Abstract
Reporter genes, including green fluorescent protein (GFP), have been used to monitor the expression of transgenes introduced into vascular cells by gene transfer vectors. Here, we demonstrate that GFP by itself can selectively induce expression of certain genes in endothelial cells. Elevation of the cytoplasmic concentration of GFP in endothelial cells, specifically, resulted in a robust upregulation of heat shock protein 70 (HSP70). GFP induced both mRNA and protein expression of HSP70 in a dose-dependent manner. GFP-mediated up-regulation of HSP70 resulted in induction of cyclooxygenase-2 (COX-2) followed by prostaglandin E2 (PGE2) production. GFP-mediated up-regulation of HSP70 is independent of mitogen-activated protein kinase and phosphatidylinositol-3-kinase signaling cascades because inhibition of these pathways had no effect on HSP70 increases. Adenoviral delivery of GFP into murine vasculature significantly enhanced blood flow, suggesting that sufficient PGE2 is produced to induce vasodilation. Identification of the molecular partners that interact with GFP will increase our understanding of the vascular-specific factors that regulate stress angiogenesis and hemostasis.
Introduction
Endothelial cells (ECs) are ideal targets for gene therapy because they are readily accessible to gene transfer vectors via the circulation and are involved in a number of normal and pathophysiologic conditions, including angiogenesis, atherosclerosis, tumor growth, myocardial infarction, and limb and cardiac ischemia.1-4 However, little is known about the direct biologic effects of gene delivery vectors on the function of ECs. Moreover, the global transcriptional perturbations that may result from overexpression of reporter genes in ECs have not been well studied.
Green fluorescent protein (GFP) is an in vivo real-time reporter molecule cloned from the jellyfish Aequorea victoria.5 It has attracted widespread interest due to its potential in the studies of gene expression and regulation.6 GFP has been used to track gene expression in a wide variety of mammalian cells,7 including ECs. However, whether GFP, as a foreign molecule, could potentially alter the biology of ECs is not known.
We have shown that E1–E4+ adenoviral vectors (Ad vectors) can directly induce genes that alter the inflammatory response of the ECs.8-10 Introduction of E1–E4+, but not E1–E4– Ad vectors, resulted in induction of factors that promote survival and prevent apoptosis of ECs.9 In addition, E1–E4+ vectors induce expression of proinflammatory adhesion molecules, including intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), and alter the chemokine expression profile.8 The effect of E4+ Ad vectors is specific to vascular ECs because E4+ vectors fail to induce similar types of changes on other cell types including monocytes, epithelial cells, or cancer cells. These data have set forth the intriguing possibility that vascular ECs may be specifically sensitive to the introduction of gene transfer vectors or, potentially the reporter genes such as GFP, that are expressed by these genes.
Our data indicate that whereas the GFP-expressing AdE1–E4– vector does not induce any overt phenotypic changes on ECs, it can specifically up-regulate gene of heat shock protein 70 (HSP70), which may alter the physiology of ECs. The HSPs are encoded by genes whose expression is substantially increased during stress conditions,11-13 such as heat shock, viral infections, alcohol, ischemia, oxidative stress, fever, or inflammation. In humans, there are at least 11 distinct genes that code for HSP70 isoforms, which are located on several different chromosomes.14 The 73-kDa protein (HSP73 or HSC70) is present at constitutive levels, whereas the 72-kDa protein (HSP72 or HSP70) is highly inducible and its synthesis is increased in response to multiple stressors. The transcription of inducible forms of HSP70 and HSP72 are under the control of the heat shock factor (HSF),15 as well as a variety of physiologic processes, such as cell cycle control, proliferation, and differentiation.16 The major HSPs are molecular chaperones, which play an essential role in directing the folding and assembly of polypeptides in addition to coordinating regulation of protein translocation. HSPs also limit cellular damage following stress by their ability to prevent protein aggregation and to restore the function of denatured proteins. Moreover, HSP70 can function as a chaperone and cytokine17 by up-regulating the expression of tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and IL-6 on human monocytes, and stimulate cells of the innate immune system.18
In the present study, we demonstrate that introduction of GFP by various gene transfer vectors into the ECs selectively induces HSP70 mRNA and protein resulting in the up-regulation of cyclooxygenase-2 (COX-2) expression, leading to enhanced production of the vasoactive prostaglandin E2 (PGE2). These studies suggest that GFP used as a reporter gene should be used with caution because it may alter the physiology and stress response of ECs during gene transfer experiments. Identification of the endothelial-specific factors that GFP interacts with will also increase our understanding of the mechanism by which ECs respond to physiologic stressors.
Materials and methods
Cell culture and treatment conditions
Human umbilical vein endothelial cells (HUVECs) and human umbilical smooth muscle cells (HuSMCs) were isolated from umbilical cord veins with collagenase and were cultured in EC medium (M199 medium containing 10% [vol/vol] fetal bovine serum, 20 μg/mL EC growth factor, 50 μg/mL heparin, 100 μg/mL penicillin, and 100 μg/ml streptomycin) in a humidified incubator at 37°C with air/5% CO2.19 HUVEC monolayers from passages 2 to 4 were used in these studies. A549 (human lung carcinoma cells), HL60 (leukemic cell lines), and HeLa (human cervix carcinoma cells) were purchased from the American Type Culture Collection (Rockville, MD). Human pulmonary microvascular endothelial cells (HMVECs) and human dermal microvascular endothelial cells (HDMECs) were obtained from Clonetics (Walkersville, MD). Bone marrow endothelial cells (BMECs) were obtained from an immortalized human BMEC line.19 Cell viability was assayed by the trypan blue exclusion method, indicating that fewer than 5% of the cells took up the dye both before and after the infection of Ad vectors.
Construction of Ad vectors
The Ad vectors used in this study included E1–E4+ AdNull (E1–, E3–, E4+, cytomegalovirus early-immediate promoter/enhancer [CMV], no transgene in the expression cassette)20 ; E1–E4+ Adβgal (E1–, E3–, E4+; CMV promoter driving the Escherichia coli β-galactosidase β-gal] gene)21 ; E1–E4+ AdGFP (identical to E1–E4+ Adβgal, but with a modified form of the A victoria GFP cDNA [GFP] in place of β-gal)22 ;E1–E4+ AdYFP (E1–, E3–, E4+; CMV promoter driving the E coli yellow fluorescent protein cDNA [YFP] gene); E1–E4–AdGFP (same as AdGFP, but with a complete deletion of the E4 gene, using the GFP cDNA gene as a spacer in the E4 region); and AdLuciferase (E1–, E3–, E4+; CMV promoter driving the luciferase gene).23 Ad vector stocks were purified by cesium chloride centrifugation and dialysis and quantified by plaque-forming units (pfu) in 293 cells, as previously described.24 All Ad vectors had a particle-pfu ratio of approximately 100.
Microarray analysis
Cells were harvested and total RNA was extracted by RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was synthesized by using Superscript choice kit (Invitrogen, Carlsbad, CA) with a T7-(dT)24 primer incorporating a T7 RNA polymerase promoter. The cRNA was prepared and biotin labeled by in vitro transcription (Enzo Diagnostics, Farmingdale, NY). Labeled cRNA was fragmented by incubation at 94°C for 35 minutes in the presence of 40 mM Tris (tris(hydroxymethyl)aminomethane) acetate, pH 8.1, 100 mM potassium acetate, and 30 mM magnesium acetate. Fragmented cRNA (15 μg) was hybridized 16 hours at 45°C to a U95a array (Affymetrix, Santa Clara, CA). After hybridization, the gene chips were automatically washed and stained with streptavidin-phycoerythrin by using a fluidics station. Finally, probe arrays were scanned at 3-μm resolution using the Genechip System confocal scanner. Affymetrix Microarray Suite 4.1 was used to scan and analyze the relative abundance of each gene from the average difference of intensities.
Western blot analysis
Cells were lysed in RIPA buffer (50 mM Tris, 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate, and 10 g/mL aprotinin). Insoluble debris was pelleted, and the protein concentration of the supernatant was determined with a DC protein assay kit (Bio-Rad, Hercules, CA). Fifty micrograms of each protein sample was separated on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. The protein samples were transferred to nitrocellulose membrane. Protein expression was confirmed by immunoblotting with the following antibodies: HSP70, HSC70, heme oxgenase-1 (HO-1; StressGen, Victoria, BC, Canada), HSP90, COX-2 (Santa Cruz Biotechnology, Santa Cruz, CA), and GFP (Invitrogen). After incubation with the appropriate primary and horseradish peroxidase–conjugated secondary antibodies, the membranes were developed with enhanced chemiluminescence reagent (Amersham Pharmacia Biotechnology, Piscataway, NJ).
Northern blot analysis
Total RNA (10 μg) was extracted from HUVECs with TRIzol reagent (Invitrogen) and was separated on 1.0% agarose gels containing 2.2 M formaldehyde, transferred to nylon membranes, and UV cross-linked.25 Probes for cDNA of HSP70b (StressGen) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were radiolabeled with [γ-32P]dCTP using a random primers DNA-labeling kit. Northern hybridization was done using QuickHyb (Stratagene, Carlsbad, CA) as per the manufacturer's protocol. After hybridization, the membranes were washed twice in 0.2% standard sodium citrate (SSC) containing 0.1% (wt/vol) SDS at room temperature for 20 minutes twice and finally in 0.1% SSC/0.1% SDS at 42°C for 45 minutes. The membranes were exposed to a Kodak film. To verify equivalency of RNA loading in the different lanes, the blot was stripped of radioactivity and rehybridized to determine the levels of GAPDH.
Transfection and assays of reporter gene activity
HUVEC monolayers were plated in 6-well plates 24 hours before transfection. Cells were transfected with the reporter plasmid HSP70b promoter expressing β-galactosidase using Polyfect transfection reagent (Qiagen) according to the manufacturer's directions. After overnight incubation with the transfection mixture, cells were infected with control or AdNull or AdGFP vectors. Cells were harvested 48 hours after infection with Ad vectors. Galactosidase activity was assayed with a luminescent substrate (Galacton Plus) according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). Results represent means of 3 experiments ± SD.
PGE2 release
HUVEC monolayers were cultured in X-vivo medium 48 hours after infection with and without AdGFP or AdNull. The supernatants were harvested and stored at –70°C until they were assayed. The amount of PGE2 in the supernatant was measured by enzyme-linked immunosorbent assay (ELISA).25
Laser-Doppler measurements of mice ear blood flow
The pinnas of the ears of the mice were injected with AdGFP (1 × 108 multiplicity of infection [MOI]), AdNull (1 × 108 MOI), or phosphate-buffered saline (PBS; control). Three days after injection, blood flow of the ear was assessed by laser-Doppler with a laser flowmeter (model ALF21/21D, Advance, Tokyo, Japan).
Results
To identify genes that were induced by GFP expression, we compared the gene expression profiles of control uninfected ECs with AdNull- or AdGFP-infected HUVECs by using Affymetrix U95 high-density oligonucleotide genechips. AdGFP infection of ECs for 48 hours resulted in an 80-fold increase in HSP70 mRNA. AdGFP also induced, to a lesser degree, other stress-related genes including HO-1 (Table 1).
Stress-related genes altered after infection with AdGFP
GenBank no. . | Gene description . | Fold change* . | Signal . |
|---|---|---|---|
| X51757 | Human heat-shock protein HSP70B gene | 88 | 2007.1 |
| D85429 | Heat shock protein 40 | 6.4 | 2481.5 |
| D85730 | HSPA1L mRNA for heat shock protein 70 testis | 4.5 | 50.2 |
| L26336 | Human heat shock protein HSPA2 | 2.4 | 782.8 |
| L12723 | Human heat shock protein 70 (hsp70) | 1.8 | 749.5 |
| Z23090 | 28-kDa heat shock protein | 1.6 | 9016 |
| J04988 | Human 90-kD heat shock protein | 1.5 | 8160 |
| Y17782 | Heat shock protein B3 | 1.2 | 3.6 |
| AF088982 | Heat shock protein hsp40-3 | 1.1 | 125.7 |
| Z82244 | Heme oxygenase 1 | 11.3 | 4052.1 |
GenBank no. . | Gene description . | Fold change* . | Signal . |
|---|---|---|---|
| X51757 | Human heat-shock protein HSP70B gene | 88 | 2007.1 |
| D85429 | Heat shock protein 40 | 6.4 | 2481.5 |
| D85730 | HSPA1L mRNA for heat shock protein 70 testis | 4.5 | 50.2 |
| L26336 | Human heat shock protein HSPA2 | 2.4 | 782.8 |
| L12723 | Human heat shock protein 70 (hsp70) | 1.8 | 749.5 |
| Z23090 | 28-kDa heat shock protein | 1.6 | 9016 |
| J04988 | Human 90-kD heat shock protein | 1.5 | 8160 |
| Y17782 | Heat shock protein B3 | 1.2 | 3.6 |
| AF088982 | Heat shock protein hsp40-3 | 1.1 | 125.7 |
| Z82244 | Heme oxygenase 1 | 11.3 | 4052.1 |
Control uninfected ECs and cells infected with AdGFP (200 MOI) for 48 hours. Microarray analysis was determined by Affymetrix genechip as described in “Materials and methods.”
AdGFP versus control.
AdGFP-induced HSP70 expression is EC specific
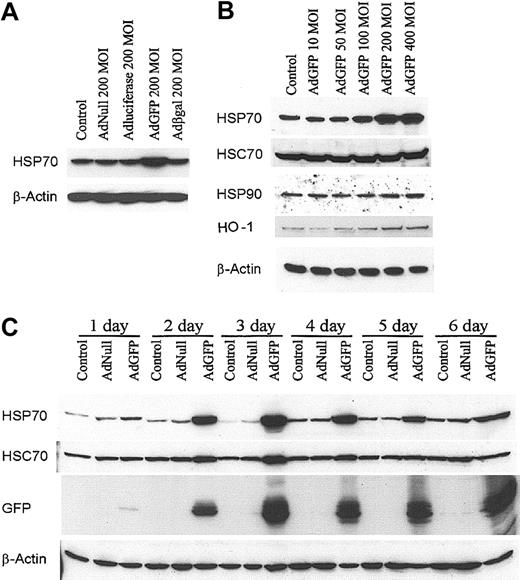
The expression of HSP70 by the ECs after AdGFP infection was assessed by immunoblot analysis. As shown in Figure 1A, AdGFP effectively induced HSP70 protein expression in HUVECs. However, AdNull, Adβ-galactosidase, and AdLuciferase failed to induce HSP70 expression in HUVECs (Figure 1A). AdGFP infection of confluent monolayers of HUVECs induced a dose-dependent expression of HSP70 with a peak effect at 400 MOI (Figure 1B). Although AdGFP also induced the expression of HO-1, it had no effect on the expression of HSP90 and HSC70 (Figure 1B). The time course of HSP70 induction by AdGFP was assessed by Western blot analysis. Maximal induction of HSP70 occurred after 2 to 3 days of infection with AdGFP (Figure 1C) and consistently high expression of HSP70 was detected after 6 days in parallel with GFP expression (Figure 1C). Of note, there was a slight up-regulation of HSC70 expression 2 to 3 days after AdGFP infection. These data suggest introduction of GFP in ECs results in a robust up-regulation of HSP70, whereas enhanced synthesis of HSC70 is less prominent and transient.
GFP Ad vector induced HSP70 protein expression in HUVECs. (A) Cells were treated with culture medium (control), AdNull 200 MOI, AdLuciferase 200 MOI, AdGFP 200 MOI, and Adβ-galactosidase (Adβ-gal) 200 MOI, and cultured in growth factor–free medium for 48 hours. Cellular extracts were prepared from HUVECs, and HSPs were detected by Western blot analysis using antibodies specific for HSP70 and β-actin. (B) HUVECs were infected for 48 hours with control or AdGFP at the indicated concentrations ranging from 50 to 400 MOI. Immunoblots were probed with antibodies specific to HSP70, HSC70, HSP90, HO-1, and β-actin. (C) HUVECs were infected with control, AdNull (200 MOI), or AdGFP (200 MOI) for the indicated time periods. Immunoblots were probed with antibodies specific for HSP70, HSC70, GFP, and β-actin. The results shown represent one sample experiment from a total of 3 independent experiments.
GFP Ad vector induced HSP70 protein expression in HUVECs. (A) Cells were treated with culture medium (control), AdNull 200 MOI, AdLuciferase 200 MOI, AdGFP 200 MOI, and Adβ-galactosidase (Adβ-gal) 200 MOI, and cultured in growth factor–free medium for 48 hours. Cellular extracts were prepared from HUVECs, and HSPs were detected by Western blot analysis using antibodies specific for HSP70 and β-actin. (B) HUVECs were infected for 48 hours with control or AdGFP at the indicated concentrations ranging from 50 to 400 MOI. Immunoblots were probed with antibodies specific to HSP70, HSC70, HSP90, HO-1, and β-actin. (C) HUVECs were infected with control, AdNull (200 MOI), or AdGFP (200 MOI) for the indicated time periods. Immunoblots were probed with antibodies specific for HSP70, HSC70, GFP, and β-actin. The results shown represent one sample experiment from a total of 3 independent experiments.
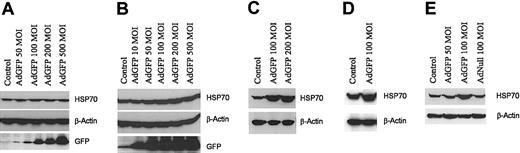
To assess whether the induction of HSP70 by AdGFP is only restricted to ECs, HSP70 expression was evaluated in HuSMCs, A549, HL60, and HeLa cell lines following infection with AdGFP. Although AdGFP was efficient in transducing more than 90% of the cells, there was no induction of HSP70 in HuSMCs, A549 (Figure 2A-B), HL60, or HeLa cell line (data not shown). Moreover, GFP not only induced HSP70 expression on the HUVECs, but also other microvascular ECs, including HDMECs (Figure 2C), BMECs (Figure 2D), and HMVECs (Figure 2E). These data suggest that AdGFP induction of HSP70 is EC specific and is not dependent on the origin of the ECs.
GFP-induced HSP70 protein expression specific in ECs. Non-ECs: HuSMCs (A) orA549 cell line (B) were infected with AdGFP (50-500 MOI) for 48 hours. ECs: HDMECs (C), BMECs (D), or HMVECs (E) were infected with AdGFP or AdNull at the indicated concentrations ranging from 50 to 200 MOI for 48 hours. Cell lysates were then assayed for HSP70, GFP, and β-actin by Western blotting.
GFP-induced HSP70 protein expression specific in ECs. Non-ECs: HuSMCs (A) orA549 cell line (B) were infected with AdGFP (50-500 MOI) for 48 hours. ECs: HDMECs (C), BMECs (D), or HMVECs (E) were infected with AdGFP or AdNull at the indicated concentrations ranging from 50 to 200 MOI for 48 hours. Cell lysates were then assayed for HSP70, GFP, and β-actin by Western blotting.
GFP directly induces HSP70 transcription
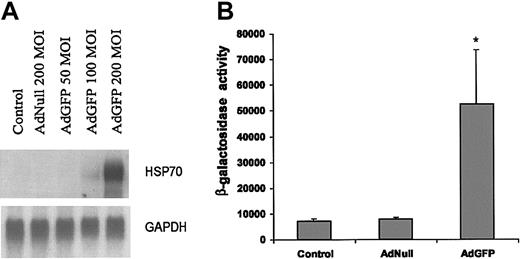
The expression of HSPs is regulated at both transcriptional and posttranscriptional levels. To further elucidate the mechanism responsible for the changes in amounts of HSP70 protein, we measured the mRNA levels of HSP70 by Northern blotting after EC infection with AdNull or AdGFP. As shown in Figure 3A, HSP70b mRNA levels were up-regulated in response to AdGFP, but not by AdNull. This result was consistent with the GFP-mediated induction as detected by microarray analysis (Table 1) and Western blotting (Figure 1). We next investigated the effect of GFP overexpression on HSP70b promoter activity in ECs. ECs were transfected with reporter vector expressing β-galactosidase (β-gal) from HSP70 promoter and then infected with AdGFP for 48 hours. Figure 3B demonstrates that in comparison to control vectors, there was an 8-fold increase in the β-gal activity in the AdGFP-treated ECs.
AdGFP-induced transcriptional activation of HSP70 promoter and RNA expression. (A) Northern analysis of HSP70 gene expression in ECs. Total RNA (10 μg/lane) was isolated from uninfected control cells and cells infected with AdNull (200 MOI) or AdGFP (50-200 MOI) for 48 hours. The blot was hybridized to a 32P-labeled human HSP70b cDNA probe and control 32P-labeled GAPDH probes to normalize RNA loading. (B) AdGFP activates HSP70 promoter activity. HUVECs were transfected with 2 μg HSP70 reporter plasmid containing the β-galactosidase for 12 hours, and then treated for 48 hours under the following conditions: control, AdNull 200 MOI, or AdGFP 200 MOI. β-Galactosidase reporter activities were measured in cellular extract. The columns are the means, and the bars are the SD; *P < .001 compared with control. The results are representative of 3 separate experiments.
AdGFP-induced transcriptional activation of HSP70 promoter and RNA expression. (A) Northern analysis of HSP70 gene expression in ECs. Total RNA (10 μg/lane) was isolated from uninfected control cells and cells infected with AdNull (200 MOI) or AdGFP (50-200 MOI) for 48 hours. The blot was hybridized to a 32P-labeled human HSP70b cDNA probe and control 32P-labeled GAPDH probes to normalize RNA loading. (B) AdGFP activates HSP70 promoter activity. HUVECs were transfected with 2 μg HSP70 reporter plasmid containing the β-galactosidase for 12 hours, and then treated for 48 hours under the following conditions: control, AdNull 200 MOI, or AdGFP 200 MOI. β-Galactosidase reporter activities were measured in cellular extract. The columns are the means, and the bars are the SD; *P < .001 compared with control. The results are representative of 3 separate experiments.
GFP induction of HSP70 is independent of gene transfer vectors
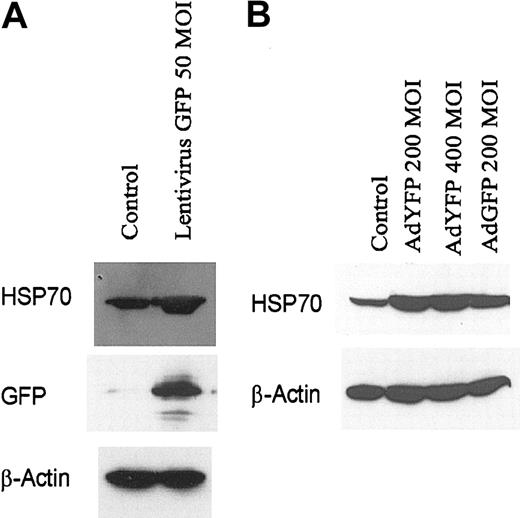
We have previously shown that the E4 gene of adenoviral gene transfer vectors can alter the survival and apoptotic potential of ECs.1,8 To rule out the possibility that the E4 gene or any other adenoviral genes can directly induce HSP70 expression, we examined HSP70 expression in HUVECs infected with lentivirus-expressing GFP. Exposure of HUVECs to lentivirus-GFP induced the synthesis of protein of HSP70 (Figure 4A) in endothelial monolayers. Moreover, we found that YFP,26 a structurally similar molecule to GFP, at 200 MOI also strongly induced HSP70 expression in HUVEC monolayers (Figure 4B). These data suggest that GFP and its homologues can induce expression of HSP70 independent of the gene transfer vectors.
Increased HSP70 expression in HUVECs after engraftment with lentivirus-transduced GFP or AdYFP. (A) HUVECs infected with lentivirus GFP (MOI 50) for 48 hours. (B) HUVECs infected with AdNull (200 MOI), AdYFP (200 MOI), AdYFP (400 MOI), and AdGFP (200 MOI) for 48 hours. Immunoblots were probed with antibody specific for HSP70, GFP, and β-actin.
Increased HSP70 expression in HUVECs after engraftment with lentivirus-transduced GFP or AdYFP. (A) HUVECs infected with lentivirus GFP (MOI 50) for 48 hours. (B) HUVECs infected with AdNull (200 MOI), AdYFP (200 MOI), AdYFP (400 MOI), and AdGFP (200 MOI) for 48 hours. Immunoblots were probed with antibody specific for HSP70, GFP, and β-actin.
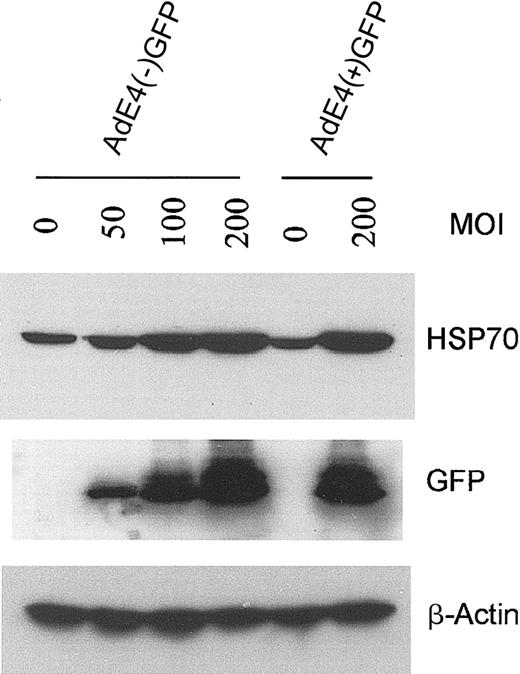
Because E4+ Ad vectors are known to modulate EC survival and activation status,9 we compared the effect of E1–E4– AdGFP with that of E1–E4+ AdGFP on HSP70 expression in HUVECs. Both forms of AdGFP induced HSP70 expression and this induction correlated with the levels of GFP expression (Figure 5). These data suggest that GFP induction is not dependent on the coexpression of the E4 gene products.
Effect of E4+ GFP Ad vector or E4– GFP Ad vector on HSP70 expression in HUVECs. Confluent ECs infected with the E1–E4– AdGFP or E1–E4+ AdGFP vector, or uninfected controls, were cultured in growth factor–free medium for 48 hours. Immunoblots were probed with antibody specific for HSP70, GFP, and β-actin.
Effect of E4+ GFP Ad vector or E4– GFP Ad vector on HSP70 expression in HUVECs. Confluent ECs infected with the E1–E4– AdGFP or E1–E4+ AdGFP vector, or uninfected controls, were cultured in growth factor–free medium for 48 hours. Immunoblots were probed with antibody specific for HSP70, GFP, and β-actin.
GFP induces HSP70 independent of MAPK and PI3K signaling cascades
To dissect the mechanism by which GFP induces HSP70 expression, we explored whether GFP activates signaling cascades, which lead to enhanced HSP70 transcription. Mitogen-activated protein kinase (MAPK), a Ser/Thr protein kinase, has been shown to participate in heat stress response.27,28 The c-Jun NH2-terminal kinase (JNK) represents one subgroup of MAPKs that is activated primarily by cytokines and exposure to environmental stress in mammalian cells.13 However, our evaluation of treatment with MEK inhibitor PD98059, p38 inhibitor SB203580, JNK inhibitor SP600125, or phosphatidylinositol-3-kinase (PI3K) inhibitor LY 294002 showed no significant disruption of AdGFP-induced HSP70 protein expression, indicating that MAPK and PI3K pathways are not affected by GFP in HUVECs (Figure 6).
Effects of kinase inhibitors on the HSP70 protein expression in HUVECs. Cells were infected with AdGFP (200 MOI) or uninfected control and cotreated with either PD98059 (PD), SB203580 (SB), SP600125 (SP), or LY294002 (LY) for 48 hours. Cells were harvested and lysed for Western blot analysis of HSP70.
Effects of kinase inhibitors on the HSP70 protein expression in HUVECs. Cells were infected with AdGFP (200 MOI) or uninfected control and cotreated with either PD98059 (PD), SB203580 (SB), SP600125 (SP), or LY294002 (LY) for 48 hours. Cells were harvested and lysed for Western blot analysis of HSP70.
GFP induces COX-2 expression and PGE2 production
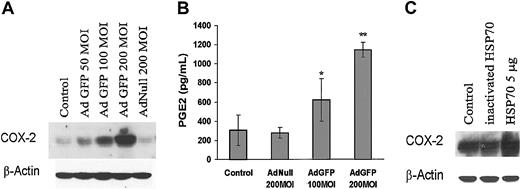
Among the known factors that regulate endothelial cell physiology, COX-2 induction has been shown to modulate EC function, including angiogenesis and inflammation. To identify the molecular pathways activated by overexpression of HSPs that alter EC function, we examined the effect of GFP on the expression of COX-2 levels. Figure 7A shows that AdGFP, but not AdNull, induced COX-2 protein expression in a dose-dependent manner. Moreover, treatment of HUVECs with AdGFP significantly increased the synthesis of PGE2, which is catalyzed by COX-2 (Figure 7B). To determine whether HSP70 causes induction of COX-2 expression in HUVECs, we evaluated the effect of HSP70 protein on COX-2 expression. As shown in Figure 7C, HSP70 protein induced COX-2 protein expression in HUVECs. These data suggest that GFP-induced HSP70 augments COX-2 expression and PGE2 production.
Effects of GFP on COX-2 expression and PGE2 production in ECs. (A) Control uninfected cells and cells infected with AdGFP (50-200 MOI) or AdNull (200 MOI) for 48 hours. Cell lysates were probed for COX-2 expression by Western blotting and (B) culture supernatants were analyzed for PGE2. Production of PGE2 was determined by enzyme immunoassay as described in “Materials and methods.” The columns are the means, and the bars are the SD (n = 4); *P < .05 compared with control; **P < .01 compared with control. (C) Cells were treated without or with recombinant human HSP70 (5 μg/mL) or heat-inactivated HSP70 (5 μg/mL) for 24 hours. Immunoblots were probed with antibody specific for COX-2.
Effects of GFP on COX-2 expression and PGE2 production in ECs. (A) Control uninfected cells and cells infected with AdGFP (50-200 MOI) or AdNull (200 MOI) for 48 hours. Cell lysates were probed for COX-2 expression by Western blotting and (B) culture supernatants were analyzed for PGE2. Production of PGE2 was determined by enzyme immunoassay as described in “Materials and methods.” The columns are the means, and the bars are the SD (n = 4); *P < .05 compared with control; **P < .01 compared with control. (C) Cells were treated without or with recombinant human HSP70 (5 μg/mL) or heat-inactivated HSP70 (5 μg/mL) for 24 hours. Immunoblots were probed with antibody specific for COX-2.
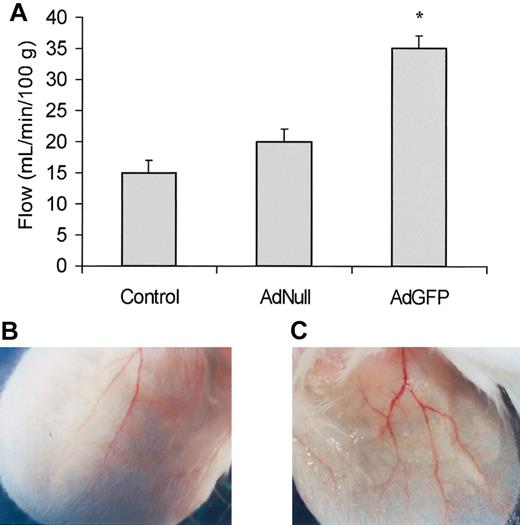
To examine the physiologic significance of GFP-mediated PGE2 up-regulation, the pinnas of the ears of the mice were injected with AdGFP and blood flow was measured by Doppler after 24 hours. As compared to AdNull-treated mice, AdGFP-treated mice showed significant increase in blood flow most likely mediated by an increase in GFP up-regulation of PGE2 expression (Figure 8). Collectively, these data suggest that elevation of GFP induces production of sufficient PGE2 to alter the regional blood flow.
Effects of AdGFP on ear blood flow and vasodilation. (A) AdGFP (1 × 108 MOI/ear), AdNull (1 × 108 MOI/ear), or control (PBS) was injected in the pinna of the mouse's ear. After 3 days following injection, blood flow was measured using a laser-Doppler. The columns are the means, and the bars are the SD (n = 3); *P < .05 compared with control or AdNull. As compared to the AdNull injected mice (B), 3 days after AdGFP injection (C) there was an increase in the number of grossly vasodilated vessels.
Effects of AdGFP on ear blood flow and vasodilation. (A) AdGFP (1 × 108 MOI/ear), AdNull (1 × 108 MOI/ear), or control (PBS) was injected in the pinna of the mouse's ear. After 3 days following injection, blood flow was measured using a laser-Doppler. The columns are the means, and the bars are the SD (n = 3); *P < .05 compared with control or AdNull. As compared to the AdNull injected mice (B), 3 days after AdGFP injection (C) there was an increase in the number of grossly vasodilated vessels.
Discussion
Successful gene therapy strategies targeted to the blood vessels depend on delivering the transgene of interest without inflicting unnecessary vascular toxicity. In this regard, identifying the factors or reporter genes that may alter the biology of the ECs promises to improve the reliability of the gene therapy strategies. In the present study, we demonstrate that introduction of the GFP reporter gene by adenoviral or lentiviral vectors selectively induces expression of HSP70 in human ECs. Up-regulation of HSP70 is associated with induction of COX-2 and synthesis of PGE2 in vitro and regional increase in blood flow in vivo. The GFP-induced expression of HSP70 is observed only on the ECs and not on other cell types such as smooth muscle cells or cancer cell lines. These data suggest that expression of GFP specifically results in induction of genes in the vascular ECs, which alters the biologic response of ECs.
GFP-mediated induction of HSP70 in ECs was not due to the introduction of the E4 adenoviral gene as the E4– vectors (AdE4–GFP) also induced HSP70 expression. Moreover, lentiviral vectors expressing GFP induced the expression of inducible HSP70. The induction of HSP70 was dose dependent with a higher level of HSP70 being induced as escalating doses of GFP were used. It is intriguing that the constitutive isoform of HSP70 was also slightly induced when high doses of GFP were introduced.
HSP70 has been shown to protect against TNF-induced lethal inflammatory shock and to have a protective effect on the myocardium against more severe physiologic stressors.12,29,30 Substantial evidence indicates that HSPs are capable of protecting cells, tissues, organs, and animals from a subsequent, normally lethal heating, as well as from other types of noxious conditions, including hypoxia and ischemia/reperfusion.31,32 Here, we demonstrate that HSP70 can also induce the expression of COX-2 in the ECs. Recombinant HSP70 induced the expression of COX-2 when the ECs were incubated with this protein. Up-regulation of COX-2 was associated with increased expression of PGE2.
Exogenous HSP70 can bind with high affinity to the cell surface17 and activate nuclear factor-κB (NF-κB) and up-regulate the expression of proinflammatory cytokines TNF-α, IL-1β, and IL-6. COX-2 can be regulated in response to proinflammatory cytokines. This could be a possible mechanism for the exogenous HSP70-mediated effects on COX-2 expression in ECs.
The mechanism by which GFP promotes the expression of HSP70 may be mediated through induction of HSP70 transcription. This hypothesis is supported by the data demonstrating that GFP can turn on the HSP70 promoter. HSP70 is regulated at the transcriptional level by heat shock transcription factor 1 (HSF1). Activated HSF1 binds to a specific DNA recognition sequence (heat shock element) in the HSP70 promoter.13 HSP70 may interact with important kinases of the mitogen-activated signal cascades, such as Src kinases, tyrosine receptor kinases, Raf, and MAPKs.33 To rule out the possibility that GFP may alter the HSP70 expression through activation of signaling cascades, we challenged the cells expressing GFP with several inhibitors that selectively block Erk1, p38 MAPK, PI3K, and cJun N-terminal kinase (Jnk). None of these inhibitors blocked the GFP-mediated up-regulation of HSP70, suggesting that GFP may not interact with those signaling pathways. It is possible that HSF1 is activated by mechanisms that are independent of phosphorylation or that dephosphorylation of inhibitory phosphorylation sites stimulates the transcriptional activity of HSF1.13
We have shown that the effect of E4+ adenoviral vectors in mediating survival and proliferation is restricted to ECs.9 Presently, the exact mechanism whereby E4+ genes selectively alter the biology of vascular cells is not known. Similarly, GFP failed to induce HSP70 in smooth muscle cells and carcinoma as well as the leukemia cells, suggesting that expression of certain permissive factors may be unique to the ECs. It is conceivable that GFP, through interaction with endothelial-specific proteins, induces the expression of HSP70.
GFP has been used in many in vivo gene transfer experiments without any apparent toxicity. Based on our data, it is possible that the dose of GFP may be the major determinant in induction of HSP70. Indeed, we show that only at relative high doses of GFP is there an induction of HSP70. This high dose can only be achieved when high-copy adenoviral vectors or lentiviral vectors with strong promoters drive GFP expression.34 In this regard, the toxicity of GFP can be diminished by adjusting the dose of the adenoviral or lentiviral constructs used in gene therapy studies.
Nonetheless, because COX-2 and PGE2 can modulate vasodilator properties of vascular ECs,35-37 the use of GFP as a reporter gene in adenoviral vectors that carry angiogenic factors should be used with extreme caution. Because GFP-mediated up-regulation of COX-2 and PGE2 may act synergistically with the angiogenic factor expressed as a transgene, such as adenoviral vectors expressing VEGF, then the outcome of these studies may not represent the true potential of the transgene of interest being tested.
Our data also set the stage for studies to understand the mechanism by which GFP alters the physiologic repertoire of ECs. If GFP, by promoting expression of HSP70 and COX-2, conveys signals that may increase the survival of ECs, GFP may be used to identify molecular factors that support survival and angiogenic potential of ECs.
Prepublished online as Blood First Edition Paper, June 12, 2003; DOI 10.1182/blood-2003-01-0049.
Supported by grants from National Heart, Lung and Blood Institute (NHLBI), R01s HL61849, HL66592, and HL67839, and American Cancer Society-101396 (S.R.), and the Leukemia and Lymphoma Society. F.Z. is recipient of Programs of Excellence in Gene Therapy fellowships from NHLBI. These studies were also supported by P01 HL59312 and GenVec, Inc, Gaithersburg, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Scott Avecilla for help in preparing this manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal