 2.9 | 47.6 ± 4.4 | 45.0 ± 6.2 |
| DOX | |||
| Survivors | 26.2 ± 4.9 | 47.2 ± 2.8 | 55.1 ± 9.7 |
| Nonsurvivors | 30.5 ± 6.8* | 33.3 ± 10.6* | 93.0 ± 9.3* |
| Hfe+/− | |||
| Saline | 31.9 ± 4.3† | 46.1 ± 1.8 | 67.0 ± 9.0† |
| DOX | |||
| Survivors | 25.8 ± 2.9 | 47.8 ± 3.7 | 54.0 ± 8.0 |
| Nonsurvivors | 30.6 ± 6.8 | 33.4 ± 5.8* | 94.5 ± 18.0* |
| Hfe−/− | |||
| Saline | 41.5 ± 3.7† | 42.4 ± 2.1‡ | 98.0 ± 7.4† |
| DOX | |||
| Survivors | 39.3 ± 8.3 | 41.3 ± 7.4‡ | 95.0 ± 7.7 |
| Nonsurvivors | 36.3 ± 10.2 | 36.6 ± 10.0 | 99.5 ± 8.1 |
Data are presented as means ± SD.
P < .001, comparison between saline-treated and DOX-treated mice.
P < .0001, comparison between wild-type and mutant mice with the same treatment.
P < .05, comparison between wild-type and mutant mice with the same treatment.
Iron concentration in the heart of nonsurvivors was found to be significantly elevated in all strains after chronic DOX treatment (wild-type, 441 ± 17 μg Fe/g dry wt, P < .01; Hfe+/-, 542 ± 49 μg Fe/g dry wt, P < .0001; Hfe-/-, 495 ± 64 μg Fe/g dry wt, P < .0005). Among the survivors, only Hfe+/- and Hfe-/- mice had significantly increased iron concentrations in the heart (Hfe+/-, 418 ± 61 μg Fe/g dry wt, P < .05; Hfe-/-, 615 ± 203 μg Fe/g dry wt, P < .0005).
In the liver, iron levels were found to be augmented in nonsurviving Hfe+/- mice (1.5-fold increase over saline-treated Hfe+/- mice) and in both surviving and nonsurviving Hfe-/- mice (2-fold increase). Finally, in the spleen, a 2- to 3-fold elevation of total iron was observed in wild-type and Hfe+/- DOX-treated mice, whereas in Hfe-/- mice, total iron content remained similar to that in matched saline-treated controls (data not shown).
Prussian blue staining of liver sections from chronically treated mice revealed a similar distribution of iron as reported previously with acute treatment. Heterozygous Hfe-deficient mice had iron in both Kupffer and parenchymal cells surrounding the portal areas (data not shown).
Overall, chronic DOX treatment induced significant alterations in iron homeostasis demonstrated by increases in plasma and tissue iron levels. These changes were consistently more prominent in Hfe-/- mice compared with wild-type mice, whereas heterozygous Hfe-deficient mice showed intermediate modifications.
Mitochondrial damage and iron deposits in chronically treated mice
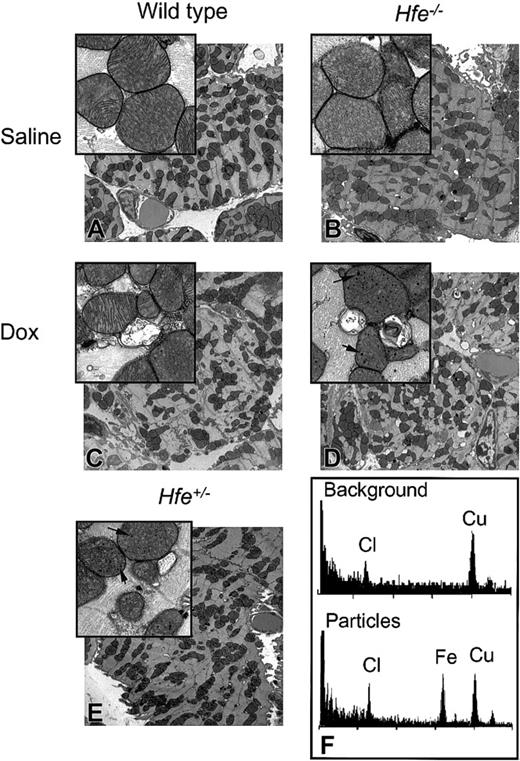
Mitochondrial damage and the disruption of myofibrillar organization are hallmarks of chronic DOX administration and cardiotoxicity.33,34 We, therefore, assessed ultrastructural changes in the mitochondria of heart tissue of DOX-treated mice by TEM. No differences were observed in cellular structure between saline-treated wild-type and Hfe-/- mice (Figure 5A-B). The DOX-treated Hfe-/- mice displayed more extensive mitochondrial degeneration in association with marked swelling, cristae disorganization, and myofibril degeneration (Figures 5D and 6B) compared with intermediate levels found in DOX-treated Hfe+/- (Figures 5E and 6C) and in wild-type mice (Figures 5C and 6A).
Ultrastructural changes in the mouse heart after chronic DOX treatment. TEM images of ultrathin sections of mouse hearts from saline-treated (A-B) and DOX-treated (C-E), nonsurviving wild-type (C), Hfe-/- (D), and heterozygous Hfe+/- (E) mice. Damaged mitochondria and electron-dense particles (magnified inset, D-E) are evident in DOX-treated mice (arrows). Original magnifications: × 5000 (A-E); × 25 000 (insets). (F) Analysis of the particles by energy-dispersive X-ray microanalysis in nonosmicated ultrathin sections obtained from the heart tissue from the same mouse shown in panel D. EDS spectra from background (top panel) and particles (bottom panel). Cl (chlorine) and Cu (copper) were detected in all samples; Fe (iron) was detected exclusively in the particles.
Ultrastructural changes in the mouse heart after chronic DOX treatment. TEM images of ultrathin sections of mouse hearts from saline-treated (A-B) and DOX-treated (C-E), nonsurviving wild-type (C), Hfe-/- (D), and heterozygous Hfe+/- (E) mice. Damaged mitochondria and electron-dense particles (magnified inset, D-E) are evident in DOX-treated mice (arrows). Original magnifications: × 5000 (A-E); × 25 000 (insets). (F) Analysis of the particles by energy-dispersive X-ray microanalysis in nonosmicated ultrathin sections obtained from the heart tissue from the same mouse shown in panel D. EDS spectra from background (top panel) and particles (bottom panel). Cl (chlorine) and Cu (copper) were detected in all samples; Fe (iron) was detected exclusively in the particles.
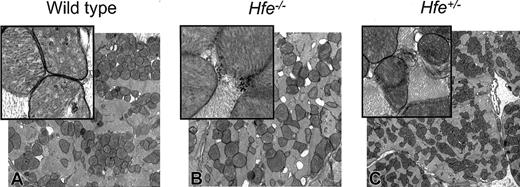
Electron micrograph of mouse heart from DOX-treated survivors. (A) Wild-type mice. (B) Hfe-/- mice. (C) Heterozygous Hfe+/- mice. Damaged mitochondria (magnified inset, B-C) are evident. Original magnifications: × 10 000 (A-C); × 50 000 (insets).
Electron micrograph of mouse heart from DOX-treated survivors. (A) Wild-type mice. (B) Hfe-/- mice. (C) Heterozygous Hfe+/- mice. Damaged mitochondria (magnified inset, B-C) are evident. Original magnifications: × 10 000 (A-C); × 50 000 (insets).
Electron-dense nano-particles were observed within the mitochondria in DOX-treated, nonsurviving Hfe-/- and Hfe+/- mice (Figures 5D-E, insets). Microanalysis of these particles in nonosmicated ultrathin section confirmed the presence of iron (Figure 5F), suggesting the formation of particles containing iron in the mitochondria of the heart tissue of DOX-treated mice. In addition to the iron peak at 6.4 keV, the energy-dispersive spectra exhibited 2 peaks: a chlorine peak at 2.6 keV and a copper peak at 8.0 keV (Figure 5F). The copper peak is from the grid. Chlorine is not associated with the iron, as it is present in both the background and mitochondria.
These results suggest that increased mitochondrial damage and myofibril degeneration may be linked to alterations in mitochondrial iron homeostasis.
Discussion
Recent advances in the field of iron metabolism have led to the identification of an impressive number of genes involved in cellular iron uptake, release, and storage. With them, an increasing number of mutations in genes involved in iron metabolism have been reported.35 Many of those genetic alterations heighten the risk of developing moderate to severe iron overload.36
The toxic potential of iron has been studied extensively, and it is now well established that iron plays a key role in the generation of ROS, which ultimately causes oxidative damage. The perception that iron-based oxidative stress is an important pathway mediating tissue damage has now extended from obvious primary and secondary iron overload conditions to many other diseases in which neurologic and/or cardiac involvement are prominent.35,37 Not surprisingly, the question is naturally now emerging as to whether genetic alterations associated with iron metabolism affect drug interactions, especially in those cases in which interference with iron metabolism has been observed.
In the current study, we addressed this question by using DOX, an anthracycline antibiotic widely administered in cancer therapy. DOX toxicity to the heart has been related to its capacity to interfere with iron metabolism, and it is the limiting factor for this otherwise very effective chemotherapeutic agent. We now report a detailed analysis of DOX toxicity in the Hfe-deficient mouse model of HH representing the most common form of genetic iron overload found in populations of European descent.
Acute DOX treatment leads to significant increases in serum iron and TS, as well as iron concentrations in the heart, liver, spleen, and pancreas of wild-type mice, demonstrating that it normally interferes with systemic iron metabolism. The elevation of iron concentration in these tissues was much more accentuated in DOX-treated Hfe-/- mice, particularly in the heart and liver, and cannot be explained solely by the pre-existing iron overload. The already saturated plasma transferrin and, more importantly, the inability to store iron in the spleen may account for the sharp increase in iron levels in other organs. This observation is in agreement with previous observations that in HH, reticuloendothelial macrophages have a defect in iron storage, both in human and mouse models.17,19,20,38,39 Consistently, cellular iron distribution in Hfe-/- mice after DOX treatment remained different from wild-type mice. Wild-type animals accumulated iron in their Kupffer cells, whereas Hfe-/- mice accumulated it in parenchymal cells. This iron distribution is characteristic of HH and extends to all HH mouse models reported so far.17,19-21
Serum CK and AST were significantly higher in DOX-treated Hfe-/- mice, indicating greater sensitivity to acute DOX toxicity. Serum iron and TS after DOX administration were similar in both mouse strains, suggesting that the observed damage in Hfe-/- mice results from high pre-existing TS levels in the serum and iron levels in the heart. The ability of DOX and its main metabolite, doxorubicinol (DOXol), to form stable complexes with iron has been implicated in its toxic effects. DOXol has been shown to contribute to cardiotoxicity by irreversibly inactivating iron regulatory proteins (IRPs), essential regulators of transferrin receptor and ferritin mRNA levels, in less differentiated cardiac cell lines.25 In differentiated myocardial cells, the iron-DOX complexes, but not DOX itself, were shown to both decrease active IRP-RNA binding activity and the total cellular IRP pool,26 indicating that the formation of iron-DOX complexes can directly affect cellular iron metabolism. The consequences of reduced IRP activity and total cellular IRP pool would be the inability of the affected cells to sense modifications in iron availability26 and, therefore, adequately respond by regulating the levels of transferrin receptor and ferritin.40 Eventually, increased systemic iron levels may very well favor the formation of such highly toxic iron complexes and consequently elicit tissue damage once DOXol or DOX-iron complexes are formed in the heart. In fact, iron loading has been shown to potentiate anthracycline toxicity.9,10
Chronic treatment of Hfe-/- mice with DOX leads to significantly decreased body weight and increased mortality compared with wild-type mice along with enhanced alterations in iron metabolism and mitochondrial damage. The survival rate after DOX treatment was 88% for wild-type, 57% for Hfe+/-, and 38% for Hfe-/- mice. Most importantly, Hfe+/- mice carrying a single mutated allele are also more susceptible to DOX toxicity than are wild-type mice. The importance of this observation is highlighted by the fact that about 10% of the population of European descent are heterozygous and may have nonsymptomatic mild iron overload, evidenced by slightly but significantly elevated levels of serum ferritin, serum iron, and TS.41 In this study, we found that, as reported previously,20 control heterozygous Hfe-deficient mice also have significantly elevated serum iron and transferrin saturation compared with control wild-type mice. Heterozygosity for the HFE mutations may, therefore, be a common genetic marker of lifelong moderate iron overload, and such a condition could very well be associated with heightened toxicity.
Eventually, screening for HFE mutations, alone or in combination with iron indices, in patients with cancer before chemotherapy may help identify those at a high risk of developing DOX-related cardiomyopathy. This screening would have the advantage of singling out patients who would mostly benefit from combined treatment with anthracyclines and iron chelators. In fact, iron chelators such as dexrazoxane have been proven to be efficient in reducing cardiotoxicity,8 both in animal models42,43 and in patients given anthracycline-based chemotherapy.44,45 Further retrospective studies in patients with cancer who received DOX-based therapy are necessary to assess if patients who have suffered from anthracycline cardiotoxicity show an increased prevalence of HFE mutations compared with patients who received comparable doses of DOX without showing evidence of cardiotoxicity.
In summary, our results in DOX-treated Hfe-/- and Hfe+/-mice support the view that HFE mutations may be responsible for more DOX-induced toxicity than expected in the presence of normal iron metabolism. Whether HFE mutations, and possibly other genetic alterations associated with defects in iron handling31,35 may alter toxicity to other drugs, needs further investigation.
Prepublished online as Blood First Edition Paper, June 12, 2003; DOI 10.1182/blood-2003-03-0869.
Supported by grants from the Canadian Institutes of Health Research (CIHR; grant MOP44045), the Canadian Breast Cancer Research Initiative (CBRCI; grant 013597), and the Fundação para a Ciência e a Tecnologia (FCT; grant CBO/33485/99-00). M.M.S. is the recipient of a CIHR New Investigator award. N.C.A. is an Associate Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Christian Dallaire for his help with atomic absorption spectroscopy, Deborah Braun for comments, and Ovid da Silva for his editorial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal