Abstract
The G-protein–coupled receptors of the endothelial differentiation gene (EDG) family mediate pro-angiogenic activities, such as endothelial cell proliferation, chemotaxis, and vessel morphogenesis. We synthesized and tested the effects of a 9-amino acid peptide (KRX-725), derived from the second intracellular loop of S1P3 (EDG3). KRX-725 mimics the effects of sphingosine 1-phosphate (S1P), the natural ligand of S1P3, by triggering a Gi-dependent MEK-ERK (mitogen-activated protein kinase kinase and extracellular signal-regulated kinase) signal transduction pathway. Using aortic rings as an ex vivo model of angiogenesis, vascular sprouting was assessed in the presence of KRX-725 or S1P. KRX-725 induced extensive and dense vascular sprouts, which contain an elaborated organization of endothelial and smooth muscle layers, including lumen formation. When KRX-725 or S1P was combined with proangiogenic factors, such as basic fibroblast growth factor (bFGF), stem cell factor, or vascular endothelial growth factor, the effect was synergistic, leading to further enhancement of vascular sprouting. KRX-725 also initiated neovascularization in a mouse corneal pocket assay in vivo and showed synergism with bFGF. The specificity of KRX-725 was demonstrated via peptide-induced receptor internalization of S1P3 but not S1P1. The ability of a short peptide to stimulate extensive angiogenesis and to synergize with pro-angiogenic factors suggests that KRX-725 may serve as a useful agent in treating pathologic conditions such as peripheral vascular disease, cardiac ischemia, or tissue grafts.
Introduction
Angiogenesis, the process by which sufficient blood supply is ensured to normal tissues through sprouting of new blood vessels, is one of the most fundamental events in tissue remodeling. This process involves intercellular cooperation between the endothelial inner layer and surrounding smooth muscle cells and pericytes. Many soluble factors are known to induce angiogenesis under various physiologic and pathologic conditions, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and angiopoietins.1 Among them, sphingosine 1-phosphate (S1P) has been shown to be a major pro-angiogenic factor in the serum.2,3 S1P is released from platelets on their activation and contributes to wound healing processes. S1P belongs to a family of lysophospholipids, which regulates many important cellular functions, such as proliferation, differentiation, survival, chemotaxis, and cytoskeletal rearrangement.4-6 S1P acts both intracellularly, as a second messenger, and extracellularly, as a ligand for a family of G-protein coupled receptors (GPCRs), the endothelial differentiation gene (EDG).7 To date, 5 receptors for S1P have been identified in mammals: S1P1 (EDG1), S1P3 (EDG3), S1P2 (EDG5), S1P4 (EDG6), and S1P5 (EDG8).8
S1P3 is expressed in many cell types, including endothelial and vascular smooth muscle cells.4,9,10 The importance of S1P3 in vasculogenesis or angiogenesis had not been fully elucidated. In contrast to the knock-out model of S1P1 in mice and zebrafish, which causes embryonic lethality accompanied by various vascular abnormalities,11,12 disruption of S1P3 in mice does not result in gross phenotypic changes.13 However, antisense mRNA to S1P3 abolishes important angiogenic functions such as adherens junction formation in endothelial cells and in vivo neovascularization in a matrigel implant assay.4
In the present study we took a rational drug-design approach to modulate S1P3 activity in a specific manner and thereby highlight its biologic role. To that end we took advantage of the knowledge about the fine structure of GPCRs. The second intracellular (i2) loop of GPCRs is highly conserved both in terms of its size and shared structural motifs. The i2 loop of various GPCRs has been implicated in the mediation of cellular interactions via G proteins, arrestins, dynamin, and GPCR kinases.14-18 Site-directed mutagenesis of selected residues of the i2 loop has been shown to directly impair G-protein coupling.15,19-21 Furthermore, peptides derived from this region have been used to modulate GPCR signaling.17,18,22,23 In the present study, we report on the biologic effects of a synthetic myristoylated 9 amino-acid peptide (KRX-725) derived from the C-terminal end of the i2 loop of S1P3. We have found the effects of KRX-725 in various experimental models of angiogenesis to be similar to that of the natural ligand of S1P3. Gi-dependent extracellular signal-regulated kinase (ERK) activation, ex vivo sprouting induction, neovascularization in vivo, and S1P3 internalization by KRX-725 were shown. Moreover, a synergistic effect was obtained when KRX-725 or S1P was combined with the major angiogenic factors, including VEGF, basic fibroblast growth factor (bFGF), and stem cell factor (SCF).
Materials and methods
Reagents
S1P, mouse recombinant VEGF, recombinant basic FGF, heparin, sucrose octasulfate aluminum complex (Sucralfate), poly (2-hydroxyethyl) methacrylate (Hydron), dimethylsulfoxide (DMSO), bovine serum albumin (BSA), and paraformaldehyde were obtained from Sigma-Aldrich, Jerusalem, Israel; mouse recombinant SCF, U0126, avidin-FITC (fluorescein isothiocyanate), and pertussis toxin (PTX) were obtained from Calbiochem, La Jolla, CA; crystal violet was obtained from Merck, Darmstadt, Germany; fetal calf serum (FCS) and charcoal-stripped FCS (c-FCS), BIO-MPM-1 (multipurpose serum-free medium for adherent cells), 10 × minimal essential Eagle medium, penicillin-streptomycin, phosphate-buffered saline (PBS), and trypsin were obtained from Biological Industries, Kibbutz Beit-Haemeq, Israel; Ham F12K was obtained from GibcoBRL, United Kingdom; endothelial mitogen (BT-203) was obtained from Biomedical Technologies, Stoughton, MA. S1P was dissolved in methanol and diluted in PBS containing 0.1% BSA immediately prior to use.
Peptides
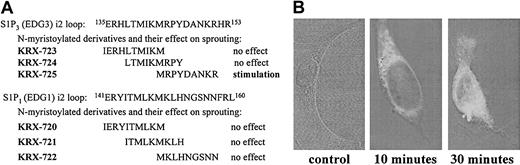
Peptides used in this study were synthesized by Fmoc solid-phase synthesis, either by Novetide (Haifa Bay, Israel) or Genemed Synthesis (San Francisco, CA), and purified by reverse-phase–high-performance liquid chromatography (RP-HPLC). Six peptides derived from the i2 loop of S1P3 and S1P1 (Figure 1) were synthesized. Myristoyl-glycine was attached to the N-terminus of the peptides, and their C-terminus was amidated. Identity and purity were confirmed by HPLC–mass spectroscopy. The purity of the peptides was more than 85%, whereas KRX-725 was purified to more than 95%. Stock solutions of peptides (20 mM) were prepared in DMSO and diluted to 800 μM concentration in PBS containing 0.1% BSA.
Screening of synthetic peptides derived from the second intracellular (i2) loops of S1P1 and S1P3. (A) Sequences of i2 loop–derived peptides and their activities in aortic ring assay. (B) Penetration into the cell. HUVECs were incubated with biotin-tagged KRX-725 for 10 and 30 minutes. Nonbiotinilated KRX-725 was used as control. Fixed and permeabilized cells were reacted with FITC-conjugated extravidin. Visualization was done using confocal microscope (axiovert M135; Zeiss). Micrographs were taken under × 63 objective.
Screening of synthetic peptides derived from the second intracellular (i2) loops of S1P1 and S1P3. (A) Sequences of i2 loop–derived peptides and their activities in aortic ring assay. (B) Penetration into the cell. HUVECs were incubated with biotin-tagged KRX-725 for 10 and 30 minutes. Nonbiotinilated KRX-725 was used as control. Fixed and permeabilized cells were reacted with FITC-conjugated extravidin. Visualization was done using confocal microscope (axiovert M135; Zeiss). Micrographs were taken under × 63 objective.
Cell cultures
Human umbilical vein endothelial cells (HUVECs; CRL-1730) and human aortic vascular smooth muscle cells (HA-VSMC; CRL-1999) were obtained from the American Type Culture Collection (ATCC; Manassas, VA). Cells were grown in Ham F12K containing endothelial mitogen (30 μg/mL), heparin (5 U/mL), 10% FCS, penicillin (100 U/mL), and streptomycin (0.1 mg/mL) at 37°C in a humidified 5% CO2 atmosphere.
Inhibition of S1P1 and S1P3 expression by small interfering RNA (siRNA)
Human S1P1- and S1P3-specific 21-nucleotide siRNA (Xeragon, German-town, MD) (AGCUGAAUAUCAGCGCGGAdTdT and CUGCCUGCACAAUCUCCCUdTdT, respectively) were targeted 99 and 529 nucleotides downstream of the start codon. HUVECs were transfected with the duplexes using OligofectAMINE (Invitrogen, Karlsruhe, Germany) according to the manufacturer's recommendations. Forty-eight hours after transfections cells were starved for further experiments.
Western blotting
HUVECs were starved in serum-free and BT-203–free medium for 12 hours and incubated with PTX (500 ng/mL) 3 hours prior to peptide, VEGF, or S1P addition. Peptides at final concentration of 20 μM and vehicle (0.1% DMSO) were added for 3 hours. VEGF (10 ng/mL) and S1P (1 μM) were added for the last 10 minutes. HA-VSMCs were incubated in the same conditions with peptides and S1P. Cells were washed with cold PBS and lysed on ice in 400 μL lysis buffer containing 20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl pH 7.4, 10% glycerol, 1 mM EDTA (ethylenediaminetetraacetic acid), 1 mM EGTA (ethyleneglycoltetraacetic acid), 0.5% Triton X-100, 0.5 mM sodium orthovanadate, 10 mM β-glycerophosphate, 5 mM sodium pyrophosphate, 50 mM sodium fluoride, and protease inhibitor cocktail (Aldrich-Sigma, St Louis, MO). The lysates were centrifuged (14 000g, 20 minutes), and the resulting supernatants subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to nitrocellulose membranes. The membranes were immunoblotted with either monoclonal antidiphosphorylated p42/44 mitogen-activated protein kinase (MAPK; clone MAPK-YT; Aldrich-Sigma) to detect ERK1 and ERK2 phosphorylation, rabbit polyclonal anti-ERK2 antibody (sc-154; Santa Cruz, Santa Cruz, CA) to detect total ERK protein, monoclonal anti–EDG3-NT (Exalpha Biologicals, Boston, MA) to evaluate S1P3 level and anti-beta actin (Aldrich-Sigma). The blots were then incubated with peroxidase-conjugated goat antirabbit antibodies (Jackson ImmunoResearch, West Grove, PA) or horseradish peroxidase–coupled donkey antimouse immunoglobulin A (IgA; Southern Biotechnology, Birmingham, AL). Immunoreactive proteins were visualized using an enhanced chemiluminescence (ECL) detection system (Pierce Chemical, Rockford, IL).
Analysis of intracellular penetration of KRX-725
To enable visualization, we used a C-terminus biotin-tagged peptide, Myristyl-G-MRPYDANKRK@-NH2 (K@ = Lys-ϵ amino-biotin). HUVECs were grown in chamber slides, 30 000 cells/slide, and incubated for 10, 30, and 60 minutes with 10 μM biotin-conjugated peptide or nontagged control peptide (KRX-725). Slides were fixed with 4% formaldehyde and permeabilized by 0.2% Triton X-100, and Avidin-FITC diluted 1:1000 in 0.5% BSA/PBS was added for 30 minutes, followed by washing with 0.5% BSA/PBS. Slides were viewed using fluorescent confocal microscopy (axiovert M135; Zeiss, Jena, Germany).
Immunofluorescence receptor localization assay
HUVECs were grown with 2% c-FCS for 24 hours and then incubated with 20 μM KRX-725 or 1 μM S1P for 45 minutes. Cells were fixed with 4% paraformaldehyde, blocked in 1% BSA in PBS (blocking solution), and permeabilized with 0.2% Triton X-100 and 1% BSA in PBS. Incubation with anti–EDG3-NT and EDG1 antibodies (Exalpha) 1:100 in blocking solution was followed by incubation with 1:100 of FITC-conjugated donkey antimouse and 1:400 of Cy3-conjugated goat antirabbit, respectively (Jackson ImmunoResearch). Cells were viewed using Axiovert M135 confocal microscope (Zeiss).
Rat-tail collagen extraction
Rat-tail collagen was prepared as previously described.24 Briefly, tendons were removed from rat tails, washed twice with PBS, and placed in Petri dishes containing 70% ethanol for sterilization and dehydration. Collagen was dissolved by suspending pieces of tendons in 0.05% acetic acid in double distilled water (DDW), and stirred slowly for 48 hours at 4°C. Undissolved pieces of tendons were removed by centrifugation at 16 000g to 20 000g for 30 minutes at 4°C. The resulting supernatants, which contained soluble collagen, were adjusted to a collagen concentration of 1 mg/mL by optical density at 280 nm wavelength and stored at –20°C until used.
Aortic ring assay
All animal procedures were approved by the animal care and use committee of the Hebrew University. As previously reported,25 thoracic aortas were dissected from 8- to 10-week-old male C57BL/6 and BALB/c mice, FVB/N-TgN(TIE2GFP)287Sato mice26 (Jackson Laboratories, Bar Harbor, ME) or Sprague-Dawley rats (250 g male). The aortas were immediately transferred to Petri dishes containing BIO-MPM-1. The adventitia and small vessels around the aorta were carefully removed under a dissecting microscope, and transverse cuts of 0.5 mm were made. The resulting aortic rings were extensively rinsed and incubated overnight in BIO-MPM-1 containing penicillin-streptomycin. Subsequently, the rings were embedded in 600 to 800 μL collagen mix (7 parts collagen, 1 part 10 × Eagle minimal essential medium [MEM], and 2 parts 0.15 M sodium bicarbonate) in 24-well plates (Nunc, Rochester, NY). Solidification of the collagen solution was achieved by raising the pH to neutral and the temperature to 37°C. In each well, 3 murine or 1 rat aortic rings were embedded. Medium (400 μL BIO-MPM-1 containing penicillin-streptomycin and the tested factors or peptides) was added to the embedded rings, and the plates were incubated at 37°C in a humidified 10% CO2 atmosphere. Medium containing factors and/or peptides was replaced 3 times a week. After 7 to 14 days, the rings were fixed with 4% formaldehyde for 24 hours, followed by staining with crystal violet (0.02%) dissolved in ethanol and extensive washing to remove excessive stain. The effect of factors and peptides was examined in 3 wells (9 rings) for mouse and 4 wells (4 rings) for rat aortic rings per assay. Micrographs of representative rings were taken using a digital camera (Nikon, Tokyo, Japan). Morphometric analysis of sprouting was performed manually using Image-Pro 4.5 software (Media Cybernetics, Silver Spring, MD) according to Nissanov et al.27 Nonstained rings were subjected to paraffin sections and hematoxylin and eosin (H&E) staining.
Whole-mount immune staining of aortic rings
Rings embedded and grown in collagen (as described in “Aortic ring assay”) were fixed with 1% paraformaldehyde in PBS for 30 minutes at room temperature and left overnight in PBS. Blocking and permeabilization of the fixed rings was achieved by incubation with PBS containing 1% BSA and 0.01% Triton-X 100 for 8 hours at 4°C. The rings were incubated with FITC-conjugated anti alpha smooth muscle actin antibody (ab8211, diluted 1:200; Abcam, Cambridge, England) in PBS containing 1% BSA for 8 hours. After removal of excess unbound antibody by several washes with PBS during the next 8 to 10 hours, the samples were visualized using a fluorescent microscope (Axiovert 200M; Zeiss).
Corneal pocket assay
Angiogenic responses to KRX-725 and bFGF were examined as previously described.28 Briefly, pellets were prepared by dissolving sucralfate (500 mg/mL) in 20 mM sodium citrate, 1 mM EDTA, and 9% sucrose solution, and 20 μL of this solution was added to 5 mg KRX-725 or 40 μg bFGF. Single pellets contained approximately 1:450 of these amounts. Following addition of 20 μL Hydron polymer in ethanol (120 mg/mL), the mixtures were applied to a 15 × 15-mm2 piece of synthetic mesh (Sefar America, Kansas City, MO) and coated by Hydron. The pellets were allowed to air dry, and fibers of the mesh were pulled apart. Corneal micropockets were created in both eyes of anesthetized C57BL/6 mice (8-10 weeks old). Incisions were made after local administration of lidocaine, using a Von-Graefe cataract knife. Pellets (0.2 × 0.2 × 0.3 mm) were impregnated into the corneal micropockets at a distance of 1 to 2 mm from the vascularized limbus. In case of peptide and bFGF combinations, 2 pellets were inserted to the same incision. Polymyxin-B-neomycin-bacitracin (Bamyxin) ophthalmic ointment was applied to the eyes to prevent infection. Responses were recorded and photographed after 8 days using stereoscopy. Vessel development was monitored twice weekly until full vessel regression occurred. Each peptide and factor tested in this assay was examined in 4 animals (4-6 eyes).
Statistical analysis
Significance of differences between groups was evaluated using Wilcoxon test. P < .05 was considered significant.
Results
Pro-angiogenic activity of a peptide derived from the second intracellular loop of S1P3
Angiogenesis is a complex intercellular process that involves proliferation, migration, and organization of endothelial cells, smooth muscle cells, and pericytes. Therefore, it is preferable to use assay systems in which the role of specific factors is determined in the context of a whole tissue as exemplified by the ex vivo aortic ring model. In this model, pieces of thoracic aorta are embedded in a 3-dimensional collagen matrix and exposed to agents tested for their ability to enhance sprouting of vessellike structures. Two sets of 3 overlapping peptides covering the entire sequence of the i2 loop of S1P1 and S1P3 were synthesized, to test the ability of designer peptides to modulate S1P signaling in a specific manner (Figure 1A). The C-terminus of the peptides was amidated, and myristoyl-Gly moiety was attached at their N-terminus to facilitate intracellular entry and enhance stability. Although there is a high degree of similarity between S1P1 and S1P3 at the N-terminal half of their i2 loop, there are significant differences in the sequence at the C-terminal half. Of the 6 peptides tested, only KRX-725 induced significant sprouting of vessellike structures in the aortic ring assay, as described in detail in Figure 4. The corresponding sequence derived from S1P1 (KRX-722) had no activity (data not shown). Because of its pro-angiogenic activity, KRX-725 was selected for further studies. Intracellular permeabilization of KRX-725 was illustrated using a biotin-tagged peptide. In Figure 1B we demonstrate that 10 minutes of incubation are sufficient for cell permeabilization of the peptide.
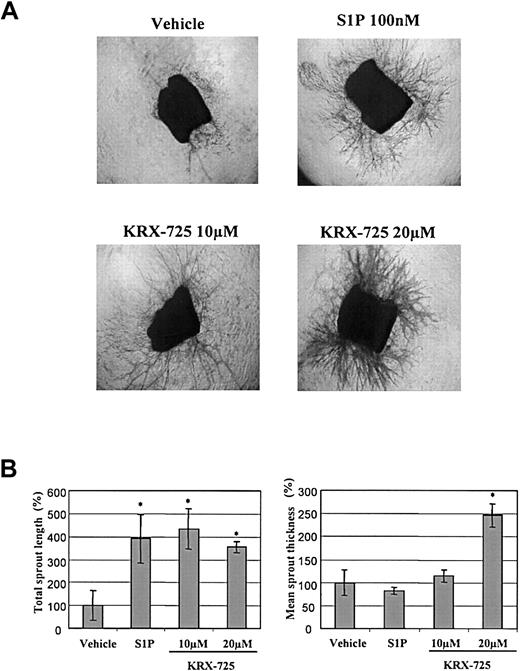
The effect of KRX-725 and S1P on aortic ring sprouting. (A) Aortic rings from C57BL/6 mice embedded in collagen matrix were exposed to KRX-725 (10 and 20 μM), S1P (100 nM), and vehicle (0.1% DMSO) for 9 days. The rings were then fixed and stained with crystal violet (0.02%) to illustrate sprouting. Representative micrographs of each arm of the experiment are shown. Micrographs were taken under × 4 objective. (B) Statistical morphometric analysis of the sprout length and thickness of 4 repeats from panel A. Mean ± SEM is presented relative to the control rings (100%), treated with vehicle. * indicates statistical significance (P < .05, Wilcoxon test) of treatment versus control.
The effect of KRX-725 and S1P on aortic ring sprouting. (A) Aortic rings from C57BL/6 mice embedded in collagen matrix were exposed to KRX-725 (10 and 20 μM), S1P (100 nM), and vehicle (0.1% DMSO) for 9 days. The rings were then fixed and stained with crystal violet (0.02%) to illustrate sprouting. Representative micrographs of each arm of the experiment are shown. Micrographs were taken under × 4 objective. (B) Statistical morphometric analysis of the sprout length and thickness of 4 repeats from panel A. Mean ± SEM is presented relative to the control rings (100%), treated with vehicle. * indicates statistical significance (P < .05, Wilcoxon test) of treatment versus control.
KRX-725 induces Gi-dependent ERK activation similar to S1P
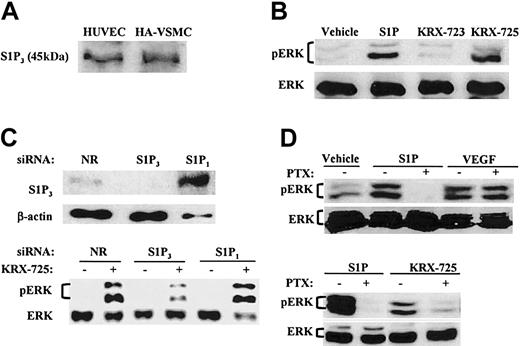
Because S1P is a ligand for the GPCR subfamily to which S1P3 belongs, we examined whether the mechanism by which KRX-725 exerts its effect is similar to that of S1P. ERK is phosphorylated following stimulation of endothelial cells with S1P.5,29 In transfected cells, S1P3 was shown to activate Gi,30 and S1P3-dependent ERK activation was found to be PTX sensitive, implicating Gi coupling.29 Expression of S1P receptors in HUVECs and in aortic smooth muscle cells was previously shown.9,10,31 The expression of S1P3 was confirmed by immunodetection of the protein in HUVECs and HA-VSMCs (Figure 2A).
KRX-725 and S1P induce Gi-dependent ERK phosphorylation in human endothelial and vascular smooth muscle cells. (A) Expression of S1P3 in HA-VSMCs and HUVECs. Lysates of cells were subjected to Western blotting with anti-EDG3 NT antibody. (B) Levels of phosphorylated ERK1/2 (pERK) and ERK2 (ERK) in HA-VSMCs exposed to either S1P (1 μM, 10 minutes), the control peptide KRX-723 (20 μM, 3 hours), KRX-725 (20 μM, 3 hours), or vehicle (0.1% DMSO, 3 hours). (C) Effect of siRNAs directed against the sequence of S1P1 or S1P3 on ERK phosphorylation induced by KRX-725. HUVECs were transfected with S1P1, S1P3, and nonrelevant (NR) 21-nucleotide siRNA. ERK phosphorylation was detected following overnight starvation and KRX-725 stimulation (20 μM, 1 hour). S1P3 protein level was assessed in the same assay conditions. (D) Phospho-ERK1/2 and ERK1/2 levels in HUVECs exposed to vehicle (0.1% DMSO, 3 hours), S1P (1 μM, 10 minutes), VEGF (10 ng/mL, 10 minutes), or KRX-725 (20 μM, 3 hours). Samples were preincubated with PTX (500 ng/mL) 3 hours prior to addition of the stimulant. Cell lysates were subjected to Western blotting with antiphospho-ERK (pERK) and reprobed with anti-ERK2 (ERK) antibodies.
KRX-725 and S1P induce Gi-dependent ERK phosphorylation in human endothelial and vascular smooth muscle cells. (A) Expression of S1P3 in HA-VSMCs and HUVECs. Lysates of cells were subjected to Western blotting with anti-EDG3 NT antibody. (B) Levels of phosphorylated ERK1/2 (pERK) and ERK2 (ERK) in HA-VSMCs exposed to either S1P (1 μM, 10 minutes), the control peptide KRX-723 (20 μM, 3 hours), KRX-725 (20 μM, 3 hours), or vehicle (0.1% DMSO, 3 hours). (C) Effect of siRNAs directed against the sequence of S1P1 or S1P3 on ERK phosphorylation induced by KRX-725. HUVECs were transfected with S1P1, S1P3, and nonrelevant (NR) 21-nucleotide siRNA. ERK phosphorylation was detected following overnight starvation and KRX-725 stimulation (20 μM, 1 hour). S1P3 protein level was assessed in the same assay conditions. (D) Phospho-ERK1/2 and ERK1/2 levels in HUVECs exposed to vehicle (0.1% DMSO, 3 hours), S1P (1 μM, 10 minutes), VEGF (10 ng/mL, 10 minutes), or KRX-725 (20 μM, 3 hours). Samples were preincubated with PTX (500 ng/mL) 3 hours prior to addition of the stimulant. Cell lysates were subjected to Western blotting with antiphospho-ERK (pERK) and reprobed with anti-ERK2 (ERK) antibodies.
The ability of S1P and KRX-725 to induce ERK activation was tested by Western blotting with antibody against the activated double-phosphorylated p42/44 MAPK. In HA-VSMCs, ERK1 and ERK2 were phosphorylated following exposure to S1P and KRX-725 for 10 minutes and 3 hours, respectively (Figure 2B). KRX-723, a myristoylated control peptide (Figure 1) that did not induce sprouting of vessels in the aortic ring assay, did not activate ERK (Figure 2B).
siRNA is a powerful analytical tool that enables sequence-specific, posttranscriptional gene silencing.32 In HUVECs transfected with siRNA derived from unique sequence of S1P3, we show that the level of S1P3 decreases, and, correspondingly, KRX-725–induced ERK phosphorylation is inhibited (Figure 2C). In contrast, no decrease in either S1P3 levels or in KRX-725–induced ERK activation was observed in cells transfected with S1P1-specific or nonrelevant siRNA (Figure 2C). It is noteworthy that S1P3 levels were markedly higher in the presence of S1P1 duplex. Likewise, S1P1 levels were increased in cells transfected with S1P3 siRNA duplex (data not shown).
Pretreatment with the Gi inhibitor PTX completely abolished S1P-induced phosphorylation of ERK, whereas VEGF-induced ERK activation was not affected (Figure 2D), as previously reported.33 As in case of S1P, KRX-725-induced ERK phosphorylation was also PTX sensitive (Figure 2D). To summarize, we show here that KRX-725 induces ERK activation, which is mediated by S1P3 and Gi.
KRX-725 selectively induces S1P3 internalization
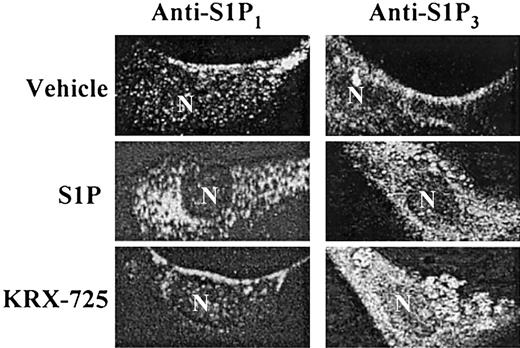
Following the interaction of a GPCR with its corresponding ligand, the receptor undergoes internalization, a process related to receptor desensitization.34 To further examine the specificity of KRX-725 with respect to S1P3, we examined the ability of the peptide to induce receptor internalization. With the use of cultured HUVECs, we traced the cellular localization of the receptor under various conditions, via immunofluorescence staining of the receptor. Both S1P1 and S1P3 are localized at the cell surface in the nonactivated state, whereas their native ligand, S1P, induces internalization of both receptors within 45 minutes (Figure 3). However, the S1P3-derived peptide KRX-725 selectively induces internalization of S1P3 without effecting S1P1 internalization (Figure 3). These findings not only point to the receptor specificity of the designer peptide but also suggest that KRX-725 simulates S1P3 activation toward the intracellular components of the internalization process.
KRX-725-induced internalization of S1P3. HUVECs were grown overnight with 2% c-FCS and incubated with vehicle (0.1% DMSO), S1P (1 μM), and KRX-725 (20 μM) for 45 minutes. Cells were fixed with 4% paraformaldehyde, permeabilized, blocked, and immunostained with anti-EDG1 and EDG3-NT antibodies, followed by Cy3- and FITC-labeled secondary antibodies, respectively. Visualization was done using confocal microscope using × 63 objective.
KRX-725-induced internalization of S1P3. HUVECs were grown overnight with 2% c-FCS and incubated with vehicle (0.1% DMSO), S1P (1 μM), and KRX-725 (20 μM) for 45 minutes. Cells were fixed with 4% paraformaldehyde, permeabilized, blocked, and immunostained with anti-EDG1 and EDG3-NT antibodies, followed by Cy3- and FITC-labeled secondary antibodies, respectively. Visualization was done using confocal microscope using × 63 objective.
KRX-725 induces vessellike sprout formation in a dose-dependent manner
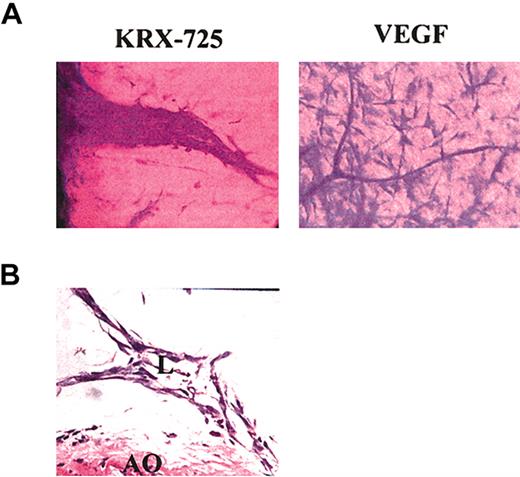
In the aortic ring assay, the number of newly formed sprouts and their morphology indicate the pro-angiogenic capabilities of specific factors. In the presence of KRX-725 (10 μM) and S1P (100 nM), aortic rings from C57BL/6 mice developed numerous sprouts, whereas almost none were observed with the vehicle by itself (Figure 4). Morphometric analysis of length and thickness of the induced sprouts reveals that doubling the concentration of KRX-725 from 10 to 20 μM contributes mainly to vessel thickness (Figure 4B). Similar results were obtained with aortic rings from Sprague-Dawley rats (data not shown). Pro-angiogenic responses were observed after 4 to 5 days and peaked following 7 to 10 days of incubation with KRX-725. Although the responses induced by KRX-725 resemble those evoked by 100 nM S1P, responses to S1P were seen earlier: within 2 to 3 days. KRX-725 induced well-developed and thick vessellike sprouts, as opposed to the thin capillaries induced by VEGF (Figure 5A). In addition, cross-section of aortic rings with further H&E staining showed that newly formed vessels are lumenized (Figure 5B). Moreover, in most of our experiments, the vessellike structures created by KRX-725 appear more like a normal vascular tree than the vessels created by S1P, which contain many fusionlike structures or other abnormal-looking vessels (see also Figures 6, 7, 8).
Detailed structure of sprouts induced by KRX-725. (A) Sprouts induced by KRX-725 (20 μM) or VEGF (25 ng/mL) in Sprague-Dawley rat aortic rings. After 10 days, the rings were fixed with formaldehyde, stained with crystal violet (0.02%), and examined microscopically. Representative micrographs of 6 repeats are depicted. (B) H&E staining of cross-section (5 μm) of paraffin-blocked BALB/c aortic rings incubated with KRX-725 (20 μM, 7 days). Micrographs were taken under × 40 objective. L indicates lumen; AO, aortic ring.
Detailed structure of sprouts induced by KRX-725. (A) Sprouts induced by KRX-725 (20 μM) or VEGF (25 ng/mL) in Sprague-Dawley rat aortic rings. After 10 days, the rings were fixed with formaldehyde, stained with crystal violet (0.02%), and examined microscopically. Representative micrographs of 6 repeats are depicted. (B) H&E staining of cross-section (5 μm) of paraffin-blocked BALB/c aortic rings incubated with KRX-725 (20 μM, 7 days). Micrographs were taken under × 40 objective. L indicates lumen; AO, aortic ring.
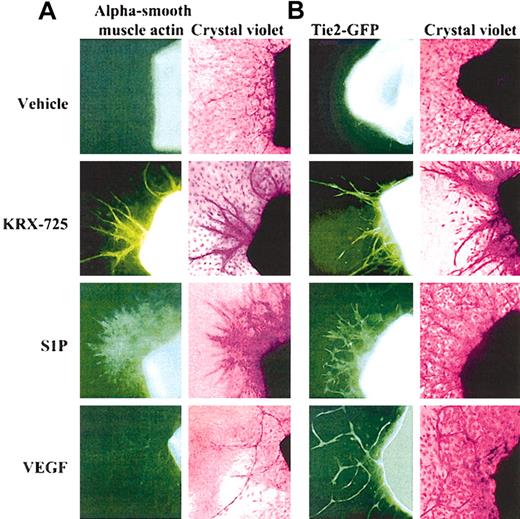
Presence of vascular smooth muscle and endothelial cells in aortic ring sprouts induced by KRX-725, S1P, and VEGF. (A) Involvement of vascular smooth muscle cells in sprouts. Aortic rings of BALB/c mice were incubated with vehicle (0.1% DMSO), KRX-725 (20 μM), S1P (200 nM), and VEGF (20 ng/mL) for 8 days. The rings were then fixed with paraformaldehyde and stained with FITC-conjugated alpha smooth muscle actin antibody (left column), followed by 0.02% crystal violet (right column). (B) Involvement of endothelial cells in sprouts. Aortic rings of FVB/N-TgN(TIE2GFP)287Sato mice were incubated with vehicle (0.1% DMSO), KRX-725 (20 μM), S1P (200 nM), and VEGF (20 ng/mL) for 8 days. Fluorescent micrographs were taken prior to fixation and staining. Fluorescence emanates from expression of green fluorescent protein (GFP) under endothelial-specific promoter (tie-2). Each micrograph is a representative of 9 repeats. Micrographs were taken under × 10 objective.
Presence of vascular smooth muscle and endothelial cells in aortic ring sprouts induced by KRX-725, S1P, and VEGF. (A) Involvement of vascular smooth muscle cells in sprouts. Aortic rings of BALB/c mice were incubated with vehicle (0.1% DMSO), KRX-725 (20 μM), S1P (200 nM), and VEGF (20 ng/mL) for 8 days. The rings were then fixed with paraformaldehyde and stained with FITC-conjugated alpha smooth muscle actin antibody (left column), followed by 0.02% crystal violet (right column). (B) Involvement of endothelial cells in sprouts. Aortic rings of FVB/N-TgN(TIE2GFP)287Sato mice were incubated with vehicle (0.1% DMSO), KRX-725 (20 μM), S1P (200 nM), and VEGF (20 ng/mL) for 8 days. Fluorescent micrographs were taken prior to fixation and staining. Fluorescence emanates from expression of green fluorescent protein (GFP) under endothelial-specific promoter (tie-2). Each micrograph is a representative of 9 repeats. Micrographs were taken under × 10 objective.
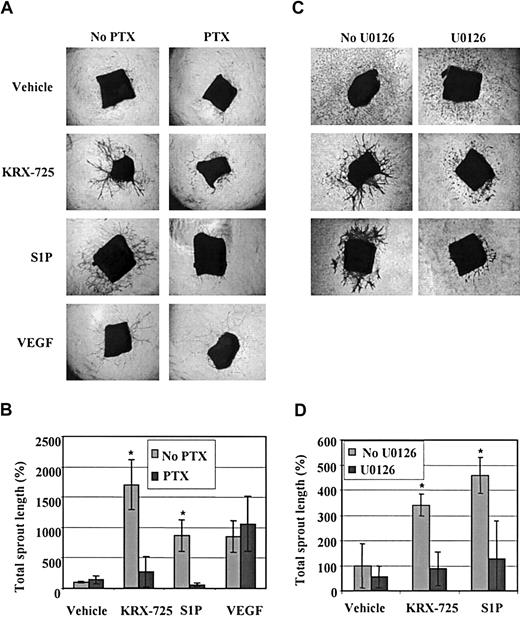
Sprout formation induced by KRX-725 and S1P is mediated via the Gi-MEK-ERK pathway. (A) Aortic rings from C57BL/6 mice were maintained for 10 days with vehicle (0.1% DMSO), KRX-725 (20 μM), S1P (200 nM), and VEGF (10 ng/mL) in the presence or the absence of PTX (200 ng/mL). Representative micrographs of rings of each arm of the experiment are shown. (B) Morphometric analysis of sprout length of 4 rings for each group described in panel A; mean ± SEM is presented relative to the control rings (100%). * indicates statistical significance (P < .05, Wilcoxon test) of treatment without PTX versus treatment with PTX. (C) Aortic rings from BALB/c mice were maintained for 7 days with vehicle (0.1% DMSO), KRX-725 (20 μM), and S1P (200 nM) in the presence or the absence of the MEK inhibitor U0126 (10 μM). Representative micrographs of rings from 9 repeats of each arm of the experiment are shown. (D) Morphometric analysis of sprout length of 4 rings in each group described in panel C; mean ± SEM is presented relative to the control rings (100%). * indicates statistical significance (P < .05, Wilcoxon test) of treatment without U0126 versus treatment with U0126. Original magnification of all micrographs is × 4.
Sprout formation induced by KRX-725 and S1P is mediated via the Gi-MEK-ERK pathway. (A) Aortic rings from C57BL/6 mice were maintained for 10 days with vehicle (0.1% DMSO), KRX-725 (20 μM), S1P (200 nM), and VEGF (10 ng/mL) in the presence or the absence of PTX (200 ng/mL). Representative micrographs of rings of each arm of the experiment are shown. (B) Morphometric analysis of sprout length of 4 rings for each group described in panel A; mean ± SEM is presented relative to the control rings (100%). * indicates statistical significance (P < .05, Wilcoxon test) of treatment without PTX versus treatment with PTX. (C) Aortic rings from BALB/c mice were maintained for 7 days with vehicle (0.1% DMSO), KRX-725 (20 μM), and S1P (200 nM) in the presence or the absence of the MEK inhibitor U0126 (10 μM). Representative micrographs of rings from 9 repeats of each arm of the experiment are shown. (D) Morphometric analysis of sprout length of 4 rings in each group described in panel C; mean ± SEM is presented relative to the control rings (100%). * indicates statistical significance (P < .05, Wilcoxon test) of treatment without U0126 versus treatment with U0126. Original magnification of all micrographs is × 4.
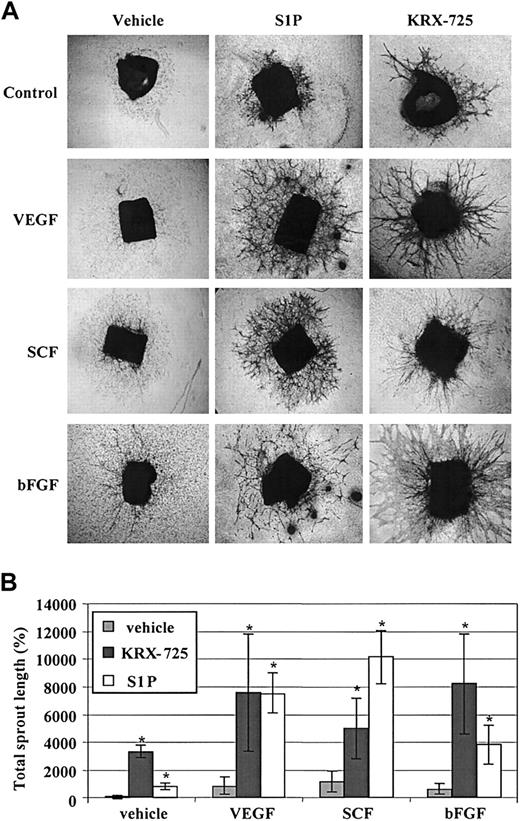
KRX-725 and S1P synergize with protein angiogenic growth factors in sprout formation. BALB/c aortic rings were cultured with vehicle (0.1% DMSO), VEGF (10 ng/mL), SCF (30 ng/mL), and bFGF (20 ng/mL) alone or in combination with S1P (200 nM) and KRX-725 (20 μM) for 10 days. The rings were then fixed, stained with 0.02% crystal violet, and examined microscopically. (A) Representative micrographs of rings of each arm of the experiment. Original magnification × 4. (B) Morphometric analysis of sprout length from 4 repeats; mean ± SEM is presented relative to the control rings (100%). * indicates statistical significance (P < .05, Wilcoxon test) of the combined treatments with KRX-725 or S1P versus the controls (vehicle or protein factors alone).
KRX-725 and S1P synergize with protein angiogenic growth factors in sprout formation. BALB/c aortic rings were cultured with vehicle (0.1% DMSO), VEGF (10 ng/mL), SCF (30 ng/mL), and bFGF (20 ng/mL) alone or in combination with S1P (200 nM) and KRX-725 (20 μM) for 10 days. The rings were then fixed, stained with 0.02% crystal violet, and examined microscopically. (A) Representative micrographs of rings of each arm of the experiment. Original magnification × 4. (B) Morphometric analysis of sprout length from 4 repeats; mean ± SEM is presented relative to the control rings (100%). * indicates statistical significance (P < .05, Wilcoxon test) of the combined treatments with KRX-725 or S1P versus the controls (vehicle or protein factors alone).
Sprouts induced by KRX-725 involve recruitment of vascular smooth muscle cells
The involvement of smooth muscle cells in vascularization was examined on aortic rings incubated in medium containing KRX-725, S1P, or VEGF for 8 days. When these preparations were stained with FITC-conjugated antibodies directed against vascular smooth muscle actin, an increase in the presence of smooth muscle cells in the aortic ring sprouts was observed following incubation with KRX-725 or S1P (Figure 6A). Sprouts induced by KRX-725 involve better organization of smooth muscle cells and the sprout structure appears more mature, whereas S1P-treated vessels are less structured and smooth muscle cells are more scattered. The thin, one-layered sprouts induced in the presence of VEGF displayed only weak staining. Cells not involved in sprout formation were not stained by the antivascular smooth muscle actin antibody. Specificity of the antibody was verified by the staining of cultured vascular smooth muscle cells (HA-VSMCs) as compared with endothelial cells (HUVECs) that showed no staining (data not shown). In aortic rings obtained from the transgenic FVB/N(IgNTIE2GFP)287Sato mice, which express GFP under endothelial-specific promoter (tie-2), newly formed sprouts in the presence of either KRX-725, S1P, or VEGF exhibited fluorescence, which indicates the presence of endothelial cells in the sprout structure (Figure 6B). Thus, the vessellike sprouts in aortic rings induced by S1P or KRX-725 do contain both endothelial and smooth muscle cells, whereas sprouts induced by VEGF contain mainly endothelial cells. The stability of KRX-725–induced vessels, containing a smooth muscle cell layer, was demonstrated by the fact that sprouts did not regress within 3 weeks, as opposed to the regression with time of capillaries induced in controls and in the presence of VEGF (data not shown). These findings suggest that KRX-725 enhances vessel stability by recruiting smooth muscle cells.
Involvement of Gi and ERK in ex vivo sprout formation induced by KRX-725 and S1P
Following our initial observation that KRX-725 induces ERK activation mediated by Gi (Figure 2), we further examined whether Gi and ERK are also involved in KRX-725– and S1P-induced vessel sprouting. Aortic rings were incubated with KRX-725, S1P, or VEGF in the presence or absence of the Gi inhibitor PTX. In Figure 7A we show that sprout formation in the presence of KRX-725 and S1P was strongly inhibited by PTX (8- to 13-fold). As expected, PTX had no effect on VEGF-induced sprouting. An involvement of the mitogen-activated protein kinase kinase (MEK)–ERK pathway was tested using the specific MEK inhibitor U0126. As demonstrated in Figure 7B, U0126 strongly inhibited KRX-725– and S1P-induced sprout formation. These findings indicate a significant role for a Gi-dependent MEK-ERK pathway in vessel sprouting induced by KRX-725 and S1P.
KRX-725 and S1P act synergistically with pro-angiogenic protein growth factors
S1P was shown to synergize with various protein growth factors in angiogenesis-related assays both in vitro and in vivo.3,4 In our study, KRX-725 as well showed strong synergistic effects with protein growth factors. In mice aortic rings, a synergistic effect of KRX-725 or S1P and bFGF, SCF, and VEGF was detected. Although these growth factors by themselves exhibited little or no effect on sprouting, their combination with KRX-725 or S1P induced a substantial enhancement of sprout formation (Figure 8). No synergistic effect on aortic sprouts was observed with PDGF and EGF when incubated with either S1P or KRX-725 (data not shown). With respect to the magnitude of the response of aortic rings to KRX-725 alone, the results varied among different experiments. However, in the presence of angiogenic factors like VEGF or bFGF, more unified and enhanced responses were obtained. The variations in the effect of KRX-725 alone might be related to differences in the basal endogenous levels of growth factors among preparations of rings as previously suggested.35,36
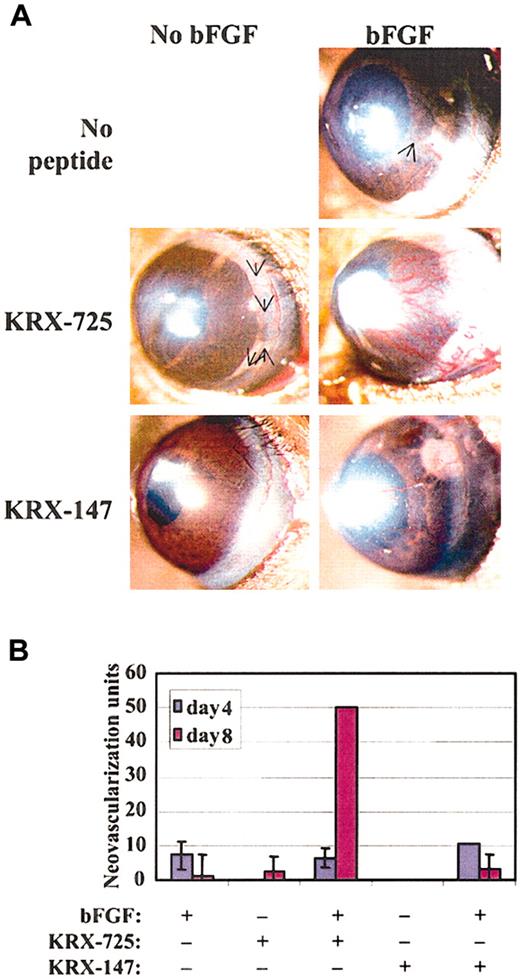
We then tested synergy at the in vivo level and compared the effect of combining bFGF and KRX-725 with the effect of each factor alone in the mouse corneal pocket model. Although both KRX-725 and bFGF, at the tested doses, exhibit limited proangiogenic activity in corneal pockets when applied alone, their combination gave rise to a large amount of vessels that covered almost the entire circumference of the cornea (Figure 9A). The synergistic effect appears on day 8 from implantation, when the effect of bFGF is starting to decay (Figure 9B). A myristoylated control peptide had no effect when applied alone, and its combination with bFGF gave neovascularization that can be attributed to the bFGF activity only. A 25-day follow-up revealed that the vessels induced by the combination of bFGF and KRX-725 remained stable, whereas in all others systems they regressed completely (data not shown). Our results are very similar to those obtained by English et al3 regarding S1P and bFGF combination, in the same experimental model.
Synergistic effect of KRX-725 and bFGF in the corneal pocket assay. (A) Hydron pellets containing bFGF (80 ng), KRX-725 (10 μg), or myristoylated control peptide (10 μg) were implanted into micropockets within the corneas of C57BL/6 mice. Blood vessel formation was assessed by stereoscopy. Angiogenic response after 8 days is presented (representative pictures from 4 repeats in each experimental group). Arrows indicate weakly visible vessels. (B) Quantitation of the response at days 4 and 8 after implantation, using the formula (vessel length) × (clock hour of vessel sprouting from limbus) × 2 π/(pellet distance from limbus).
Synergistic effect of KRX-725 and bFGF in the corneal pocket assay. (A) Hydron pellets containing bFGF (80 ng), KRX-725 (10 μg), or myristoylated control peptide (10 μg) were implanted into micropockets within the corneas of C57BL/6 mice. Blood vessel formation was assessed by stereoscopy. Angiogenic response after 8 days is presented (representative pictures from 4 repeats in each experimental group). Arrows indicate weakly visible vessels. (B) Quantitation of the response at days 4 and 8 after implantation, using the formula (vessel length) × (clock hour of vessel sprouting from limbus) × 2 π/(pellet distance from limbus).
Discussion
Angiogenesis is a complex developmental process induced by soluble factors, released from a tissue in need for vascular reinforcements. S1P3 belongs to a family of receptors that regulates the angiogenic process and is selectively expressed at higher levels in endothelial cells and vascular smooth muscle cells. In this study we demonstrated the pro-angiogenic activity of a designer short peptide, KRX-725, whose sequence is derived from the i2 loop of S1P3. When tested in an ex vivo aortic ring assay, it exhibited distinct pro-angiogenic effects similar to that of S1P3's native ligand, S1P. KRX-725 induced lumenized, well-developed multilayered sprouts (Figures 4, 5) that showed an increase in the presence of smooth muscle cells (Figure 6). At the molecular level, both KRX-725 and S1P induced ERK phosphorylation and are PTX sensitive (Figure 2). With the use of siRNA, we showed that KRX-725–induced ERK activation was S1P3 dependent, whereas no inhibition of ERK activation was detected using siRNA targeted against S1P1 (Figure 2C). The relevance of this signaling pathway to the angiogenic process is evident by the abrogation of sprout formation induced by KRX-725 or S1P via the Gi inhibitor PTX or MEK inhibitor U0126 (Figure 7). KRX-725 showed synergistic effects with pro-angiogenic factors, including VEGF, SCF, and bFGF (Figure 8). Moreover, the combination of KRX-725 and bFGF evoked remarkable neovascularization in the in vivo model of the corneal pocket assay (Figure 9).
Substantial experimental evidence has shown that the i2 loop of GPCRs plays an important role in G protein coupling and receptor activation.37 Site-specific mutations in selected residues of the i2 loop in many receptors have been shown to directly impair G protein coupling.15,19-21 The i2 loop is also involved in G protein selectivity and has a role in correct folding of the receptor.38 The modulation of G-protein–mediated response by peptides derived from GPCR intracellular loops is supported by several studies. In a cell-free system, peptides corresponding to the whole i2 loop of the 5-HT1A (5-hydroxytryptamine1A) receptor can directly bind to Gi proteins, mimic receptor activation, and, thus, inhibit adenylyl cyclase activity.23 Similarly, peptides derived from the i2 loop of δ- and μ-opioid receptors inhibit adenylyl cyclase activity via coupling to Gi.22 However, synthetic peptides derived from this region in rhodopsin act as competitors of rhodopsin-Gt interaction.39 In this study, we demonstrate that a myristoylated peptide whose sequence is derived from the c-terminus end of i2 loop, unique to S1P3, mimics the ligand effect on angiogenesis. The precedent for such a phenomenon has been demonstrated in a recently published report in which palmitoylated peptides derived from the 3rd intracellular loop (i3) of the GPCRs PAR1 and MC4 were shown to activate their coupled G proteins and to mimic natural ligand effects in intact cells.40 Similar to our study, this group showed the need for intact receptor to induce activation by the peptide. As there are data indicating also a role of i3 loop in G protein activation in several GPCRs, including EDG,41,42 further work may examine a potential activity of i3 loop-derived peptides in relation to S1P1 and S1P3.
The ability to design a receptor-specific agonist is of great analytical and clinical importance. Although S1P activates all family of S1P receptors, our work indicates that KRX-725 is S1P3 specific. The siRNA data already demonstrate the specific need for S1P3 to obtain KRX-725–mediated activation of ERK (Figure 2C). In addition, KRX-725 leads to receptor internalization of S1P3 but not S1P1 (Figure 3). This last finding also provides an insight regarding KRX-725 mechanism of action. One possible explanation is a tethering of the peptide to a high-affinity site within the intracellular machinery of GPCR signaling, thereby stabilizing the active-state conformation of the receptor. Alternatively, the peptide might mimic a dimerization state that is necessary for the activation of some GPCRs (similar arguments with respect to the i3 loop are found in Covic et al40 ). The ability of KRX-725, an i2 loop–derived peptide, to cause receptor internalization is compatible with other works that assign a critical role for the i2 loop in receptor internalization and desensitization.14,16,43 Thus, our findings suggest a specific mode of action for KRX-725, which is S1P3 dependent.
Vessel maturation during angiogenesis is dependent on appropriate recruitment of pericytes and vascular smooth muscle cells, covering the newly formed endothelium.44 Multiple angiogenic factors are involved in this process with S1P-mediated EDG signaling having a major role in endothelial layer integrity and VSMC recruitment to form stable vascular structures.45,46 A detailed analysis of S1P receptor location in the human aorta revealed that, although S1P1 expression is confined to endothelial cells, S1P3 expression is largely distributed in smooth muscle cells.9 In S1P1 knock-out mice, VSMC recruitment to maturing vessels is impaired,11 and S1P modulates migration of aortic smooth muscle cells in vitro, which affects their recruitment to newly formed vessels.47 S1P, through S1P1 and S1P3, affects the integrity of the endothelial barrier, which is essential for vessel stability.48 S1P-treated vessels were resistant to the edema-inducing agent, thrombin. Such resistance is important because new vessels, produced in the presence of VEGF, tend to be leaky, and the resulting edema limits the use of VEGF in pro-angiogenic therapy.49 It is possible that VEGF alone is insufficient to induce adequate angiogenesis, and there may be a requirement for the addition of a stabilizing factor, such as S1P, for the completion of the process. The fact that protein and lipid growth factors can display synergy is not unusual. In vivo, synergism occurs between bFGF and S1P in the avascular cornea model3 and the matrigel implant assay.4 Lee et al4 also showed synergistic effect of S1P and VEGF. Both responses were abolished by S1P1 and S1P3 antisense mRNA. Microscopic examination of the matrigel implants revealed that vessels generated by S1P together with protein growth factors appear intact and well developed, whereas vessels generated in the presence of protein growth factors alone were leaky.50 In this work we demonstrated that both KRX-725 and S1P induce the assembly of vascular smooth muscle cells on the sprouts of aortic rings, creating multilayer well-developed lumenized vessellike structures (Figures 5 and 6). The synergistic effect obtained by the combination of KRX-725 and protein angiogenic growth factors (Figure 8) suggests a therapeutic value for the coadministration of KRX-725 with such factors. Moreover, the local secretion of endogenous angiogenic factors under hypoxic conditions51 might act synergistically with KRX-725 administrated alone to ischemic tissues.
The above findings suggest that KRX-725 may serve as a therapeutic agent for the treatment of vascular disorders in which angiogenesis is desirous, such as peripheral vascular disease, myocardial ischemia, tissue grafts, and diabetic wound healing. The advantage of using KRX-725, as compared with S1P, is the possibility of designing a product that is degraded slowly and acts locally. KRX-725 may also be more selective, being specifically derived from the S1P3 receptor, in contrast to S1P that binds to 5 members of the S1P receptor family and acts independently as an intracellular second messenger as well. As S1P mediates multiple physiologic processes, including opening of ion channels in the heart muscle,52 activating endothelial nitric oxide synthase,53 or elevating blood pressure,54 drugs based on S1P may be undesirable. Additional work is required to elucidate further the molecular mechanism by which KRX-725 exerts its pro-angiogenic effect.
Prepublished online as Blood First Edition Paper, May 22, 2003; DOI 10.1182/blood-2002-12-3634.
Supported by a grant from Keryx Biopharmaceuticals, Israel.
T.L. and H.R. are employed by a company (Keryx Biopharmaceuticals), whose potential product was studied in the present work. S.A.B.-S. was a scientific founder of Keryx, which licensed the drug design technology, but disconnected all day-to-day ties with the company as of November 30, 2001. S.A.B.-S. previously held financial interest in Keryx but has sold the majority of his shares in the company. S.A.B.-S. still maintains a financial interest in the future of Keryx through an indirect interest in shares, warrants, and potential royalties issued or due to Children's Medical Center (CMCC), the institution from which Keryx licensed the technology. S.A.B.-S. assumes full academic responsibility regarding the content of this article.
T.L. and L.T. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Catherine Butterfield and Dr Karen Moulton, Department of Surgical Research, Children's Hospital, Boston, for their help in the corneal pocket assay and the whole mount immunostaining of the aortic rings. We thank Drs Masha Niv and Ilan Stein from Keryx Biopharmaceuticals for helpful assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal