Abstract
We have previously found that P210BCR-ABL increases the adhesion of hematopoietic cell lines to fibronectin by a mechanism that is independent of tyrosine kinase activity. To investigate the pathway(s) by which P210BCR-ABL influences cell adhesion, we used a quantitative cell adhesion device that can discern small changes in cell adhesion to assay P210BCR-ABL with mutations in several critical domains. We expressed P210BCR-ABL mutants in 32D myeloblast cells and found that binding to fibronectin is mediated primarily by the α5β1 integrin. We performed a structure/function analysis to map domains important for cell adhesion. Increased adhesion was mediated by 3 domains: (1) the N-terminal coiled-coil domain that facilitates oligomerization and F-actin localization; (2) bcr sequences between aa 163 to 210; and (3) F-actin localization through the C-terminal actin-binding domain of c-abl. We compared our adhesion results with the ability of these mutants to cause a chronic myelogenous leukemia (CML)–like disease in a murine bone marrow transplantation assay and found that adhesion to fibronectin did not correlate with the ability of these mutants to cause CML. Together, our results suggest that F-actin localization may play a pivotal role in modulating adhesion but that it is dispensable for the development of CML.
Introduction
P210BCR-ABL, the oncogene formed by a balanced rearrangement between the c-abl gene on chromosome 9 and the bcr gene on chromosome 22, leads to the formation of the Philadelphia (Ph) chromosome, a diagnostic marker of chronic myelogenous leukemia (CML).1,2 Expression of P210BCR-ABL in hematopoietic progenitor cells causes deregulated cell proliferation, decreased programmed cell death, and abnormal adhesion to bone marrow (BM) stroma (for a review, see Wertheim et al3 ). Together, these processes are thought to lead to the defects in cell number and maturation that characterize CML. Defective cellular adhesion may enhance cell cycling and egress of leukemia cells out of the BM into peripheral blood, allowing cells to home to extramedullary sites.4-6
P210BCR-ABL is found primarily in the cytoplasm, closely associated with F-actin through a C-terminal actin-binding domain.7,8 Additionally, P210BCR-ABL phosphorylates several proteins of the focal adhesion complex.9 Modification of linkages between cancerous cells and the extracellular matrix and interactions between P210BCR-ABL and the cell cytoskeleton may be important for its oncogenic functions. P210BCR-ABL likely plays a direct role in modifying cell adhesion; however, the specific functional properties of P210BCR-ABL that cause defective adhesion have not been identified.
Phosphorylation of intracellular signaling cascades by P210BCR-ABL tyrosine kinase is critical for cell transformation and development of a CML-like disease in mice.10 Clinical trials with the specific c-abl tyrosine kinase inhibitor STI-571 (imatinib mesylate [Gleevec]) show that hematologic and cytologic remission can be achieved by attenuating the P210BCR-ABL tyrosine kinase activity indicating that the tyrosine kinase domain is essential for disease pathogenesis.11 P210BCR-ABL phosphorylates several proteins involved in cell adhesion—including paxillin, FAK, and CRKL— that are associated with focal adhesion contacts.9,12 However, neither the attenuation of tyrosine kinase activity by STI-571 nor a lysine-to-arginine point mutation at amino acid (aa) 1176 rendering the tyrosine kinase domain inactive can correct for the adhesive defect caused by P210BCR-ABL expression in transformed human and murine myeloid cell lines.13
The fusion of bcr to the N-terminal portion of c-abl leads to cell transformation by deregulating c-abl functions that are normally tightly controlled.7 Recent results suggest that the N-terminal coiled-coil (C-C) domain may not be required to activate the P210BCR-ABL tyrosine kinase because mutants lacking this domain exhibit elevated tyrosine kinase activity in cell lines.14,15 Nonetheless, the C-C region contributed by bcr leads to dimerization and tetramerization16,17 of P210BCR-ABL that may enhance the c-abl tryosine kinase or that may be required for actin binding.7
Although the c-abl actin-binding function is necessary for F-actin localization and transformation of Rat-1 fibroblasts,16 the contribution of F-actin localization to either P210BCR-ABL–induced adhesion defects or CML remains unclear. Failure to oligomerize P210BCR-ABL by deletion of the bcr C-C domain severely retards the development of myeloproliferative disease (MPD) in mice, most likely because of a decrease in tyrosine kinase activity.14,15 However, loss of F-actin binding is an alternative explanation because the effect of F-actin localization on P210BCR-ABL–induced disease has not been studied in a murine BM transplantation model of CML.
We used a quantitative cell adhesion device that can discriminate between changes in cell adhesion by specifically measuring the strength of binding between a population of adherent cells and fibronectin, a major component of the BM extracellular matrix.18 Using this device, we have shown that expression of P210BCR-ABL in a myeloblast cell line, 32D, leads to increased cell adhesion in accordance with several published studies using qualitative plate and wash assays.4,13,19 We also found that expression of a mutant lacking tyrosine kinase activity failed to normalize cell adhesion and that treatment of Meg-01 cells, a P210BCR-ABL–expressing cell line from a CML patient in blast crisis, with STI-571 had no effect on binding to fibronectin, suggesting that P210BCR-ABL does not enhance adhesion through inside-out signaling originating at its tyrosine kinase.13
We constructed several mutants with domain deletions or point mutations in key regions in P210BCR-ABL and expressed these mutants in 32D cells to determine the region in P210BCR-ABL that is responsible for enhancing adhesion to fibronectin. We identified 3 regions that are necessary for P210BCR-ABL to cause elevated adhesion: the N-terminal C-C domain that facilitates oligomerization and F-actin localization, the bcr sequences between aa 163 to 210, and the C-terminal actin-binding domain of c-abl. We were particularly interested in the relationship between F-actin localization, elevated cell adhesion to fibronectin, and MPD development in our mouse model. Here we report that deletion of the C-terminal actin-binding domain had no effect on development of the MPD. Together, our findings indicate that localization of P210BCR-ABL to the actin cytoskeleton is responsible for abnormal adhesion to fibronectin; however, F-actin binding, and potentially deregulated cell adhesion to fibronectin, is not a primary determinant for murine CML development.
Materials and methods
Plasmid construction and cell preparation
To create Δ(1-63) BCR-ABL lacking the N-terminal C-C domain, a 1.4-kb HpaI/BamHI fragment was released from MigP210BCR-ABL.20 An ATG start codon was inserted upstream of aa 64 by generating a polymerase chain reaction (PCR) fragment using the 5′ primer CT CTC GAGGTTAAC ATG GCC AAG GAA AAG AAG AGC (boldface and underlined sequences are recognized by XhoI and HpaI, respectively) and the 3′ primer ACG TAG AAG GGC TTC TCG. This fragment was digested with HpaI and BamHI and was ligated to HpaI/BamHI-digested MigP210BCR-ABL. The resultant plasmid was cut with BamHI, and the 1.4-kb fragment of P210BCR-ABL was inserted in the correct orientation to construct MigΔ(1-63) BCR-ABL. Δ(1-63) BCR-ABL was released with HpaI and EcoRI and was ligated to the analogous site in the pK1 vector, a murine stem cell leukemia virus (MSCV)–based vector that is identical to MigRI except that an internal ribosomal entry site (IRES)–puromycin resistance gene was inserted in place of the IRES-green fluorescence protein (IRES-GFP) (kind gift from Karen Ehrmann and Stephen Emerson, University of Pennsylvania, Philadelphia).
The (1-162) BCR-ABL mutant was cloned through the release of full-length BCR-ABL from MigP210BCR-ABL with EcoRI. The c-abl portion was isolated by digestion with HincII and was cloned into the HpaI–EcoRI site of the pK1 vector. The BCR-ABL breakpoint at aa 162 was generated through sequential PCR amplification of a fragment containing the first 486 bp bcr and 3′ overlapping abl sequences (fragment 1, 5′ primer 7779114 TCA CTC CTT CTC TAG GC and 3′ primer GCCGCTGAAGGGCTTTGC GGA TCC GCT CGA AG, abl sequences underlined) and a second fragment (fragment 2, 5′ primer CTT CGA GCG GAT CCG CAAAGCCCT TCAGCGGC and 3′ primer 7779314 AGC AGA TAC TCA GCG GC) containing 5′ overlapping bcr sequences. Fragments 1 and 2 were used as templates to generate a fragment that contained the BCR-ABL breakpoint at aa 162 using primers 77791 and 77793. This fragment was digested with HincII and was ligated in the correct orientation into the HpaI site of pK1 already containing the c-abl fragment. The BCR-ABL cassettes in MigP210BCR-ABL, MigRI 1-210 BCR-ABL,14 MigRI (1-63) BCR-ABL,14 and MSCV 2.2 ΔActin BCR-ABL21 were released with EcoRI and were cloned into the pK1 vector at the corresponding site.
High-titer retroviral supernatants were generated by transfection into Bosc23 cells.20 Viral titers were normalized by GFP expression of transduced NIH 3T3 cells (MigRI constructs) or colony formation of puromycin-resistant NIH 3T3 cells (pK1 constructs).14 32D cells22 were maintained in 32D culture media13 and were transduced by spinoculation.23 At 48 hours after transduction, 32D cells transduced with vectors containing the puromycin resistance gene were selected with puromycin (1.5 μg/mL) for 4 days and maintained in 32D media containing 10% WEHI supernatant. 32D cells transduced with GFP as a surrogate marker for P210BCR-ABL expression were sorted for GFP expression (MigRI) or were selected for growth in the absence of interleukin-3 (IL-3) (MigP210BCR-ABL) for 3 to 4 days, after which WEHI was reintroduced into the culture medium to maintain consistent growth rates.
Before they were spun, all cells were washed and transferred to a Tris-based buffer as described.13 Cells were treated with control or adhesion blocking reagents for 10 minutes before incubation with fibronectin. Functional blocking antibody PS/2 (anti-α4 integrin) was a gift from C. Buck (Wistar Institute, Philadelphia, PA) and L. Terracio (University of South Carolina, Columbia).24,25 BMA5 (anti-α5 integrin) was obtained from Chemicon (Temecula, CA).26
Adhesion assays
Circular glass coverslips (Fisher Scientific, Hampton, NH) were adsorbed with 10 μg/mL fibronectin (Becton Dickinson, Franklin Lakes, NJ) and were blocked with 1% bovine serum albumin (BSA). The cell detachment device was operated as described.27 Adhesion experiments were carried out in the absence of growth factors. Coverslips were analyzed by recording the adherent fraction of cells at various radial distances from the center. The shear stress, τ, imparted on the cells was calculated by the equation τ = 0.800 r (ρμω3)1/2, where τ is proportional to r, the radial distance; μ and ρ are the fluid dynamic viscosity and density, respectively; and ω is the angular velocity of the spinning disk. The fluid flow that is established by a spinning disk has been characterized,28,29 and the fraction of beads or cells under shear flow that remain bound to an adhesive surface is known to follow a sigmoid-shaped model.30 Adhesion profiles for this study were prepared27 and were accepted for R2 ≥ 0.70. This is an arbitrary value to ensure that the cell distribution data conform to the numeric model; however, increasing or decreasing this value does not significantly affect the measurement of cell adhesion (data not shown). This criterion was used for all conditions with the exception of runs using cells incubated with reagents that severely block cell adhesion or when cells were incubated on matrices of BSA alone because of the few cells that remained attached after a spin.
The critical shear stress, τ50, was measured as the surface shear stress, τ, which is at an adherent fraction of 0.50 along the fitted curve. Determination of significance between mean values of critical shear stress, τ50, was determined using a 2-tailed Student t test with significance defined as P ≤ .050. Data are presented as mean ± SD.
Bone marrow infection/transplantation
Donor BM was harvested from femurs and tibias of C57BL/6 mice that had been treated with 1 mg/kg 5-flurouracil (5-FU) 4 days previously. BM was transduced with titer-matched retroviral supernatants, recipient mice were killed when they exhibited evidence of disease, and tissues were processed for analysis as described.14,20
Western blots and flow cytometry
Western blots were performed on lysates from 32D cells or from primary mouse tissue. 32D cells were incubated overnight in 32D medium with 10% WEHI, and Western blots were prepared as described using the 8E9 antibody (PharMingen, San Diego, CA) to reveal BCR-ABL.13
For flow cytometry analysis, 32D cells transduced with the control vector MigRI or MigP210BCR-ABL were handled according to the same procedure used to prepare cells for adhesion experiments except that cells were not preselected for GFP or P210BCR-ABL expression. Integrin expression was assessed in triplicate for each population. Three populations of MigRI cells and 2 populations of MigP210BCR-ABL were transduced separately. Anti-α4 (R-2; PharMingen) and anti-α5 (5H10-27; PharMingen) antibodies were used to stain for integrins, and cells were analyzed by flow cytometry.13
Microscopy
NIH 3T3 cells were transduced with retroviral constructs encoding P210BCR-ABL, ΔActin, Δ(1-63) BCR-ABL, (1-63) BCR-ABL, or the pK1 control plasmid and were selected for 4 days with puromycin. Cells were stained to reveal F-actin, BCR-ABL, and the cell nucleus using confocal microscopy.13
Results
P210BCR-ABL leads to increased adhesion in 32D cells through fibronectin-integrin bonds
The spinning disk cell detachment device uses fluid flow to detach cells bound to a monolayer of fibronectin. Detachment force can easily be determined by the properties of the buffer and distance of the cells from the center of the coverslip (see “Materials and methods”). The spinning disk cell detachment device measures discrete changes in cell adhesion and has been used to characterize individual activation states in α5β1 and αvβ3 integrins in cells bound to fibronectin and other extracellular matrix molecules.30-33
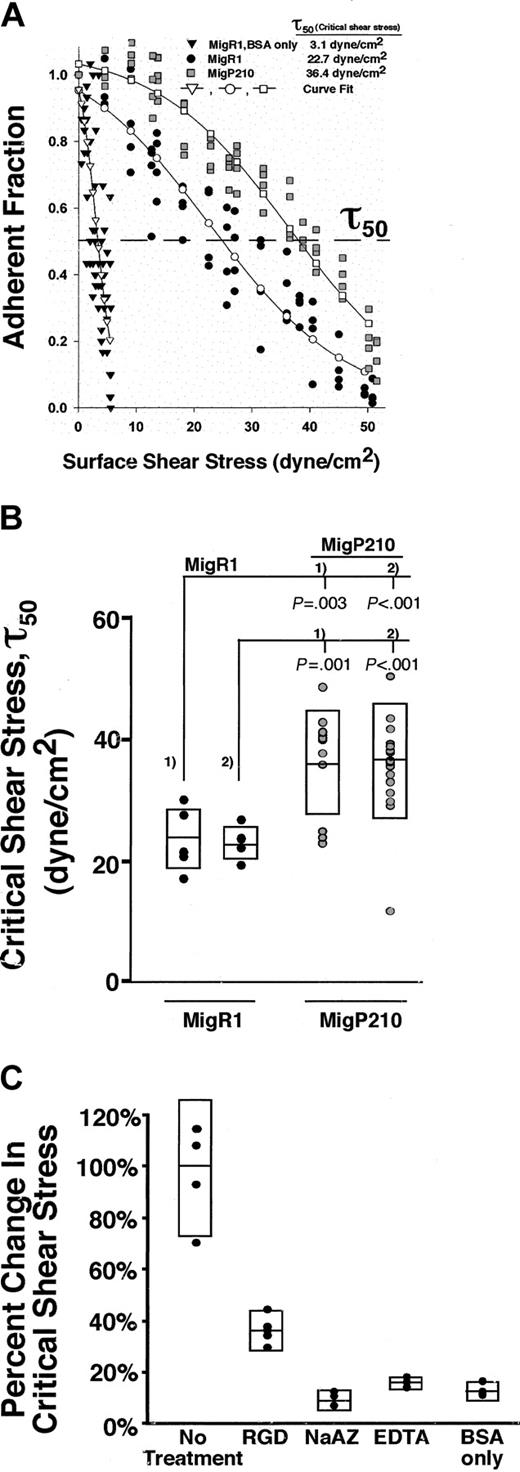
To determine which integrin receptors primarily mediate adhesion in P210BCR-ABL-expressing cells, we transduced 32D cells with a retrovirus coexpressing P210BCR-ABL and GFP (Mig P210BCR-ABL) or GFP alone (MigRI). Incubation of control MigRI cells on BSA coverslips without fibronectin results in few cells remaining bound after a spin and gives a τ50 of 3.1 dyne/cm2 (Figure 1A). This condition represents background or nonspecific adhesion, which is low compared with that for cells bound to fibronectin. In contrast, the τ50 of MigRI-transduced cells incubated on fibronectin-coated coverslips is 22.7 dyne/cm2, greater than a 7-fold increase in adhesion compared with that for BSA alone (Figure 1A). The increased binding is observed as a rightward shift in the adhesion profile as cells remain attached at higher shear stress (Figure 1A). Expression of MigP210BCR-ABL in 32D cells leads to a further rightward shift in the adhesion profile and an increase in the τ50 to 36.4 dyne/cm2 (Figure 1A). Comparison of 2 separate populations of MigP210BCR-ABL and MigRI cells shows that MigP210BCR-ABL leads to a 1.5- to 1.6-fold increase in fibronectin binding (P ≤ .003; Figure 1B). This increase in adhesion is similar to that observed in our previous report that P210BCR-ABL caused a 1.7-fold increase in binding between 32D cells and fibronectin when P210BCR-ABL was expressed from the pK1 vector containing the puromycin resistance gene in place of GFP.13
P210BCR-ABL leads to increased integrin-mediated adhesion to fibronectin. (A) Each set of points makes up an individual spin and is the number of cells (adherent fraction) at a particular location on the coverslip that experiences a known shear stress relative to the count at the center where the shear stress is zero. Expression of MigP210BCR-ABL (▦) allowed a higher fraction of cells to remain attached compared to vector control MigRI cells (•), indicating that MigP210BCR-ABL–expressing cells bound more tightly to fibronectin. Coverslips coated with BSA alone did not support significant cell binding to either MigRI (▾) or MigP210BCR-ABL (data not shown) cells. Binding of MigRI or MigP210BCR-ABL cells to BSA-coated surfaces was comparable, and at times so few cells remained attached after a spin that a detachment profile could not be generated. Curves fitted to the experimental points for MigRI-expressing cells on BSA-only–coated coverslips (▿), MigRI cells on fibronectin-coated coverslips (○), and MigP210BCR-ABL on fibronectin-coated coverslips (□). The dashed line represents an adherent fraction of 0.5. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve. (B) τ50 was determined for spins with 2 populations each of cells expressing MigRI (•) or MigP210BCR-ABL (○), indicating that an average of 1.5 to 1.6 times as much force is needed to detach 50% of MigP210BCR-ABL–expressing cells from fibronectin in our system. Each circle represents τ50 for a particular spin, and rectangles denote standard deviations above and below the mean. Significance is shown and is determined by comparing the MigRI population (#1 or #2) with the corresponding MigP210BCR-ABL population (#1 or #2). (C) MigRI cells were treated with a GRGDSP (RGD) peptide (P = .001), 0.02% sodium azide (NaAZ) (P =.001), or 1 mM EDTA (EDTA) (P = .001) and were assayed for the ability to bind fibronectin compared with untreated MigRI cells. MigRI cells were incubated on coverslips coated with BSA only as a negative control (P = .001). The percentage change in critical shear stress is relative to untreated MigRI cells.
P210BCR-ABL leads to increased integrin-mediated adhesion to fibronectin. (A) Each set of points makes up an individual spin and is the number of cells (adherent fraction) at a particular location on the coverslip that experiences a known shear stress relative to the count at the center where the shear stress is zero. Expression of MigP210BCR-ABL (▦) allowed a higher fraction of cells to remain attached compared to vector control MigRI cells (•), indicating that MigP210BCR-ABL–expressing cells bound more tightly to fibronectin. Coverslips coated with BSA alone did not support significant cell binding to either MigRI (▾) or MigP210BCR-ABL (data not shown) cells. Binding of MigRI or MigP210BCR-ABL cells to BSA-coated surfaces was comparable, and at times so few cells remained attached after a spin that a detachment profile could not be generated. Curves fitted to the experimental points for MigRI-expressing cells on BSA-only–coated coverslips (▿), MigRI cells on fibronectin-coated coverslips (○), and MigP210BCR-ABL on fibronectin-coated coverslips (□). The dashed line represents an adherent fraction of 0.5. The critical shear stress (τ50) is defined as the point on the abscissa (shear stress) that corresponds to an adherent fraction of 0.5 along the fitted curve. (B) τ50 was determined for spins with 2 populations each of cells expressing MigRI (•) or MigP210BCR-ABL (○), indicating that an average of 1.5 to 1.6 times as much force is needed to detach 50% of MigP210BCR-ABL–expressing cells from fibronectin in our system. Each circle represents τ50 for a particular spin, and rectangles denote standard deviations above and below the mean. Significance is shown and is determined by comparing the MigRI population (#1 or #2) with the corresponding MigP210BCR-ABL population (#1 or #2). (C) MigRI cells were treated with a GRGDSP (RGD) peptide (P = .001), 0.02% sodium azide (NaAZ) (P =.001), or 1 mM EDTA (EDTA) (P = .001) and were assayed for the ability to bind fibronectin compared with untreated MigRI cells. MigRI cells were incubated on coverslips coated with BSA only as a negative control (P = .001). The percentage change in critical shear stress is relative to untreated MigRI cells.
Binding of integrins to fibronectin requires intracellular energy stores and extracellular divalent cations as cofactors. To show that cells specifically bind fibronectin, control MigRI cells were preincubated with a soluble Arg-Gly-Asp (RGD)–containing peptide that occupies the fibronectin binding site on integrins that recognize the RGD sequence. Incubation of cells with the RGD-containing peptide led to a significant decrease (P = .001) in adhesion to fibronectin. Cells were also treated with 0.02% sodium azide (NaAZ) to reduce energy stores by blocking oxidative phosphorylation or 1 mM EDTA (ethylenediaminetetraacetic acid) to chelate free cations. Both treatments decreased binding (10% of control for NaAZ and 16% of control for EDTA) to levels achieved with BSA alone (13% of control), indicating that adhesion in our system requires energy and divalent cations, properties of all integrin-fibronectin linkages.
32D cells express α4β1 and α5β1 integrins; however α5β1 is the predominant integrin-mediating binding
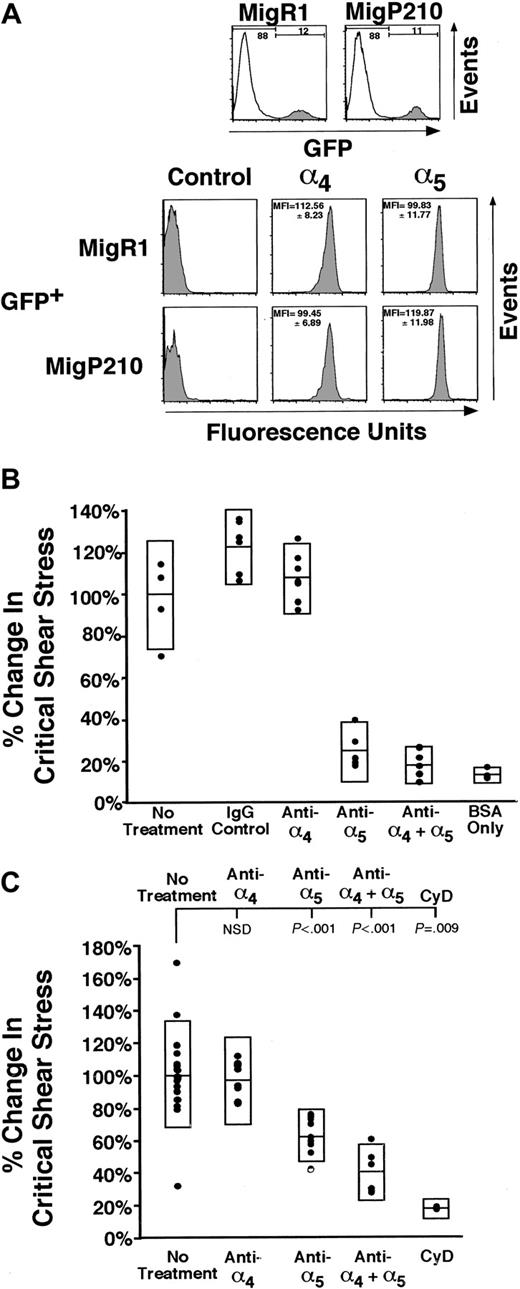
The α4β1 and α5β1 integrins are the primary cell surface receptors that bind fibronectin. To determine whether P210BCR-ABL influences the relative expression of these integrins, unselected 32D cells transduced with MigP210BCR-ABL or MigRI were stained with antibodies to the extracellular region of the α4 or α5 integrin subunits (Figure 2A). Comparison between GFP+ MigP210BCR-ABL and GFP+ MigRI cells suggests that P210BCR-ABL may cause a 1.2-fold increase in α5β1 expression; however, this change was not statistically significant across all populations of cells tested. Expression of α4β1 was essentially unchanged. These findings are comparable with those of other published reports that suggest P210BCR-ABL expression may lead to minimal changes in integrin expression.4,19 These small alterations in integrin levels may contribute to, but are unlikely to account for, the entire increase in adhesion in P210BCR-ABL cells.13
α5β1 primarily mediates cell adhesion in MigR1 and MigP210BCR-ABL cells, though α5β1 and α4β1 are present at similar levels on 32D cells. (A) Three populations of 32D cells expressing MigR1 and 2 populations of MigP210BCR-ABL cells were assessed for the level of integrin expression. Cell populations were transduced independently and subsequently stained in triplicate without selection. Cells stained with the secondary phycoerythrin-conjugated antibody alone are shown as negative controls. Representative histograms are shown for a single population, and the average mean fluorescence index (MFI)13 for all populations and standard deviations determined by a propagation of error are also indicated. Significant differences in α4 expression are observed neither between MigP210BCR-ABL GFP+ and MigR1 GFP+ cells (P = .13) nor between MigP210BCR-ABL GFP– and GFP+ cells (P = .26, not shown). Likewise, significant differences in α5 expression are observed neither between MigP210BCR-ABL GFP+ and MigR1 GFP+ cells (P = .13) nor between MigP210BCR-ABL GFP– and GFP+ cells (P = .09, not shown). (B) The ability of MigR1 cells to bind fibronectin was determined by leaving cells untreated (No treatment) or treating cells with a control Rat immunoglobulin G (IgG) antibody, a monoclonal antibody directed against the fibronectin-binding region of α4β1 (anti-α4), a monoclonal antibody directed against the fibronectin-binding region of α5β1 (anti-α5), both antibodies (anti-α4 + α5), or untreated cells incubated on coverslips coated without fibronectin (BSA only). The percentage change in critical shear stress is relative to that of untreated cells. (C) MigP210BCR-ABL 32D cells were treated with the same anti-integrin antibodies as in panel B. Additionally, MigP210BCR-ABL cells were treated with 1 μM cytochalasin D (CyD), which abrogates cell adhesion to levels that are comparable to coverslips coated with BSA only. NSD indicates not significantly different.
α5β1 primarily mediates cell adhesion in MigR1 and MigP210BCR-ABL cells, though α5β1 and α4β1 are present at similar levels on 32D cells. (A) Three populations of 32D cells expressing MigR1 and 2 populations of MigP210BCR-ABL cells were assessed for the level of integrin expression. Cell populations were transduced independently and subsequently stained in triplicate without selection. Cells stained with the secondary phycoerythrin-conjugated antibody alone are shown as negative controls. Representative histograms are shown for a single population, and the average mean fluorescence index (MFI)13 for all populations and standard deviations determined by a propagation of error are also indicated. Significant differences in α4 expression are observed neither between MigP210BCR-ABL GFP+ and MigR1 GFP+ cells (P = .13) nor between MigP210BCR-ABL GFP– and GFP+ cells (P = .26, not shown). Likewise, significant differences in α5 expression are observed neither between MigP210BCR-ABL GFP+ and MigR1 GFP+ cells (P = .13) nor between MigP210BCR-ABL GFP– and GFP+ cells (P = .09, not shown). (B) The ability of MigR1 cells to bind fibronectin was determined by leaving cells untreated (No treatment) or treating cells with a control Rat immunoglobulin G (IgG) antibody, a monoclonal antibody directed against the fibronectin-binding region of α4β1 (anti-α4), a monoclonal antibody directed against the fibronectin-binding region of α5β1 (anti-α5), both antibodies (anti-α4 + α5), or untreated cells incubated on coverslips coated without fibronectin (BSA only). The percentage change in critical shear stress is relative to that of untreated cells. (C) MigP210BCR-ABL 32D cells were treated with the same anti-integrin antibodies as in panel B. Additionally, MigP210BCR-ABL cells were treated with 1 μM cytochalasin D (CyD), which abrogates cell adhesion to levels that are comparable to coverslips coated with BSA only. NSD indicates not significantly different.
To determine the extent to which α4β1 and α5β1 are involved in binding to fibronectin in our system, 32D cells selected for the expression of MigRI (Figure 2B) or MigP210BCR-ABL (Figure 2C) were incubated with blocking antibodies to α4β1, α5β1, or both integrins simultaneously. Blocking α4β1 function in either MigRI or MigP210BCR-ABL cells had no effect on cell adhesion (MigRI, P = .439; Figure 2B) (MigP210BCR-ABL, P = .537; Figure 2C). In contrast, attenuation of adhesion by the α5β1 integrin reduced binding to 25% and 63% of untreated MigRI (P < .001; Figure 2B) and MigP210BCR-ABL cells (P < .001; Figure 2C), with nonspecific adhesion representing approximately 13% and 17% of adhesion, respectively. Blocking both α4β1 and α5β1 did not significantly decrease binding to fibronectin in MigRI cells beyond that attenuated by inhibiting α5β1 alone (P = .163); however, adhesion in MigP210BCR-ABL cells was significantly lower than that achieved using only a blocking antibody to α5β1 (P = .007). It is likely that α4β1 plays a minor role in adhesion in MigP210BCR-ABL cells and is low compared with the involvement of α5β1.
Neither the coiled-coil domain, Y177, nor the actin-binding domain of BCR-ABL is required for factor-independent growth
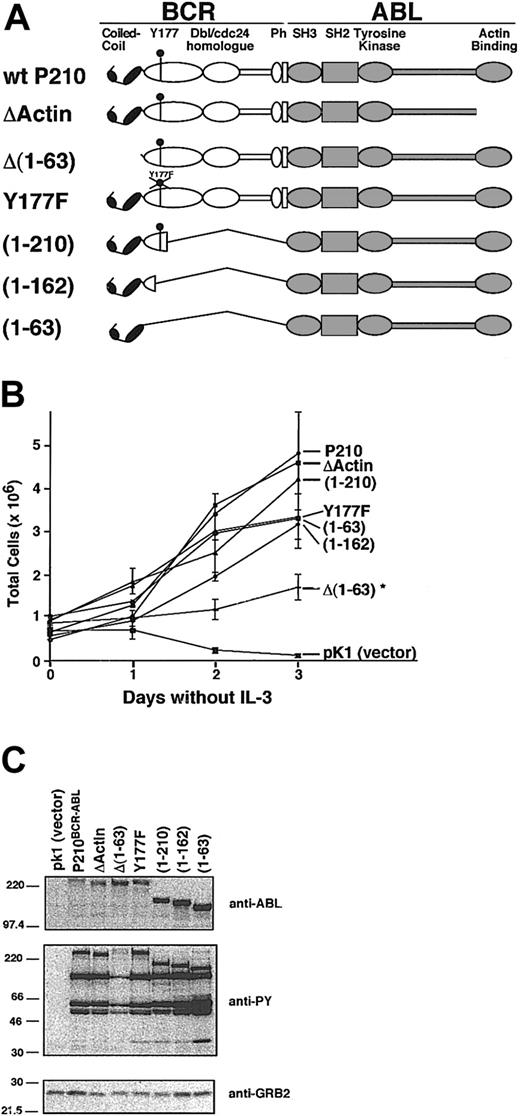
We compared P210BCR-ABL mutants that lack functional C-C, Y177, or actin-binding domains to test the contribution of these regions on adhesion to fibronectin (Figure 3A). Many of these mutants were tested in our murine CML model, and we found that the deletion of the C-C domain or the mutation of Y177 was sufficient to prevent induction of the wild-type MPD.14 Expression of P210BCR-ABL in 32D cells alleviates the requirement of IL-3 for survival.34 After selection in puromycin for 4 days, each cell population was assessed for the ability to grow in IL-3–free media (Figure 3B). All mutants survived without IL-3 supplementation; however, cells transduced with the control vector pK1 rapidly died within 48 hours, as assessed by trypan blue inclusion. Growth of 32D cells transduced by all mutants except Δ(1-63) BCR-ABL was similar to wild-type P210BCR-ABL. Deletion of the C-C domain in Δ(1-63) prevents oligomerization of P210BCR-ABL.14,16 Although able to grow in IL-3–free media, the proliferation rate of cells transduced with this mutant was decreased relative to wild-type P210BCR-ABL (Figure 3B). This mutant also expressed lower tyrosine kinase activity, suggesting that this may be a cause of decreased growth (Figure 3C).
Characterization of P210BCR-ABL mutants. (A) Schematic representation of wild-type P210BCR-ABL and corresponding mutants with deletions or point mutations in critical functional domains. The C-C domain mediates protein oligomerization, Y177 binds GRB2, and Ph stands for pleckstrin homology. (B) Each construct was cloned into the pK1 vector that coexpresses the mutant of interest along with the puromycin resistance gene as a bicistronic message through an internal ribosomal entry site. Each construct was expressed in 32D cells and selected for growth in puromycin-treated medium in the presence of IL-3. A representative plot of 32D-cell proliferation is shown in which IL-3 was withdrawn from the media at day 0. All mutants and wild-type P210BCR-ABL led to growth factor independence when expressed in this cell line. However, the Δ(1-63) mutant proliferated at a significantly lower rate (*), and cells expressing the control vector, pK1, rapidly died within 48 hours. Viability was determined by trypan blue exclusion; each sample was tested in triplicate. (C) Western blots of whole cell lysates from 32D cells expressing constructs shown in panel A were prepared using cells that had recently been transduced and selected (passage 3 or sooner after selection). Blots were assessed for BCR-ABL expression (anti-ABL), for whole cell phosphotyrosine (anti-PY), or for GRB2 expression as a loading control (anti-GRB2). The Δ(1-63) mutant shows reduced levels of phosphotyrosine, which may account for the lower proliferation rate seen in panel B.
Characterization of P210BCR-ABL mutants. (A) Schematic representation of wild-type P210BCR-ABL and corresponding mutants with deletions or point mutations in critical functional domains. The C-C domain mediates protein oligomerization, Y177 binds GRB2, and Ph stands for pleckstrin homology. (B) Each construct was cloned into the pK1 vector that coexpresses the mutant of interest along with the puromycin resistance gene as a bicistronic message through an internal ribosomal entry site. Each construct was expressed in 32D cells and selected for growth in puromycin-treated medium in the presence of IL-3. A representative plot of 32D-cell proliferation is shown in which IL-3 was withdrawn from the media at day 0. All mutants and wild-type P210BCR-ABL led to growth factor independence when expressed in this cell line. However, the Δ(1-63) mutant proliferated at a significantly lower rate (*), and cells expressing the control vector, pK1, rapidly died within 48 hours. Viability was determined by trypan blue exclusion; each sample was tested in triplicate. (C) Western blots of whole cell lysates from 32D cells expressing constructs shown in panel A were prepared using cells that had recently been transduced and selected (passage 3 or sooner after selection). Blots were assessed for BCR-ABL expression (anti-ABL), for whole cell phosphotyrosine (anti-PY), or for GRB2 expression as a loading control (anti-GRB2). The Δ(1-63) mutant shows reduced levels of phosphotyrosine, which may account for the lower proliferation rate seen in panel B.
Both the actin-binding and the coiled-coil domains are required for F-actin localization and increased adhesion to fibronectin by P210BCR-ABL
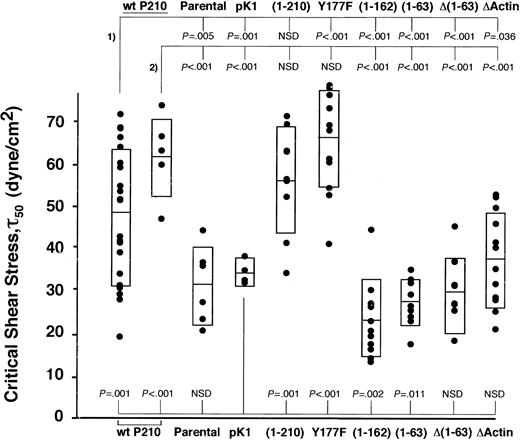
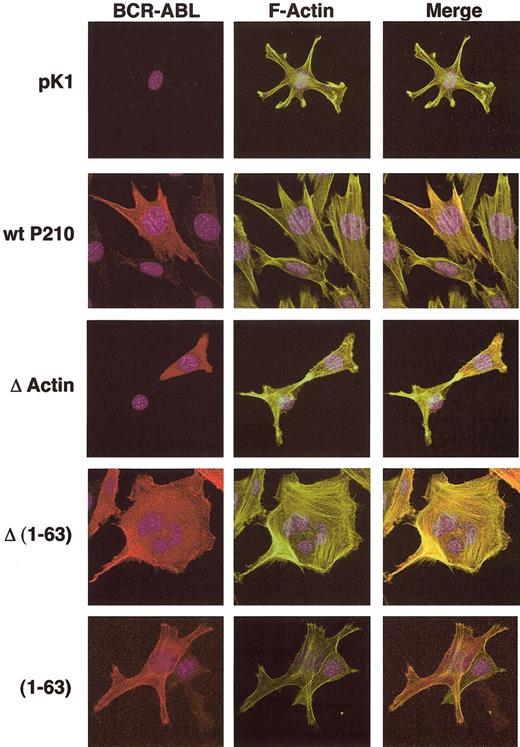
Two independently transduced populations of each mutant were repeatedly spun, and the critical shear stress of each spin is presented for 1 of the 2 populations tested (Figure 4). Of the mutations known to affect F-actin binding, both Δ(1-63) and ΔActin showed significantly lower adhesion in our assay compared with 2 populations of wild-type P210BCR-ABL (Δ(1-63), P < .001 and P < .001; ΔActin, P = .036 and P < .001; Figure 4), suggesting that association with F-actin may be critical for enhanced binding to fibronectin. To confirm that Δ(1-63) resulted in reduced localization to F-actin, the control vector pK1, Δ(1-63), and ΔActin cells were transduced into NIH 3T3 fibroblasts. Cells were stained for P210BCR-ABL localization and F-actin filaments (Figure 5). The pattern of wild-type P210BCR-ABL staining in NIH 3T3 cells resembled linear filament-like strands that overlap with F-actin. In contrast, ΔActin- and Δ(1-63)–transduced NIH 3T3 fibroblasts showed a diffuse pattern of anti-abl staining in the cytoplasm, consistent with previously published results.7 A reduction in F-actin colocalization with the ΔActin mutant had been previously demonstrated in 32D cells.21
N-terminal and C-terminal deletions abrogate increased cell adhesion. Wild-type P210BCR-ABL or mutants were expressed in 32D cells, and the magnitude of binding between each population and a fibronectin monolayer was determined. Two independently transduced populations of each mutant were tested, and the critical shear stress of individual spins for a single population is shown (•). Rectangles represent 1 SD greater than and less than the mean. Significance values are given at the top of the graph for mutants compared with 2 wild-type P210BCR-ABL populations (#1 and #2) or at the bottom of the graph when compared with vector control pK1. Wild-type P210BCR-ABL or functional mutants segregate into 2 groups that have a higher (P210BCR-ABL, Y177F, and (1-210)) or lower ((1-162), (1-63), Δ(1-63), and ΔActin) ability to bind fibronectin, suggesting that both N-terminal and C-terminal functional domains contribute to increased cell adhesion. Binding of each mutant to BSA-only–coated coverslips was similar for each mutant and was low, comparable to wild-type P210BCR-ABL or vector control cells (data not shown). (An additional population of pK1 similar to the one shown, as well as wild-type P210BCR-ABL #2, were previously published.13 ). NSD indicates not significantly different.
N-terminal and C-terminal deletions abrogate increased cell adhesion. Wild-type P210BCR-ABL or mutants were expressed in 32D cells, and the magnitude of binding between each population and a fibronectin monolayer was determined. Two independently transduced populations of each mutant were tested, and the critical shear stress of individual spins for a single population is shown (•). Rectangles represent 1 SD greater than and less than the mean. Significance values are given at the top of the graph for mutants compared with 2 wild-type P210BCR-ABL populations (#1 and #2) or at the bottom of the graph when compared with vector control pK1. Wild-type P210BCR-ABL or functional mutants segregate into 2 groups that have a higher (P210BCR-ABL, Y177F, and (1-210)) or lower ((1-162), (1-63), Δ(1-63), and ΔActin) ability to bind fibronectin, suggesting that both N-terminal and C-terminal functional domains contribute to increased cell adhesion. Binding of each mutant to BSA-only–coated coverslips was similar for each mutant and was low, comparable to wild-type P210BCR-ABL or vector control cells (data not shown). (An additional population of pK1 similar to the one shown, as well as wild-type P210BCR-ABL #2, were previously published.13 ). NSD indicates not significantly different.
Localization of P210BCR-ABL to F-actin is dependent on the presence of the coiled-coil domain and the C-terminal actin-binding domain. NIH 3T3 cells were transduced with pK1, wild-type P210BCR-ABL, ΔActin BCR-ABL, Δ(1-63) BCR-ABL, or (1-63) BCR-ABL to determine the essential regions for colocalization of BCR-ABL with F-actin. Puromycin-resistant NIH 3T3 cells were stained for BCR-ABL expression (anti-abl, cy3, red), F-actin (fluorescein isothiocyanate (FITC)–phalloidin, green), and the nucleus was visualized by staining with DAPI (blue).
Localization of P210BCR-ABL to F-actin is dependent on the presence of the coiled-coil domain and the C-terminal actin-binding domain. NIH 3T3 cells were transduced with pK1, wild-type P210BCR-ABL, ΔActin BCR-ABL, Δ(1-63) BCR-ABL, or (1-63) BCR-ABL to determine the essential regions for colocalization of BCR-ABL with F-actin. Puromycin-resistant NIH 3T3 cells were stained for BCR-ABL expression (anti-abl, cy3, red), F-actin (fluorescein isothiocyanate (FITC)–phalloidin, green), and the nucleus was visualized by staining with DAPI (blue).
A region in bcr between aa 163 and aa 210 is also necessary to support P210BCR-ABL cell adhesion
The (1-63) mutant, in which the C-C domain was fused to c-abl, had decreased adhesion compared with wild-type P210BCR-ABL (P < .001 and P < .001; Figure 4), but the staining pattern indicated that it retains the ability to colocalize with F-actin (Figure 5). This suggests that the mutant leads to lower adhesion through a mechanism that is distinct from Δ(1-63) because the c-abl sequences are conserved among the 2 mutants, but each retains distinct regions of bcr.
One possibility is that bcr sequences downstream of the C-C domain may recruit proteins to the cell cytoskeleton that are necessary for adhesion. To investigate this possibility, we attempted to map the minimal region in bcr between aa 64 and aa 927 that facilitates P210BCR-ABL cell adhesion. We found that a (1-210) BCR-ABL mutant conferred high binding, similar to wild-type P210BCR-ABL, compared with cells expressing the (1-63) mutant (Figure 4). This suggests that aa 64-210 is a critical region in bcr that leads to increased binding to fibronectin.
To narrow the critical region within aa 64-210, we constructed a (1-162) mutant that fuses the first 162 aa of bcr onto c-abl. This mutant results in transformation of 32D cells and increased whole cell phosphotyrosine levels (Figure 3B-C). When assessed for its ability to bind fibronectin in our spinning disk adhesion device, 32D cells expressing this mutant showed decreased levels of adhesion that were in the same range as the (1-63) mutant, narrowing the critical region to between aa 163 and aa 210.
Tyr177 is the only tyrosine that may bind SH2-containing proteins within this region. Mutation of tyrosine to phenylalanine (Y177F) abrogates binding to GRB214,35 ; however, similar to P210BCR-ABL, it caused increased binding to fibronectin (Figure 4). This suggests that the P210BCR-ABL–GRB2 interaction is not responsible for increased adhesion in P210BCR-ABL–expressing 32D cells.
Together, our adhesion experiments identify 3 regions in P210BCR-ABL that contribute to the ability of wild-type P210BCR-ABL to increase the magnitude of binding between 32D cells and fibronectin. The regions are the N-terminal C-C domain that facilitates oligomerization and enhances F-actin localization, the bcr sequences spanning aa 163 to 210, and the C-terminal actin-binding domain mediating F-actin localization.
ΔActin induced MPD in mice is similar to that caused by wild-type P210BCR-ABL
Although P210BCR-ABL colocalizes with F-actin, the contribution of F-actin localization to CML development has not been characterized in a BM transplantation model of CML. Evaluation of the ability of the ΔActin mutant to induce wild-type MPD may indicate whether the failure of Δ(1-63) to cause murine CML14,15 is attributed to its reduction in F-actin localization.
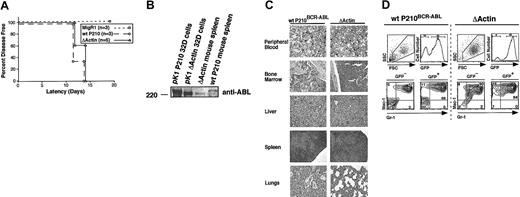
All mice given ΔActin-expressing BM developed overt disease within 14 days, indicated by elevated white blood cell (WBC) counts (109 000 ± 61 000 cells/μL) (Figure 6A). Wild-type P210BCR-ABL mice also developed disease during this interval (Figure 6A). MigRI mice remained healthy with normal WBC counts (less than 10 000 cells/μL). At necropsy, both wild-type P210BCR-ABL and ΔActin mice exhibited hepatosplenomegaly, pulmonary hemorrhage, and absent lymphadenopathy. Histologic examination showed infiltration of the BM, liver, spleen, and lungs by neutrophils (Figure 6B). MPD was similar between P210BCR-ABL and ΔActin except that pulmonary hemorrhage was greater in the P210BCR-ABL mice. The ΔActin protein could be detected in spleens of these animals at the appropriate size using an antibody to the c-abl tyrosine kinase domain, excluding the possibility of reversion to wild-type in ΔActin mice (Figure 6C). BM, spleen, and peripheral blood were collected from all mice at the time of killing, and their immunophenotypes were assessed using flow cytometry (Figure 6D). Most cells from all 3 organs of diseased mice (wild-type P210BCR-ABL and ΔActin) were GFP+, indicating that the transduced donor cells became the dominant population. In both cohorts, these cells stained predominantly GR-1+/Mac-1+, indicating they were of granulocytic lineage. These cells also stained negatively for T-cell and B-cell markers, Thy 1.2 and B220, respectively (data not shown). Increased GFP–/GR-1+/Mac-1+ cells were observed in wild-type P210BCR-ABL and ΔActin mice compared with MigRI. This is likely because of a trans effect caused by cytokines released from GFP+/GR-1+/Mac-1+ cells.10,36 Together, these results indicate that preventing the association of P210BCR-ABL with F-actin is dispensable for CML development in our mouse model but is necessary for P210BCR-ABL to enhance cell adhesion.
Myeloproliferative disease in mice receiving ΔActin BCR-ABL bone marrow is indistinguishable from wild-type P210BCR-ABL. (A) All lethally irradiated mice receiving transplanted bone marrow expressing either wild-type P210BCR-ABL (○) or ΔActin BCR-ABL (▵) bone marrow showed evidence of disease by 14 days after transplantation. The latency between wild-type P210BCR-ABL and ΔActin mice was not significantly different (P = .38), whereas mice given bone marrow expressing the MigRI control vector (□) are healthy within this interval and remain disease free for more than 300 days.14 (B) Spleens harvested from wild-type P210BCR-ABL or ΔActin mice were lysed and fractionated on a Western blot to show expression of P210BCR-ABL or ΔActin at the appropriate size. 32D cells transduced with either P210BCR-ABL or ΔActin were used as a control. Expression of BCR-ABL could not be detected in spleens of ΔActin mice using an antibody to the extreme C-terminus of BCR-ABL, confirming that the C-terminal actin-binding domain was absent in these mice (data not shown). (C) Sections of peripheral blood (40 ×), bone marrow (20 ×), spleen (4 ×), liver (20 ×), and lung (4 ×) were stained with hematoxylin and eosin or Wright stain (peripheral blood). Mature myeloid cells are present in the peripheral blood, and the normal architecture of each organ is replaced by infiltrative granulocytes. Lungs of the ΔActin mice were less hemorrhagic than of P210BCR-ABL mice. (D) A representative flow cytometry profile of peripheral blood from wild-type P210BCR-ABL and ΔActin mice is shown. Peripheral blood, bone marrow, and spleen from wild-type P210BCR-ABL or ΔActin mice were harvested and stained for the myeloid markers GR-1 and Mac-1. Most cells from each organ of mice from both cohorts stained GR-1+/Mac-1+, indicating infiltrative granulocytic disease.
Myeloproliferative disease in mice receiving ΔActin BCR-ABL bone marrow is indistinguishable from wild-type P210BCR-ABL. (A) All lethally irradiated mice receiving transplanted bone marrow expressing either wild-type P210BCR-ABL (○) or ΔActin BCR-ABL (▵) bone marrow showed evidence of disease by 14 days after transplantation. The latency between wild-type P210BCR-ABL and ΔActin mice was not significantly different (P = .38), whereas mice given bone marrow expressing the MigRI control vector (□) are healthy within this interval and remain disease free for more than 300 days.14 (B) Spleens harvested from wild-type P210BCR-ABL or ΔActin mice were lysed and fractionated on a Western blot to show expression of P210BCR-ABL or ΔActin at the appropriate size. 32D cells transduced with either P210BCR-ABL or ΔActin were used as a control. Expression of BCR-ABL could not be detected in spleens of ΔActin mice using an antibody to the extreme C-terminus of BCR-ABL, confirming that the C-terminal actin-binding domain was absent in these mice (data not shown). (C) Sections of peripheral blood (40 ×), bone marrow (20 ×), spleen (4 ×), liver (20 ×), and lung (4 ×) were stained with hematoxylin and eosin or Wright stain (peripheral blood). Mature myeloid cells are present in the peripheral blood, and the normal architecture of each organ is replaced by infiltrative granulocytes. Lungs of the ΔActin mice were less hemorrhagic than of P210BCR-ABL mice. (D) A representative flow cytometry profile of peripheral blood from wild-type P210BCR-ABL and ΔActin mice is shown. Peripheral blood, bone marrow, and spleen from wild-type P210BCR-ABL or ΔActin mice were harvested and stained for the myeloid markers GR-1 and Mac-1. Most cells from each organ of mice from both cohorts stained GR-1+/Mac-1+, indicating infiltrative granulocytic disease.
Discussion
Abnormal binding between hematopoietic progenitor cells and BM stroma has been proposed, in addition to loss of programmed cell death and enhanced cell proliferation, as an important contributor to the onset of leukemia.37 Several studies using BM from CML patients5,38 or hematopoietic cell lines4,19 expressing P210BCR-ABL have documented an adhesion defect between cells and fibronectin, a major extracellular molecule of the BM microenvironment.18 Contact between P210BCR-ABL–expressing cell lines and fibronectin promotes entry into the cell cycle, suggesting that enhanced binding may promote deregulated cell proliferation and tumor formation.4 Other studies indicate that P210BCR-ABL may assist in the extravasation of leukemia cells out of the BM and into the peripheral circulation, where abnormally high levels of WBCs are typically present in untreated CML patients.5,38 Fibroblasts expressing P210BCR-ABL support this theory by exhibiting increased motility, accumulation of intracellular F-actin, and formation of filopodia compared with parental cells cultured on fibronectin.39
In vitro studies such as these provide a context for understanding the relationship between P210BCR-ABL expression and cell adhesion and motility. What remains to be determined is the contribution of defective adhesion to development of the CML disease. To investigate this relationship, we sought to understand how the P210BCR-ABL protein regulates cell adhesion and then to determine whether this process is contributory to murine CML. We used 2 experimental techniques to link cell adhesion with disease development.
We identified 3 regions in wild-type P210BCR-ABL—the N-terminal C-C domain, aa 163-210, and the C-terminal actin-binding domain—that, when deleted, lead to reduced adhesion to fibronectin compared with wild-type P210BCR-ABL. The C-C domain enhances the c-abl tyrosine kinase and the F-actin–binding domain.7 It is unlikely, however, that the depreciation in tyrosine kinase activity in the Δ(1-63) mutant led to a decrease in adhesion to fibronectin because we have previously shown that the attenuation of tyrosine kinase activity with STI-571 or a point mutation in the tyrosine kinase domain does not diminish the effect of P210BCR-ABL on adhesion.13 Rather, the decreased ability of the Δ(1-63) mutant to colocalize with F-actin is the likely mechanism leading to decreased adhesion with this mutant.
The observation that interactions between P210BCR-ABL and the cell cytoskeleton are necessary for P210BCR-ABL–mediated increased adhesion is consistent with observations in fibroblasts that indicate that P210BCR-ABL expression leads to abnormal cytoskeletal architecture, altered F-actin formation, and increased filopodia formation.39 An intact actin cytoskeleton is necessary for binding fibronectin in our system because complete depolymerization of F-actin with cytochalasin D abrogates all binding to fibronectin (Figure 2C).
Evidence from the literature shows that P210BCR-ABL expression directly alters integrin-cytoskeletal linkages by modifying F-actin. Human megakaryoblast cells, Mo7e, expressing P210BCR-ABL or CD34+ cells from CML patients are inefficient in the formation of integrin caps, or clusters, containing α4β1 and α5β1 integrins.40 P210BCR-ABL is thought to contribute to increased F-actin polymerization that retards integrin mobility within the cell membrane, preventing integrin clustering.40 Treatment of CML CD34+ cells with low-dose cytochalasin D increased cap formation and restored adhesion to near normal levels.
Our results favor a mechanism in which P210BCR-ABL binds to F-actin filaments to directly modify F-actin or, more likely, to recruit effector molecules potentially through the aa 163-210 region of P210BCR-ABL to the cell cytoskeleton. The significance of the aa 163-210 region to cell adhesion, is unclear. The Y177 binding domain is located in this region and binds GRB2, which has been suggested to activate RAS through the guanine exchange factor SOS.41 Others and we have shown that wild-type P210BCR-ABL–GRB2 binding at this region is necessary for efficient CML development.14,15,42 However, prevention of GRB2 binding through a Y177F mutation has no effect on enhanced adhesion, suggesting that GRB2 binding to P210BCR-ABL is noncontributory to cell adhesion. Recently, the P210BCR-ABL/GRB2 interaction was shown to recruit the scaffolding adapter GAB2 to the SH3 domain of GRB2. This association enhances PI3K/AKT and RAS/ERK activation and is required for efficient myeloid transformation in murine BM cells.43 Interestingly, binding of GRB2 to P210BCR-ABL is required for fibroblast transformation44 but is generally not needed to transform hematopoietic cell lines. This is likely because of alternative pathways sufficient to activate RAS in the absence of GRB2 binding at Y177 in cell lines such as 32D cells.45 One possibility not excluded by our findings is that RAS may be activated in 32D cells expressing the Y177F mutant and may enhance adhesion through pathways that do not involve GRB2 binding at this site.
Mouse models of CML using either transgenic mice or mice that underwent BM transplantation are useful tools to evaluate how P210BCR-ABL leads to leukemia. Leukemia induced by the ΔActin mutant is indistinguishable from wild-type P210BCR-ABL. Our findings also provide insight into the role of the C-C domain and argue that the inability of the Δ(1-63) mutant to efficiently colocalize with F-actin is an unlikely explanation for its inability to cause a CML-like disease in mice. Rather, the effect on tyrosine kinase activity and protein-protein interactions46 is the likely contribution of oligomerization to the efficient induction of CML. Others have shown that complete deletion of the SH3 domain or mutation of the critical intramolecular binding motifs that regulate the tyrosine kinase inhibitory role of the SH3 domain restores the tyrosine kinase activity of a C-C mutant and enables the induction of the MPD.15,47 This suggests that the moderate reduction in tyrosine kinase activity is likely to account for the inability of the C-C mutant to cause the MPD rather than its inability to colocalize with F-actin.
Our findings contrast those from mice transgenic for the expression of P190BCR-ABL lacking the C-terminal F-actin–binding domain. These mice were characterized by an increase in leukemia latency and a decrease in development of acute B-cell leukemia.48 The reason for these disparate findings is likely attributed to the intrinsic differences between the 2 models that produce different diseases (MPD in our model and acute lymphoid leukemia in the model of Heisterkamp et al48 ). Although unlikely, differences between actin-binding mutants used for each study, such as the size of the C-terminal deletion or the additional bcr regions present in P210BCR-ABL and absent from P190BCR-ABL, may affect disease onset.
In summary, we show that the localization of P210BCR-ABL to F-actin is not required for MPD induction but is needed for the elevated adhesion to fibronectin seen in 32D cells expressing wild-type P210BCR-ABL. We used 2 independent assays to assess adhesion and oncogenicity; however, we were unable to find a consistent correlation between normalization of adhesion to fibronectin and attenuation of MPD in our murine model. This suggests that abnormal cell-fibronectin binding is unlikely to be a major independent contributor to murine CML development. Alternatively, our results implicate the dysregulated signaling caused by the P210BCR-ABL tyrosine kinase and other motifs (eg, Y177) as critical determinants of CML induction. The relative significance of the fusion of bcr sequences upstream of c-abl is best observed with the Δ(1-63) mutant, in which our results suggest that a decline in tyrosine kinase below a critical threshold blocks the ability of this mutant to induce murine CML. Together, our studies provide further evidence that even a slight disruption in the abnormal signaling patterns of P210BCR-ABL can significantly impact the emergence of leukemia and direct attention to several regions in P210BCR-ABL for targeted therapy.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2003-01-0062.
Supported by National Institutes of Health grants CA68008 (R.R.), HL18208 (D.A.H.), and CA77570 (W.S.P.) and by Scholar Awards from the Leukemia and Lymphoma Society (R.R., W.S.P.). J.A.W. was supported by the Whitaker Foundation and the Medical Scientist Training Program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Andrea Carpenter, Bill DeMuth, Kevin Forsythe, Gladys Grays-Board, Fred Karnell, Lara Lynch, Joey Plumb, and Lanwei Xu for their technical assistance. The flow cytometry studies were performed in the University of Pennsylvania Cancer Center Flow Cytometry and Cell Sorting Shared Resource (supported in part by the Lucille B. Markey Trust and the National Institutes of Health). The Gastroenterology Cell Morphology Core at the University of Pennsylvania processed tissue sections and was supported by National Institutes of Health Center grant P30-DK50306.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal