Hereditary hemochromatosis (HH) is the most common inherited disorder in humans. It is characterized by an increased absorption of iron and its excessive accumulation in various tissues. If left untreated, the iron accumulates to harmful levels in the parenchymal cells of the liver, heart, and pancreas and can cause severe damage. In the United States, it is estimated that at least a million individuals may have HH. The defect in the majority of HH cases has been linked to the HFE gene. While most individuals with HH are homozygous for the Cys282Tyr HFE mutation, it has been suggested that heterozygosity may confer a selective advantage by protecting against iron depletion and anemia. With a carrier rate of about 10%, it is estimated that 1 in 200 to 1 in 400 individuals of northern European descent are at risk of having this iron overload disorder. Early diagnosis and phlebotomy treatment can effectively reduce complications. Recent epidemiologic studies have made a case in support for and against the screening for the most common mutations of the HFE gene.
In this issue, Miranda and colleagues (page 2574) provide compelling evidence that mutations in the HFE gene may influence treatment for other disorders as well. The anthracycline, doxorubicin (DOX), is one of the most widely used and active cytotoxic agents in cancer chemotherapy. Its use however has been affected by its cardiotoxicity. The authors tested the hypothesis that toxicity to DOX may be modulated by mutations that result in genetic iron overload. Using a murine model of hemochromatosis, the Hfe knockout mouse, they show that acute DOX treatment of homozygous knockout mice results in increased iron in the serum and its accumulation in tissues. Besides the expected accumulation of iron in the liver, they also found excessive deposition in the heart.
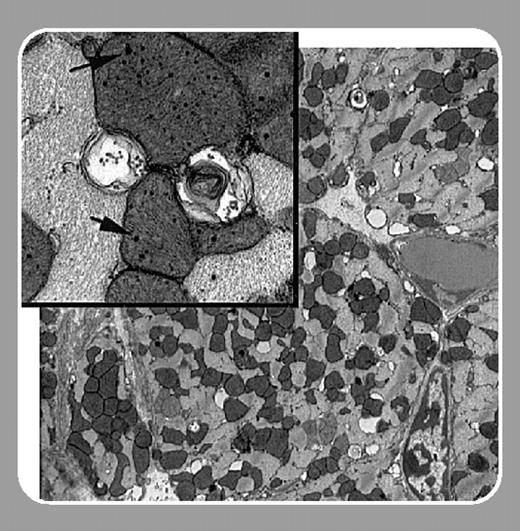
To further simulate the distribution of hemochromatosis mutations in the general population, they studied the effect of chronic administration of DOX on both heterozygous and homozygous knockout mice. Both groups of mice displayed increased mortality and excessive iron accumulation when compared with wild-type mice. Significantly, Miranda et al show that the iron accumulation results in extensive mitochondrial damage in heart tissue.FIG1
Recently, Swain et al1 reported that DOX-related cardiotoxicity and induced congestive heart failure occur at a greater frequency than was previously reported, and that patients of advanced age may be at a greater risk. As iron accumulation increases with age, this observation has profound implications when considered along with the results presented by Miranda et al. These studies warrant a closer look at the relationship between DOX cardiotoxicity and the prevalence of HFE mutations and other genetic changes that affect iron homeostasis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal