Abstract
Growth factor–induced neovascularization has received a great deal of attention because it is fundamental to the growth and metastasis of solid tumors. This multistep process requires extensive signaling through growth factor receptors and integrins. Among the integrins involved in this process, integrin αvβ3 is specific to basic fibroblast growth factor (bFGF)–induced angiogenesis. Here we show that junctional adhesion molecule 1/A (JAM-1/A) and αvβ3 form a complex in the absence of bFGF. JAM-1, which is normally localized at the cell-cell junctions of quiescent endothelial cells, redistributes to the cell surface on bFGF treatment. Blockage of the extracellular domain of JAM-1 inhibits bFGF-induced endothelial cell morphology, proliferation, and angiogenesis. Additionally, mutation in the JAM-1 cytoplasmic domain blocks bFGF-induced mitogen-activated protein (MAP) kinase activation and ablates its ability to induce endothelial cell tube formation, suggesting that signaling through JAM-1 is key to bFGF-induced signaling. Immunoprecipitation analysis suggests that bFGF signaling dissociates the JAM-1/ αvβ3 complex, allowing for signaling through JAM-1 and αvβ3. In addition, blockage of either JAM-1 or αvβ3 inhibits bFGF-induced MAP kinase activation. Thus, our results suggest that signaling through JAM-1 and αvβ3 is necessary for bFGF-induced angiogenesis.
Introduction
During angiogenesis, endothelial cells become activated and are coordinately involved in the processes of proliferation, migration, and tube formation.1-3 This neovascularization is a multistep process influenced by soluble angiogenic growth factors and a close interaction between adhesive proteins of the extracellular matrix and their integrin receptors.4,5 Recently, angiogenesis has received a great deal of attention because it is required for the growth and metastasis of solid tumors and for the restoration of tissues in various ischemic conditions.1
Among the growth factors implicated in the angiogenic process, bFGF and vascular endothelial growth factor (VEGF) are known to be potent angiogenic molecules that induce the growth of new blood vessels during wound healing and embryonic development.6,7 More recently, however, elevated bFGF and VEGF levels have been implicated in pathologic angiogenesis that aid in tumor growth and metastasis.8 For this reason, many therapeutic agents generated to block growth factor–induced angiogenesis are now in clinical trials.9,10
Among the integrin family members most widely studied in the angiogenic process are of the αv family—integrins αvβ3 and αvβ5, which are receptors for vitronectin.4,11-13 These 2 integrins regulate 2 distinct pathways of angiogenesis.12 Angiogenesis initiated by VEGF, transforming growth factor-α (TGF-α), or phorbol ester, is dependent on integrin αvβ5 because function-blocking antibody or antagonist specific to αvβ5 inhibits these growth factor–induced pathways but not those induced by bFGF or tumor necrosis factor α (TNF-α).12 The latter factors in fact signal through αvβ34,12 . Expression of αvβ3 is up-regulated on endothelial cells involved in new blood vessel formation and is required for sustained mitogen-activated protein (MAP) kinase activity leading to neovascularization induced by bFGF and TNF-α.14 Function-blocking antibody or low-molecular-weight Arg-Gly-Asp (RGD)–based antagonist specific to αvβ3 inhibits angiogenic pathways induced by these factors and these antagonists are now in clinical trials.15,16 In addition, a dual αvβ3- and αvβ5-specific integrin antagonist, SCH221153, inhibits angiogenesis and tumor growth induced by bFGF and VEGF.17 The current study highlights the essential role of these αvβ3 integrin in angiogenesis.
We found that JAM-1 (also known as JAM-A), an immunoglobulin superfamily (IgSF) member, is involved in signaling that induces αvβ3-dependent endothelial cell migration on vitronectin (Naik et al, manuscript submitted). Because bFGF-induced angiogenesis requires endothelial cell migration on vitronectin through integrin αvβ3, we sought to further explore our findings with regard to the role of JAM-1 in bFGF-induced angiogenesis. In this study, we find that the overexpression of JAM-1 mimics bFGF-induced signaling events such as endothelial cell morphology, proliferation, in vitro tube formation, and sustained MAP kinase activity. Overexpression of JAM-1 mutants failed to induce the same effects. Further, JAM-1 associates with αvβ3, and bFGF induces dissociation of this complex. Blockade of either JAM-1 or αvβ3 inhibits these events induced by bFGF. Taken together, our results suggest that signaling through JAM-1 is required for the angiogenic action of bFGF.
Materials and methods
Cell culture reagents, construction of expression vectors, and stable transfection
Human umbilical vein endothelial cells (HUVECs) and appropriate growth media were purchased from Clonetics (San Diego, CA). Construction of full-length JAM-1 in the expression vector pcDNA3.1 was described previously.18 A JAM-1 cytoplasmic deletion mutant (Δ-257) and a JAM-1 substitution mutant of cytoplasmic tyrosine 280 to phenylalanine (Y→F) were constructed using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). All constructs were stably transfected into HUVECs using SuperFect reagent (Qiagen, Santa Clara, CA). Transfectants were selected in 500 μg/mL G418-containing growth medium, and, because of a limited passage number, pools of stably transfected cells between passages 3 and 7 were used instead of a clonal population. The level of protein expression was determined by Western blotting as described.18 Densitometric analysis of band intensity was quantitated using a BioRad GelDoc 2000 (Hercules, CA).
Antibodies and reagents
Production of anti–JAM-1 function-blocking monoclonal antibody (mAb) J3F.1 was generated as described previously.19 Anti-αvβ3 (LM609; IgG1 antibody) was a kind gift from David Cheresh (Scripps Institute, La Jolla, CA). Anti-αvβ5 (P1F6, IgG1 antibody) was purchased from Chemicon International (Temecula, CA). Isotype-matched control antibody, HB67 (cIgG), was obtained from the American Type Culture Collection (Manassas, VA). Anti–calcium- and integrin-binding (CIB) protein, a control antibody, was produced as described previously.20 Antivascular endothelial-cadherin (anti-VE-cadherin) and anti-β3 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–JAM-1 polyclonal antibody was raised against the peptide (SVTVHSSEPEVRIPENNPVKLSC) and was purified using a peptide-affinity column (BioSource, Camarillo, CA). Rabbit polyclonal antibodies against ERK1/2 and phospho-ERK1/2 were purchased from New England Biolabs (Beverly, MA). bFGF was purchased from Sigma (St Louis, MO). Human vitronectin was purchased from Collaborative Biomedical Research (Bedford, MA).
Immunofluorescence
Immunofluorescence studies were performed essentially as described previously.18 In brief, HUVECs stably transfected with mock or JAM-1 constructs were allowed to spread on cover-slide chambers to form cell-cell junctions (Nalge Nunc International, Rochester, NY). Cells were then serum-starved overnight, treated with bFGF for 10 minutes where indicated, rinsed twice with phosphate-buffered saline (PBS), and fixed with freshly prepared 4% (wt/vol) paraformaldehyde. After fixation, cells were permeabilized with 0.2% (vol/vol) Triton X-100 for 5 minutes, rinsed, and blocked with 3% (wt/vol) BSA in PBS for 1 hour at room temperature. Cells were incubated with the indicated primary antibody overnight at 4°C, and then incubated with appropriate fluorescence-labeled secondary antibody for 1 hour at room temperature. Horizontal optical selections were taken through the middle of the cells to highlight cell-cell junctions using a Zeiss (Thornwood, NY) LSM510 laser-scanning microscope.
Cell morphology
Untransfected HUVECs were serum-starved overnight and harvested. Cells in suspension were treated with anti–JAM-1 for 20 minutes where indicated, then stimulated with bFGF (or PBS as control) and allowed to spread on vitronectin-coated dishes for 4 hours at 37°C. Adherent cells were stained with Diff-Quik (Dade Behring, Newark, DE) to view cellular morphology. Photographs were taken using a Nikon (Melville, NY) inverted phase-contrast microscope.
Proliferation assay
Proliferation of HUVECs pretreated with bFGF (10 ng/mL) or anti–JAM-1 (10 μg/mL) as indicated was observed by 5-bromodeoxyuridine (BrdU) incorporation assay and by counting the cell number. The BrdU incorporation experiment was performed using HUVECs transfected with mock or JAM-1 constructs that were serum-starved overnight. Cells were then pretreated with anti–JAM-1 for 20 minutes where indicated, then treated with 10 ng/mL bFGF and labeled with BrdU labeling solution (Boehringer Mannheim, Germany) for 18 hours. After fixation, cells were stained with a monoclonal anti-BrdU antibody and were read on a microwell plate reader at 495 nm. For cell counting, mock- and JAM-1–transfected HUVECs were plated in triplicate in 24-well dishes (2 × 104 cells/mL), then harvested and counted daily using a hemocytometer.21
Chick chorioallantoic membrane angiogenesis assay
A chick chorioallantoic membrane (CAM) assay was performed as described previously.15 Angiogenesis was induced in 10-day-old chick embryos with 50 ng/mL bFGF (or PBS as control). After 24 hours, anti-αvβ3 (2.5 μg/mL), anti–JAM-1 (1-10 μg/mL), or cIgG (5 μg/mL) was added topically to appropriate bFGF-stimulated CAMs The number of vessel branch points per CAM was scored as an angiogenic index.
Aortic ring assay
An aortic ring assay was performed essentially as described.22 Murine thoracic aortic rings (1 mm) were placed between 2 layers of 50 μL growth-factor–reduced Matrigel containing 20 U/mL heparin. Dulbecco modified Eagle medium (DMEM) containing 10 μg/mL anti–JAM-1 or cIgG was then added. After overnight incubation, 20 ng/mL bFGF was added to induce vessel outgrowth. The number of microvessels per aortic ring was quantified after 4 days using a Nikon phase-contrast microscope.
In vitro tube formation
HUVEC tube formation, an in vitro model for angiogenesis, was performed in a 3-dimensional growth-factor–reduced Matrigel (Becton Dickinson, San Diego, CA) as described previously.23 HUVECs mock-transfected or transfected with various JAM-1 constructs as indicated were serum-starved overnight, harvested, and plated (1 × 105 cells) on a layer of Matrigel in the presence or absence of bFGF as indicated. After 4 hours, another layer of Matrigel was added, and photographs were taken after 24 hours using a Nikon phase-contrast microscope.
Immunoprecipitation
Serum-starved JAM-1-overexpressing cells (3 × 106) were lysed with lysis buffer (1% [vol/vol] NP40, 150 mM NaCl, 50 mM Tris pH 7.5, 10 mM sodium orthovanadate, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 2 mM phenylmethylsulfonyl fluoride [PMSF], and 10 mM NaF) for 30 minutes on ice, and then centrifuged at 15 000g for 10 minutes at 4°C. Where indicated, cells were treated with 10 ng/mL bFGF for 10 minutes or 100 μg/mL RGDS for 20 minutes at 37°C and then lysed as described. Lysates were immunoprecipitated with anti–JAM-1 antibody or HB67 (cIgG) along with protein G-Sepharose beads (Amersham Biosciences, Piscataway, NJ). Immunocomplex-captured beads were washed with lysis buffer without inhibitors and were boiled in 50 μL Laemmli sample buffer. Proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotted using anti-β3 as described previously.18
MAP kinase activation
MAP kinase activation was assessed essentially as described previously.24 In brief, HUVECs mock-transfected or transfected with the indicated JAM-1 constructs (3 × 105 cells/mL) were serum-starved overnight, then treated with 10 ng/mL bFGF for 10 minutes where indicated. In some experiments, cells were preincubated with 10 μg/mL each of anti–JAM-1, anti–αvβ3, anti-CIB, or PBS as a control for 20 minutes before treatment with bFGF. Laemmli sample buffer was then added immediately after bFGF treatment, and proteins from total cell lysates were separated by 10% SDS-PAGE, immunoblotted as described, and probed with anti–phospho-ERK1/2. Blots were then reprobed with anti-ERK1/2 antibodies to show total ERK1/2. Results presented are representative of at least 3 separate experiments.
Statistical analysis
Standard statistical tests (mean value, SEM, paired Student t test) were performed for data analysis.
Results
JAM-1 is required for bFGF-induced angiogenesis
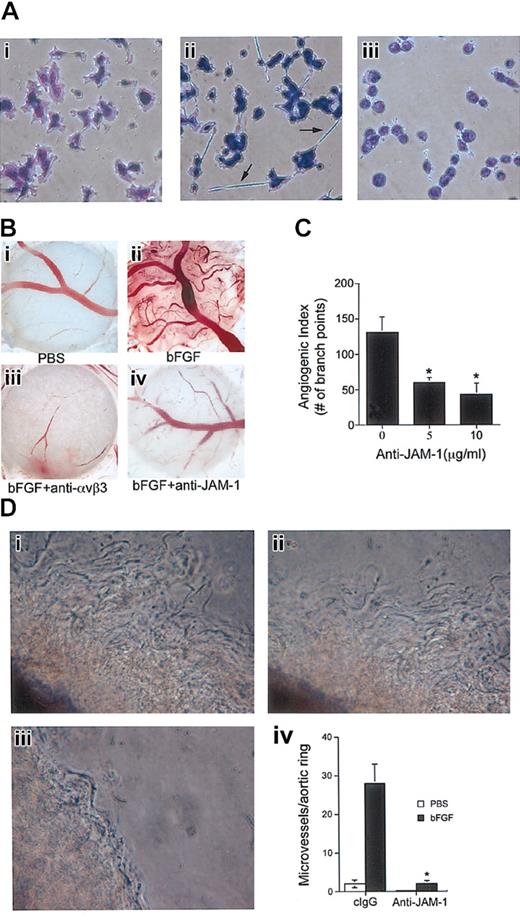
In light of our recent data that JAM-1 induces endothelial cell migration through αvβ3, we sought to examine bFGF-induced angiogenic signaling with respect to JAM-1. To do so, we first studied the effect of bFGF on HUVEC morphology. Untreated HUVECs plated on a vitronectin matrix adhered normally (Figure 1Ai). However, when stimulated with bFGF, distinct morphologic changes resulted, such as the formation of long cellular extensions that connected one cell to another (Figure 1Aii). These extensions are similar to those observed when JAM-1 is overexpressed in HUVECs plated on vitronectin (Naik et al, manuscript submitted). Therefore, we investigated whether this change in morphology induced by bFGF is dependent on JAM-1, which is endogenously expressed in these cells. Interestingly, pretreatment of HUVECs with anti–JAM-1 completely blocked the bFGF-induced morphology (Figure 1Aiii), preliminarily associating JAM-1 in the regulation of the bFGF-induced response.
JAM-1 is involved in bFGF-induced angiogenesis. (A) Serum-starved untransfected HUVECs were plated on vitronectin and stained to observe cellular morphology. bFGF-treated cells (ii) formed cellular extensions (arrows) that were blocked by anti–JAM-1 antibody (iii). (B) Low-power light micrograph of representative CAMs. (i-ii) CAM treated with PBS (control) or stimulated with 50 ng/mL bFGF. (iii-iv) bFGF-stimulated CAM was treated topically with anti-αvβ3 (2.5 μg/mL) or anti–JAM-1 (5 μg/mL) antibodies. (C) Angiogenic index of the data obtained from anti–JAM-1-treated CAMs shows dose-dependent inhibition of bFGF-induced angiogenesis (n = 8 CAMs; *P < .05). Index values were normalized by subtracting control (PBS) values. (D) Low-power light micrograph of representative murine aortic ring assay. (i) bFGF; (ii) bFGF+clgG; (iii) bFGF+anti-JAM1. (iv) Graph represents the number of microvessel sprouts per field. Anti–JAM-1 (10 μg/mL) significantly (*P < .005) inhibits bFGF-induced microvessel outgrowth (n = 6 mice). Original magnifications × 400 (A) and × 50 (B, D).
JAM-1 is involved in bFGF-induced angiogenesis. (A) Serum-starved untransfected HUVECs were plated on vitronectin and stained to observe cellular morphology. bFGF-treated cells (ii) formed cellular extensions (arrows) that were blocked by anti–JAM-1 antibody (iii). (B) Low-power light micrograph of representative CAMs. (i-ii) CAM treated with PBS (control) or stimulated with 50 ng/mL bFGF. (iii-iv) bFGF-stimulated CAM was treated topically with anti-αvβ3 (2.5 μg/mL) or anti–JAM-1 (5 μg/mL) antibodies. (C) Angiogenic index of the data obtained from anti–JAM-1-treated CAMs shows dose-dependent inhibition of bFGF-induced angiogenesis (n = 8 CAMs; *P < .05). Index values were normalized by subtracting control (PBS) values. (D) Low-power light micrograph of representative murine aortic ring assay. (i) bFGF; (ii) bFGF+clgG; (iii) bFGF+anti-JAM1. (iv) Graph represents the number of microvessel sprouts per field. Anti–JAM-1 (10 μg/mL) significantly (*P < .005) inhibits bFGF-induced microvessel outgrowth (n = 6 mice). Original magnifications × 400 (A) and × 50 (B, D).
To more directly assess the involvement of JAM-1 in bFGF-induced signaling, we used a chick CAM assay, an established model for in vivo angiogenesis.4 Species cross-reactivity of anti–JAM-1 antibody was performed before proceeding, which demonstrated that anti–JAM-1 indeed recognizes chicken JAM-1 in CAM vessels (data not shown). PBS-treated CAMs (control) were infiltrated with a basal amount of vessels (Figure 1Bi). However, when stimulated with bFGF, an abundant network of blood vessels developed (Figure 1Bii). Pretreatment with anti-αvβ3 antibody completely inhibited bFGF-induced neovascularization (Figure 1Biii), consistent with previous reports.4,15 Interestingly, anti–JAM-1 also substantially inhibited bFGF-induced angiogenesis (Figure 1Biv). An isotype-specific control antibody had little effect (data not shown). Quantitation of the extent of angiogenesis showed that anti–JAM-1 dose dependently inhibited bFGF-induced angiogenesis to a maximum of approximately 70% (Figure 1C; *P < .05). To further support these CAM data, we performed an ex vivo aortic ring assay. Murine aortic rings (1-mm diameter) were plated into a 3-dimensional growth factor-reduced Matrigel, and vessel sprouting was induced by treatment with bFGF (Figure 1Di). We found that anti–JAM-1 (Figure 1Diii) but not cIgG (Figure 1Dii) significantly inhibited bFGF-induced vessel outgrowth (Figure 1Div; *P < .005). Taken together, our data thus far suggest that JAM-1 may be necessary for bFGF-induced angiogenesis.
JAM-1 redistributes in response to bFGF treatment
Having determined the involvement of JAM-1 in bFGF-induced angiogenesis, we sought to identify its specific role in this process. JAM-1 is known to be associated with endothelial cell tight junctions.25 Because growth factors are known to disrupt cell junctions,26,27 we studied whether bFGF treatment would affect specific junctional localization of JAM-1. Immunofluorescence studies showed that endogenous JAM-1 was predominantly localized to cell-cell junctions of unactivated HUVECs, as was visible by an intense, highly localized staining pattern along the cell-cell contact (Figure 2A). However, after bFGF treatment, JAM-1 distribution changed to a broad, zipperlike pattern and appeared to relocalize diffusely along the entire cell membrane, apparently liberated from the tight junctions (Figure 2A), possibly because of junctional reorganization. To determine whether this redistribution was specific to JAM-1, we studied the effect of bFGF treatment on another cell-cell junction molecule, VE-cadherin, which is known to redistribute in response to VEGF.28 We observed that though JAM-1 again redistributed, the distribution of VE-cadherin remained essentially unaffected by bFGF treatment (Figure 2B), suggesting that this redistribution is specific to JAM-1.
bFGF treatment specifically redistributes JAM-1. (A) Immunofluorescence images of JAM-1 localization in HUVECs labeled with anti–JAM-1 and phalloidin for F-actin in the absence or presence of bFGF. On bFGF treatment, JAM-1 redistributes along the cell membrane. (B) HUVECs labeled with anti–VE-cadherin and polyclonal anti–JAM-1 in the absence or presence of bFGF. Although JAM-1 redistributes, VE-cadherin remains localized at the cell-cell junction. Scale bars represent 10 μm.
bFGF treatment specifically redistributes JAM-1. (A) Immunofluorescence images of JAM-1 localization in HUVECs labeled with anti–JAM-1 and phalloidin for F-actin in the absence or presence of bFGF. On bFGF treatment, JAM-1 redistributes along the cell membrane. (B) HUVECs labeled with anti–VE-cadherin and polyclonal anti–JAM-1 in the absence or presence of bFGF. Although JAM-1 redistributes, VE-cadherin remains localized at the cell-cell junction. Scale bars represent 10 μm.
JAM-1 induces HUVEC proliferation and in vitro tube formation
We have recently found that the overexpression of JAM-1 induces morphologic changes in HUVECs (Naik et al, manuscript submitted). This morphology is almost identical to that observed in HUVECs after bFGF treatment, as shown here (Figure 1Aii), suggesting that JAM-1 overexpression mimics bFGF-induced morphology in HUVECs. This led us to speculate that perhaps JAM-1 overexpression also mimics other bFGF actions on cell physiology. Because endothelial cell proliferation is a key initial step of angiogenesis induced by bFGF,14 it was of interest to determine whether JAM-1 overexpression would influence HUVECs in a similar manner. Overexpression of JAM-1 indeed induced proliferation compared to mock transfected cells, as quantitated by manual counting of cell number (Figure 3A). In addition, we observed that the cell proliferation induced by bFGF was significantly inhibited by the addition of anti–JAM-1, as determined by BrdU incorporation (Figure 3B; *P < .01). Note that the inhibition of bFGF-induced DNA synthesis by anti–JAM-1 also was observed in mock-transfected cells, presumably because of antibody inhibition of endogenous JAM-1 (Figure 3B; P < .05). These results indicate that JAM-1 may regulate bFGF-induced signals that contribute to endothelial cell proliferation.
JAM-1 induces HUVEC proliferation and tube formation. (A) The proliferation of HUVECs stably transfected with mock (•) or JAM-1 (○) constructs was quantified by counting cell number. (B) Cells as in panel A were treated with bFGF and assayed for BrdU incorporation. Anti–JAM-1 significantly inhibited bFGF-induced proliferation (*P < .01). (C) In vitro Matrigel tube formation assay. Serum-starved mock-transfected (i, iii), JAM-1–transfected (ii, iv), Δ-257–transfected (v), and Y→F–transfected (vi) HUVECs were assayed for tube formation in the presence or absence of bFGF as indicated. Original magnification × 100.
JAM-1 induces HUVEC proliferation and tube formation. (A) The proliferation of HUVECs stably transfected with mock (•) or JAM-1 (○) constructs was quantified by counting cell number. (B) Cells as in panel A were treated with bFGF and assayed for BrdU incorporation. Anti–JAM-1 significantly inhibited bFGF-induced proliferation (*P < .01). (C) In vitro Matrigel tube formation assay. Serum-starved mock-transfected (i, iii), JAM-1–transfected (ii, iv), Δ-257–transfected (v), and Y→F–transfected (vi) HUVECs were assayed for tube formation in the presence or absence of bFGF as indicated. Original magnification × 100.
Among the key steps required for neovascularization are cell proliferation, migration, and tube formation. In addition to its induction of proliferation illustrated above, we have recently found that JAM-1 overexpression also induces HUVEC migration specifically on vitronectin (Naik et al, manuscript submitted). Thus, we next sought to determine whether JAM-1 also induces tube formation in a 3-dimensional Matrigel.23 When embedded in the absence of bFGF, mock-transfected cells failed to form tubelike structures and instead formed evenly distributed small colonies (Figure 3Ci), whereas JAM-1–overexpressing cells formed distinct tubelike structures (Figure 3Cii). In the presence of bFGF, both mock- and JAM-1–transfected cells formed networks of tubes (Figure 3Ciii-iv); however, those formed by JAM-1–transfected cells were denser and more ordered. JAM-1 overexpression in NIH3T3 cells did not form tubelike structures in a similar context (data not shown), suggesting that JAM-1–induced tube formation is endothelial cell–specific. The cytoplasmic domain of JAM-1 is known to be tyrosyl-phosphorylated, and this phosphorylation is required for the activation of MAP kinase leading to JAM-1–induced migration on vitronectin (Naik et al, manuscript submitted). Interestingly, when overexpressed in HUVECs, a cytoplasmic domain deletion mutant (Δ-257) and a cytoplasmic substitution mutant of tyrosine 280 to phenylalanine (Y→ F) mutant of JAM-1 both failed to form tubelike structures, even in the presence of bFGF (Figure 3Cv, vi). These results suggest that perhaps signaling through JAM-1 is required for this event; we infer that bFGF-induced angiogenesis requires signaling through JAM-1.
bFGF dissociates the complex formed by αvβ3 and JAM-1
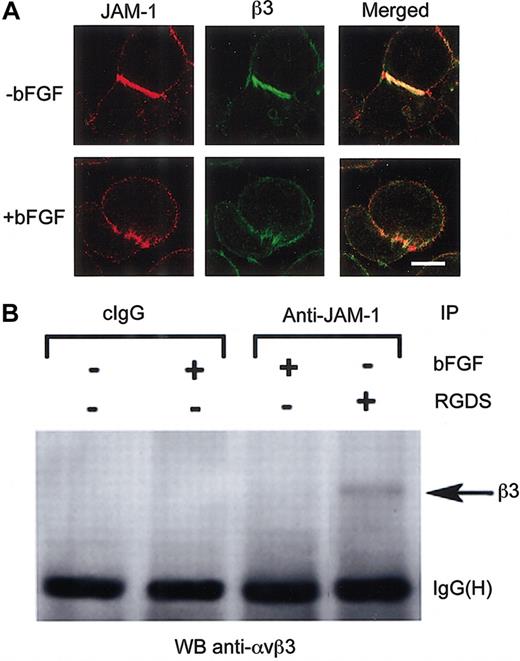
We have recently found that JAM-1–induced cell migration on vitronectin is αvβ3-dependent and that JAM-1 and αvβ3 interact, more abundantly in fact on integrin engagement of the ligand-binding site by low-molecular–weight ligand mimetic antagonists (Naik et al, manuscript submitted). Because JAM-1 appears to be functionally and physically linked to αvβ3 during cell migration, we asked whether these proteins physically associate with each other during bFGF-induced signaling events. To determine this, we first examined the distribution pattern of integrin αvβ3 with respect to JAM-1. In unactivated HUVECs, most integrin αvβ3 localized at the cell-cell junctions, coincident with JAM-1 localization (Figure 4A). However, when these cells were pretreated with bFGF, αvβ3 and JAM-1 appeared no longer to localize at the junctions, but they redistributed to localize more diffusely along the cell membrane (Figure 4A).
The integrin αvβ3/JAM-1 complex is disrupted on stimulation of HUVECs with bFGF. (A) Immunofluorescence images of serum-starved, untransfected HUVECs in the absence or presence of bFGF stained for JAM-1 and integrin β3. Scale bar represents 10 μm. (B) Coimmunoprecipitation of αvβ3 with JAM-1 from HUVECs treated with bFGF or RGDS peptide where indicated. HB67 (cIgG) was used as a control. IgG heavy chain—IgG(H)—bands indicate equal loading.
The integrin αvβ3/JAM-1 complex is disrupted on stimulation of HUVECs with bFGF. (A) Immunofluorescence images of serum-starved, untransfected HUVECs in the absence or presence of bFGF stained for JAM-1 and integrin β3. Scale bar represents 10 μm. (B) Coimmunoprecipitation of αvβ3 with JAM-1 from HUVECs treated with bFGF or RGDS peptide where indicated. HB67 (cIgG) was used as a control. IgG heavy chain—IgG(H)—bands indicate equal loading.
Because it appeared that these proteins no longer colocalized on bFGF-induced activation, we next investigated the effect of bFGF treatment on the interaction of these 2 proteins. To do so, we performed an experiment in which JAM-1 was immunoprecipitated using anti–JAM-1 and was analyzed by Western blot with anti-β3 antibody. We found that when the ligand-binding site of αvβ3 was engaged by preincubation with RGDS peptide, αvβ3 did coimmunoprecipitate with JAM-1 (Figure 4B). Interestingly, however, αvβ3 did not coimmunoprecipitate with JAM-1 from bFGF-activated HUVECs (Figure 4B), suggesting the dissociation of αvβ3 and JAM-1 on initiation of bFGF-induced signaling. An isotype-specific IgG antibody was used as a control (cIgG; Figure 4B). These data indicate that in quiescent endothelial cells, αvβ3 and JAM-1 associate with each other, and this association is disrupted on treatment with bFGF.
JAM-1 regulates bFGF/αvβ3-induced MAP kinase activation
It is reported that bFGF-induced angiogenic signaling to MAP kinase requires integrin αvβ3.14,29 Because we found that αvβ3 interacts with JAM-1, we reasoned that JAM-1 may regulate bFGF-induced signaling to MAP kinase. We have found that JAM-1 overexpression up-regulates MAP kinase activity in HUVECs plated on vitronectin (Naik et al, manuscript submitted). Thus, we investigated the effect of JAM-1 overexpression on bFGF-induced MAP kinase activation. As expected, basal levels of ERK1/2 phosphorylation in mock-transfected HUVECs were elevated by bFGF treatment (Figure 5A). Overexpression of JAM-1 resulted in an enhancement of basal and bFGF-induced ERK1/2 activity (Figure 5A). These data indicate that the bFGF-induced ERK1/2 activity may be attributed to integrin αvβ3 and JAM-1. We then sought to confirm that this bFGF-induced activation of MAP kinase required signaling through JAM-1. Interestingly, overexpression of the Δ-257 or the Y→F JAM-1 mutants not only failed to induce enhanced MAP kinase activation, their overexpression substantially inhibited bFGF-enhanced MAP kinase phosphorylation (Figure 5A), evidence that JAM-1 may be an essential component of the bFGF-induced signaling pathway to MAP kinase. To define the individual roles of integrin αvβ3 and JAM-1 in bFGF-induced MAP kinase activation, we used function-blocking antibodies for αvβ3 and JAM-1. When HUVECs were pretreated with anti–JAM-1 to block bFGF-induced signaling through JAM-1, MAP kinase activity was inhibited (Figure 5B); densitometric analysis revealed this inhibition was approximately 65% (Figure 5C; *P < .05). Additionally, when HUVECs were pretreated with anti-αvβ3, bFGF-induced activation of MAP kinase was also blocked to a similar extent (Figure 5B-C; *P < .05). An unrelated, isotype-specific antibody, anti-CIB, had no effect (Figure 5B-C). These results suggest that bFGF-induced MAP kinase activation is dependent on JAM-1 and integrin αvβ3. Blocking integrin αvβ3 or JAM-1 would, therefore, also block this signaling.
bFGF-induced MAP kinase activation requires JAM-1 and αvβ3. (A) HUVECs expressing various JAM-1 constructs were stimulated with bFGF, as indicated, and were processed to observe the amount of phospho-ERK1/2 and total ERK1/2. (B) Serum-starved JAM-1–overexpressing HUVECs were preincubated with PBS (control) or indicated antibodies, stimulated with bFGF, and then processed as in panel A. (C) Quantitation of the amount of phospho-ERK1/2 from panel B by densitometry. Anti-αvβ3 and anti–JAM-1 significantly inhibit ERK1/2 activity (*P < .05).
bFGF-induced MAP kinase activation requires JAM-1 and αvβ3. (A) HUVECs expressing various JAM-1 constructs were stimulated with bFGF, as indicated, and were processed to observe the amount of phospho-ERK1/2 and total ERK1/2. (B) Serum-starved JAM-1–overexpressing HUVECs were preincubated with PBS (control) or indicated antibodies, stimulated with bFGF, and then processed as in panel A. (C) Quantitation of the amount of phospho-ERK1/2 from panel B by densitometry. Anti-αvβ3 and anti–JAM-1 significantly inhibit ERK1/2 activity (*P < .05).
Discussion
Collectively, the data presented here show for the first time the involvement of JAM-1 in the process of bFGF-induced angiogenesis. We also find that overexpression of JAM-1 mimics bFGF-induced phenotypes in the absence of bFGF stimulation. However, these data do not imply that JAM-1 is capable of angiogenesis induction independent of bFGF. In JAM-1–overexpressed cells, endogenous JAM-1 remains sequestered at the cell-cell junction complexed with αvβ3, but additional (uncomplexed) JAM-1 is now expressed on the cell surface, free to initiate angiogenic signaling. Conversely, in vivo, where JAM-1 expression is physiological, most of JAM-1 is confined to the cell-cell junction and must be redistributed to induce angiogenic signaling. Our data suggest that this redistribution is caused by bFGF. Interestingly, TNF-α is also known to redistribute JAM-1.30-32 Because TNF-α and bFGF induce a common angiogenic signaling pathway that involves αvβ3,12 we infer that JAM-1 may also be involved in TNF-α–induced angiogenesis.
JAM-1 is the founding member of the JAM family of proteins, which includes JAM-2 and JAM-3.33 It is thus possible that JAM-2 and JAM-3 would function similarly on bFGF stimulation. Although these members are structurally similar, they have only approximately 30% to 35% similarity at the amino acid level.33 The fact that we used a JAM-1–specific antibody in our studies that does not cross-react with other family members but does in fact specifically and substantially inhibit bFGF-induced angiogenesis indicates that these family members do not function similarly in such an environment. Further, it has been previously reported that these family members are differentially localized in the vasculature,33 indicative of their distinct functional significance in vascular cells.
Much information has been published implicating the requirement of integrins αvβ3 and αvβ5 for growth-factor–induced angiogenesis by demonstrating that their inhibition blocks this process.12,17,34-36 It has been proposed that αvβ3 antagonists interfere with ligand-binding functions of this integrin,17 thereby leading to reduced cell proliferation and migration, key steps of the initiation of blood vessel formation. For this reason, potential antiangiogenic therapies have been targeted toward the inhibition of αvβ3,35,37 attesting to the general belief that αvβ3 is key to this process. Our data support these findings because the use of anti-αvβ3 inhibited bFGF-induced MAP kinase activation (Figure 5B) and angiogenesis in the CAM assay (Figure 1B). As mentioned above, our data suggest that on bFGF activation of endothelial cells, the αvβ3/JAM-1 complex dissociates, and individual components redistribute, allowing angiogenic signals to traverse these receptors and initiate angiogenesis. Antagonists, either to αvβ3 as previously reported or to JAM-1, may block dissociation of the individual components and perhaps transmission of angiogenic signals. This explanation is supported by our findings that MAP kinase activation, and a number of angiogenic steps thereafter needed for blood vessel formation, is blocked in the presence of anti-αvβ3 or anti–JAM-1 antibodies. It is also possible that unactivated bFGF receptor, αvβ3, and JAM-1 form a complex that is disrupted on bFGF receptor activation through bFGF binding. Further experimentation to identify the components of this complex would be prudent for a more conclusive evaluation of these possibilities.
It has been shown that quiescent endothelial cells that line blood vessels express minimal amounts of αvβ3, whereas angiogenic vessels show increased expression.4 The role of αvβ3 in angiogenesis is largely attributed to its induced expression4 ; however, gene ablation studies suggest otherwise. It has now been shown that, in contrast to the inhibition data discussed above, mice genetically deficient in αv, β3, or β5 integrins exhibit normal developmental angiogenesis.38-40 Even more intriguing are the data demonstrating that mice lacking β3 and β5 integrins show evidence of enhanced pathologic angiogenesis.22 Taken together, these findings challenge the previously accepted role of αvβ3 in the angiogenic process. The possibility that genetic elimination of these integrins may result in the initiation of a compensatory response by other integrins, thereby restoring angiogenic activity in their absence, has been ruled out.22 Our data demonstrate that αvβ3 antagonists inhibit a number of steps leading up to and including neovascularization. These data, along with a number of previously published data concerning inhibition studies in vitro and in vivo, provide ample evidence of the key role of αvβ3 in the regulation of this process. Further experimentation concerning genetic ablation of JAM-1 is ongoing to ascertain more definitively the precise roles of JAM-1 and αvβ3 in this process.
It has also been proposed that αvβ3 may act as a biosensor, deciding between cell survival and death as a function of extracellular matrix (ECM) composition.41,42 It is thus believed that bFGF-induced up-regulation of αvβ3 expression in the absence of its ligand or in the presence of antagonist may promote apoptosis even in the presence of growth-permissive conditions.41,42 In genetic ablation, the absence of αvβ3 may uncouple the apoptotic signaling pathway, thereby allowing for observed enhanced angiogenesis. Although our results support JAM-1 as a novel component of the pathway of bFGF-induced angiogenesis and re-emphasize the role of integrin αvβ3 in the angiogenic process, we cannot rule out the possibility of the biosensor model. Further study of the role of JAM-1 in angiogenic and apoptotic signaling pathways will help to resolve this issue.
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2003-04-1114.
Supported in part by the American Heart Association Pennsylvania and Delaware Affiliate grant 9906203U (U.P.N.) and National Institutes of Health grants HL63960 (U.P.N.), HL54229 (C.A.P.), and DK61379 (C.A.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank K. Eckfeld for her tremendous help in preparing the manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal