Abstract
In a phase 1 dose escalation study, 13 subjects with hemophilia A received by peripheral intravenous infusion a retroviral vector carrying a B-domain–deleted human factor VIII (hFVIII) gene. Infusions were well tolerated. Tests for replication competent retrovirus have been negative. Polymerase chain reaction (PCR) analyses demonstrate the persistence of vector gene sequences in peripheral blood mononuclear cells in 3 of 3 subjects tested. Factor VIII was measured in serial samples using both a one-stage clotting assay and a chromogenic assay. While no subject had sustained FVIII increases, 9 subjects had FVIII higher than 1% on at least 2 occasions 5 or more days after infusion of exogenous FVIII, with isolated levels that ranged from 2.3% to 19%. Pharmacokinetic parameters of exogenous FVIII infused into subjects 13 weeks after vector infusion showed an increased half-life (T1/2; P < .02) and area under the curve (AUC, P < .04) compared with prestudy values. Bleeding frequency decreased in 5 subjects compared with historical rates. These results demonstrate that this retroviral vector (hFVIII(V)) is safe and, in some subjects, persists more than a year in peripheral blood mononuclear cells, with measurable factor VIII levels and with increased available FVIII activity (increased T1/2 and AUC) after infusion of exogenous FVIII concentrate.
Introduction
Hemophilia A is an X-linked bleeding disorder caused by a deficiency or abnormality of factor VIII (FVIII), a necessary cofactor for the generation of thrombin. It is the most common inheritable coagulation protein deficiency, with an incidence of approximately 1 in 10 000 males.1 Individuals with severe hemophilia suffer episodes of spontaneous bleeding in joints and soft tissue, including intracranial hemorrhage. Recurrent joint bleeding leads to characteristic chronic debilitating hemophilic arthropathy. The development of joint disease and other complications of hemorrhage results in absences from school and work, and chronic pain, and often leads to disability.
Hemophilia is treated by replacement of the missing clotting factor using intravenous infusion of FVIII protein concentrates. Such infusions must be administered frequently for hemostasis after serious bleeding episodes because the half-life of FVIII protein is only 8 to 12 hours. To sustain prophylactic FVIII levels, infusions are administered every other day. While current therapeutic products for hemophilia A, including plasma-derived factor VIII concentrates and recombinant FVIII products, have significantly lowered the risk of viral transmission and offer reliable prophylactic and therapeutic efficacy,2 there are lingering concerns about potential viral contamination and transmission of other infectious agents. Furthermore, the concentrates are extremely expensive and require frequent intravenous administration, sometimes necessitating placement of venous access devices. For these and other reasons, gene therapy for hemophilia has been of considerable interest.
FVIII deficiency is a promising genetic disease to target for gene therapy for a number of reasons.3-6 Delivery and expression of the normal FVIII gene in any tissue with vascular exposure that leads to even low levels of expression of FVIII protein would be expected to prevent spontaneous bleeding. In addition, it is likely that neither tissue-specific expression nor exogenous control of gene expression is required, making this approach relatively straightforward and achievable with current gene transfer methods. The gene for FVIII is well characterized and there are excellent preclinical mouse and dog models of hemophilia. Successful FVIII gene therapy could be expected to reduce health care expenditures as well as improve the quality of life for these individuals.7,8
In this report, we describe the results of a phase 1, single-treatment, dose-escalation study of gene therapy using a retroviral vector in subjects with hemophilia A. The retroviral vector (hFVIII(V)) was based on a type C retrovirus, Moloney murine leukemia virus (MoMLV), that was rendered replication deficient and carried a B-domain–deleted gene for human factor VIII (hFVIII). The vector has been extensively modified to carry no genes encoding viral proteins, and to minimize or eliminate regions of homology between vector and producer cell sequences in order to reduce the chance of a recombination event during production. Safety of the vector was supported by preclinical studies in mice, rabbits, and normal and hemophilia A–infected dogs.9-13 The same proprietary vector system had been used previously to produce vectors carrying genes for clinical applications in cancer and human immunodeficiency virus (HIV) disease, and these preparations have been directly administered to more than 300 subjects without safety problems in phase 1 and phase 2 clinical trials.14-17 However, this study was the first using intravenous administration, and, therefore, safety and tolerability were carefully monitored. The results of the trial demonstrate the safety of this retroviral vector in human subjects with hemophilia A.
Patients, materials, and methods
This phase 1 clinical trial was designed as a multicenter, intersubject dose-escalation, single-treatment, open-label trial in adult male subjects with severe hemophilia A. We planned to enroll between 9 and 20 subjects, and to follow them closely for an initial period of 13 weeks (phase 1) when they were predicted to show first appearance of measurable FVIII activity and when they would be monitored for early adverse events related to the infusion of the retroviral construct. Subsequently, they were to be seen at less frequent intervals through 53 weeks after treatment (phase 1 extension). This report presents the results through the first 53 weeks of study. Subsequently, these subjects have been asked to enroll into a long-term safety surveillance program in accord with FDA guidelines for the follow-up of individuals who have received retroviral constructs.
Study subjects
All subjects gave signed informed consent after the study design and protocol were approved by the individual institutional review boards (IRBs; listed in “Acknowledgments”) of the participating centers. The study protocol was submitted and approved, as part of an investigational new drug (IND) application, by the Center for Biologics Evaluation and Research of the United States Food and Drug Administration and by the Recombinant DNA Advisory Committee of the Office of Biotechnology Activities of the National Institutes of Heath.
Inclusion criteria included adult males (age, > 18 years) with severe hemophilia A (< 1% FVIII, measured following ≥ 5 days without FVIII treatment) who had received 100 or more treatments with FVIII concentrates in the past, who had no history of FVIII inhibitor, and whose current inhibitor titer was less than 0.6 Bethesda units (BU). For each potential subject, a pharmacokinetic study was conducted by measuring FVIII activity following infusion of 50 IU/kg of the subject's usual FVIII product. The FVIII activity assay at 6 hours had to be higher than 30% of the 10-minute peak, and FVIII activity had to be detectable between 24 and 36 hours after FVIII treatment. While participating in the study, the subjects had to be willing and able to use “on demand” treatment only (for active bleeding episodes), rather than prophylactic treatment.
Subjects who were seropositive for HIV could be enrolled in this study if their CD4 counts were higher than 300 cells/mm3 and they were not being treated concurrently with reverse-transcriptase inhibitors. Subjects had to agree to use a barrier method of contraception throughout the study (53 weeks), and, if vector was detected in the semen, until 3 consecutive monthly semen specimens showed no detectable vector. All subjects were seropositive for hepatitis C antibody; no specified viral RNA level or liver histology by biopsy was required for participation in the study.
Exclusion criteria included any significant cardiac, pulmonary, endocrine, neurologic, or hematologic condition, other than hemophilia A; alanine transaminase (ALT) value 5 times or more than normal; abnormal laboratory values for albumin, bilirubin, or prothrombin time (PT); treatment with interferon or ribavirin within 3 months of enrollment; prior history of allergic reactions to factor VIII or prophylactic medication required to prevent such reactions; or therapy with any non-FVIII investigational agent within 30 days of enrollment. Those individuals who had received investigational FVIII within 30 days were required to have had at least 50 days of exposure to that preparation and no drug-related adverse events.
Viral vector construct
The viral vector construct, hFVIII(V) (Chiron, Emeryville, CA), in this trial consisted of a retroviral vector, based on the Moloney murine leukemia virus (MoMLV), carrying a B-domain–deleted gene for human factor VIII (hFVIII). The B-domain–deleted form of the FVIII gene was used because vectors carrying this gene consistently yielded higher titers and higher levels of protein expression per gene copy number compared with the full-length gene (Sheridan et al, unpublished data, May 1999). Experience with a commercially available recombinant form of FVIII produced from cells lacking the B-domain has shown that this alteration does not affect hemostatic ability or immunogenicity of the FVIII molecule.18,19 The vector has an amphotrophic envelope and is made in a human packaging cell line. The vector is introduced into the packaging cell line by transduction of a vesicular stomatitis virus G protein (VSVg)–pseudotyped particle made by transient transfection. This procedure produces a high titer vector that is resistant to inactivation by human complement.20,21 The vector21 carries a single gene (FVIII) and expression is driven from the promoter in the Moloney 5′ long terminal repeat (LTR). Similarly, the 3′ polyadenylation site is provided by the 3′ LTR. The vector carries no genes for viral proteins, and retroviral sequences that remain were modified to minimize or eliminate regions of homology between vector and producer cell sequences in order to reduce the chance of a recombination event during production of the vector. The vector (hFVIII(V)) was harvested from the supernatant of cells grown in the CellCube (Corning Costar, Cambridge, MA) as described and was purified using ion exchange and column chromatography.22 The final product was formulated with lactose and phosphate-buffered saline for intravenous infusion. Vector titer was determined by adding a dilution series of the vector to human fibrosarcoma cells (HT 1080) and performing limiting dilution polymerase chain reaction (PCR) analysis to determine the number of DNA vector genomes (provector copies) present in each member of the original dilution series. One provector copy was termed a transducing unit (TU).
Extensive testing of this retroviral construct in animals demonstrated safety and potential efficacy.9-12,23 The highest FVIII levels were obtained in adult animals when total doses were divided and administered over consecutive days. At doses similar to those planned for this phase 1 clinical trial, rabbits showed reproducible production of hFVIII protein and sustained expression, for as long as the rabbits were followed, of concentrations of FVIII protein of 30 to 40 ng/mL (which would be equivalent to 15%-20% FVIII activity in humans). There were 2 studies conducted in hemophilic dogs. In one study, a shortening of the whole blood clotting time (WBCT) from longer than 40 minutes to 12 to 22 minutes was observed between days 4 and 14 in 6 of 7 treated animals (normal control dogs had WBCT values of 6-10 minutes).23 In addition, between days 4 and 14 (the time the dogs showed shortening of the WBCT), hFVIII concentrations, measured by enzyme-linked immunosorbent assay (ELISA), increased to a range between 25 to 90 ng/mL. Immune complexes between vector-derived hFVIII and canine immunoglobulin G (IgG) were detected initially at low levels, coinciding with the period of partial WBCT correction. After day 14, the amounts of immune complex formation increased rapidly, coinciding with the period when most of the dogs showed return of WBCTs to pretreatment levels. In 2 animals, however, WBCT remained shortened (approximately 21 minutes) 6 to 12 months after treatment, again coinciding with decreasing amounts of canine IgG:hFVIII complexes. In a second set of experiments the vector was delivered at 3 dose levels in 3 dogs per dose level.12,13 Significant concentrations of human factor FVIII were observed in 1 of 3 dogs at each dose level and reduced bleeding and shortened partial prothrombin time (PTT) were observed in all 9 dogs. The dog experiments were complicated by the appearance of dog antihuman FVIII antibodies. In addition to these studies, extensive toxicology studies performed in rabbits and mice established that dose ranges associated with potentially therapeutic FVIII levels in plasma could be administered without significant toxicity. Detection of vector-specific sequences in tissues, primarily liver and spleen, was not associated with histopathologic changes.
Coagulation assays
Coagulation tests, including FVIII levels, inhibitor assays, prothrombin time, partial thromboplastin time, and chromogenic assays, were performed in a central reference laboratory at the Special Coagulation Laboratory, Children's Hospital of Michigan. All samples had code numbers only, and all assays were performed blinded. Assays were performed on frozen samples that had been stored at –80°C and thawed once in a 37°C waterbath. FVIII activity was measured both by a one-stage coagulation assay and by a chromogenic assay (Coatest; Chromogenix, Milan, Italy).24 Both techniques were used because B-domain–deleted FVIII has been reported to result in higher plasma FVIII activity values when measured by chromogenic assay than by coagulant assay.25 The usual lower limit of detection for the FVIII coagulant activity assays in this reference laboratory was 0.7%; however, after recalibrating the assay using additional dilutions and control curves in order to modify the assay, levels as low as 0.2% could be measured reproducibly using the one-stage assay, and as low as 0.3% using the chromogenic assay. The standard curve was established using Biopool human reference plasma (HRP) standardized against WHO standard. A B-domain–deleted factor VIII concentrate was not used to standardize assays. In recalibrating the FVIII assays the plasmas were diluted with buffer. The coefficients of variance (CVs) of the FVIII assays were 1.87% with George King B-Fact, 1.99% with George King severe hemophilia A, 5.2% with mild hemophilia A (all within runs), and 6.5% with Biopool HRP, and 6.2% with Biopool Abnormal (the latter 2 between runs). The CV for the chromogenic FVIII assay with George King Fact was 3.1% within runs. FVIII protein was measured using an enzyme immunoassay (Immuno).26 FVIII inhibitor antibodies were measured using a Nijmegen-modified Bethesda assay.27,28 The CVs of the inhibitor assays were 4.6% within a run and 7.7% between runs.
Laboratory monitoring
Baseline clinical history, physical examinations, and laboratory testing, including complete blood counts (CBCs), urinalysis, serum chemistry panels, testing for HIV and, if positive, viral load and CD4 counts, and testing for hepatitis C antibody and viral load were performed prior to and then following treatment with the FVIII construct, hFVIII(V). Safety assessments included FVIII inhibitor assays; adverse events; physical examination; standard clinical laboratory tests (complete blood count [CBC] with differential, serum chemistries [albumin, alkaline phosphatase, alanine transaminase, amylase, aspartate aminotransaminase, blood urea nitrogen, calcium, cholesterol, creatinine, direct and total bilirubin, gamma-glutamyl transferase, total protein, triglycerides, uric acid, and globulin], activated partial thromboplastin time [aPTT], prothrombin time [PT], and urinalysis); and viral serologies for HIV, hepatitis C virus (HCV), hepatitis B surface antigen (HBsAg) and surface antibody (HBsAb), and hepatitis A IgG. CD4 counts and HIV RNA by PCR were assayed in HIV-positive subjects and HCV RNA by PCR was assayed in HCV-positive subjects. Antibodies to fetal bovine serum (FBS), a possible low level contaminant of the vector preparations, were also measured. In addition, testing was performed for replication competent retrovirus (RCR) by polymerase chain reaction (PCR) assay at baseline, and 6 and 12 months after treatment, and semen samples were analyzed from baseline, and at weeks 2, 6, 9, 11, 17, 29, and 53 for detectable vector sequences by PCR.11 Unless otherwise noted, the sensitivity of individual PCR test wells was 1 copy per 1.5 × 105 diploid cell genomes.
Dose escalation and follow-up
Each subject received a single treatment of hFVIII(V), at 1 of 4 escalating levels, administered as equally divided doses on 3 consecutive days via peripheral venous access. The total dose levels were 2.8 × 107, 9.2 × 107, 2.2 × 108, 4.4 × 108, and 8.8 × 108 transducing units (TU)/kg. The total dose was divided and administered as 3 equal daily doses on 3 consecutive days via peripheral venous access.
The hFVIII(V) dose was infused each day via peripheral vein at the infusion rate of 2 mL per minute. There were 3 subjects who received the same dose and were monitored weekly for 7 weeks for FVIII inhibitor formation and safety parameters prior to treatment of additional subjects and dose escalation. The first subject receiving the next higher dose was monitored throughout the 3 infusions (72 hours) before 2 additional subjects were treated with that dose. FVIII protein expression was assessed with FVIII assays 3 times weekly. Bleeding episodes were treated with an infusion of the FVIII concentrate usually used by the subject. All FVIII concentrate infusions and bleeding episodes were reported in home diaries, which were collected weekly during the first 3 months of study and monthly throughout the rest of the year. Subjects were monitored for FVIII protein expression and safety, and, after 53 weeks, were to be enrolled in a lifelong surveillance protocol that requires annual visits and blood samples for RCR testing, according to appropriate current FDA guidelines.
Efficacy measurements included FVIII activity and protein expression and recording in home diaries any bleeding episodes and treatment for bleeding. Assays were also performed to detect antibodies to the viral vector. At week 13, a repeat 36-hour pharmacokinetic study was conducted by measuring FVIII activity concentrations following administration of 50 IU/kg of the subject's usual FVIII product.
Statistical considerations
All subjects who received hFVIII(V) were included in safety analyses. Continuous data are expressed as means ± SD and categoric data are expressed as proportions, unless otherwise specified. Descriptive statistics were used to summarize data. Pharmacokinetic parameters were calculated at baseline and at end of phase 1 (week 13 after infusion of vector) using standard noncompartmental methods and WinNonlin Professional Version 3.3 (Pharsight, Mountain View, CA). All calculations were performed prior to rounding. Differences in pharmacokinetic (PK) values for FVIII at the end of phase 1 compared with baseline were analyzed using a 2-sided paired Student t test. P ≤ .05 was considered statistically significant.
Results
Enrolled and treated with hFVIII(V) were 13 subjects. Of these, 3 each received the first 4 doses (2.8 × 107, 9.2 × 107, 2.2 × 108, and 4.4 × 108 TU/kg) and 1 received the highest dose of 8.8 × 108 TU/kg. Of the subjects, 11 completed the entire study (53 weeks), and 2 withdrew for personal reasons (refused to comply with follow-up appointments) after 3 and 6 months, respectively. One subject was noncompliant with a number of study requirements prior to withdrawal and did not have pharmacokinetic studies completed. Thus, the pharmacokinetic studies, at baseline and at 13 weeks, included a total of 12 subjects.
The subjects who participated in the trial were representative of individuals with severe hemophilia A. Baseline characteristics summarizing severity and prior therapy for hemophilia A, and HIV and HCV status of the population are shown in Table 1. The 13 subjects had an average of 3.7 ± 2.6 spontaneous bleeding episodes per month, with various joints affected. The most common bleeding sites were elbows (92%), ankles (62%), knees (31%), shoulders (7.7%), and other (7.7%). The presence of a target joint was not an exclusion criterion. Of the subjects, 5 were seropositive for HIV and all 13 were positive for the hepatitis C virus.
Baseline and demographic characteristics
Characteristic . | No. . |
|---|---|
| Demographics | |
| Age, y (range) | 37.5 ± 14.7 (18-55) |
| Weight, kg | 83.1 ± 8.8 |
| Race | 12 white, 1 black |
| HIV and HCV status (%) | |
| HIV, no. with positive serology | 5/13 (38) |
| HCV, no. with positive serology | 13/13 (100) |
| FVIII treatment schedule (%) | |
| Prophylactic | 1/13 (8) |
| On demand | 12/13 (92) |
| FVIII therapy in past 12 months (range) | |
| Number of infusions | 50 ± 40.5 (10-150) |
| FVIII usage, units | 117 076 ± 52 000 (12-450 000) |
| Spontaneous bleeding episodes per month (range) | 3.7 ± 2.6 (0.5-8.0) |
| Functional status (%) | |
| Work or attend school | 8/13 (62) |
| Disabled, hemophilia related | 5/13 (39) |
Characteristic . | No. . |
|---|---|
| Demographics | |
| Age, y (range) | 37.5 ± 14.7 (18-55) |
| Weight, kg | 83.1 ± 8.8 |
| Race | 12 white, 1 black |
| HIV and HCV status (%) | |
| HIV, no. with positive serology | 5/13 (38) |
| HCV, no. with positive serology | 13/13 (100) |
| FVIII treatment schedule (%) | |
| Prophylactic | 1/13 (8) |
| On demand | 12/13 (92) |
| FVIII therapy in past 12 months (range) | |
| Number of infusions | 50 ± 40.5 (10-150) |
| FVIII usage, units | 117 076 ± 52 000 (12-450 000) |
| Spontaneous bleeding episodes per month (range) | 3.7 ± 2.6 (0.5-8.0) |
| Functional status (%) | |
| Work or attend school | 8/13 (62) |
| Disabled, hemophilia related | 5/13 (39) |
Safety
All subjects were alive and in their usual states of health at the completion of the 53 weeks of study. The administration of vector hFVIII(V) via peripheral vein each day for 3 consecutive days was well-tolerated, with no complications associated with the infusions reported in any subject.
Safety monitoring studies indicated that the infusion of hFVIII(V) via peripheral vein at these doses in these subjects was safe and well tolerated. Adverse events considered related to hFVIII(V) were as follows: dizziness in 4, flushing in 4, increased blood pressure in 2, headache in 2, increased heart rate in 1, chest pain in 1, and positive semen PCR test for vector in 1. All were mild in severity, except for a moderately severe headache in one subject. Tests for replication competent retrovirus were performed at regular intervals during the trial and were consistently negative. There was no clinical exacerbation of pre-existing HIV- or HCV-associated disease. CD4 counts, and HIV RNA and HCV RNA titers showed no significant adverse trends. In addition, clinical parameters showed no trends that correlated with treatment or treatment doses.
No FVIII inhibitors were detected by either Bethesda assay or by FVIII recovery and pharmacokinetic studies. The HIV and HCV RNA data overall showed no trends of concern according to 2 independent reviewers. Clinical laboratory testing (CBC, chemistry, and urinalysis) also revealed no trends over the year of the study. Nonneutralizing antibodies to murine leukemia virus (MLV) were detected in all subjects after treatment. No increases over background were detected for antibodies to fetal bovine serum or to FVIII protein by ELISA.
The only study-related serious adverse event occurred at week 9 following hFVIII(V) infusion, with a semen test that resulted in a transient positive PCR signal using 2 primer sets for vector in 1 of 10 replicates (subject no. 01001) (Table 2). This subject had received dose 4 (4.4 × 108 TU). Further studies could not be performed to determine whether the signal was in the sperm or the white blood cell fraction of the semen because such studies require fresh specimens, and all 4 subsequent semen samples showed no evidence of vector. The samples during the remainder of the study showed no evidence of vector sequences. All semen samples at study completion were negative for vector.
Analysis of semen for provector sequences
. | Week . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject ID no. . | 0 . | 2 . | 6 . | 9 . | 11 . | 17 . | 29 . | 53 . | |||||||
| 02001 | NT | Neg | NA, x2 | Neg | NA | Neg | Neg | NA | |||||||
| 05001 | NT | Neg | Neg | Neg | Neg | Neg | QNS | Off study (1) | |||||||
| 05002 | NT | Neg | QNS, x2 | Neg | QNS | QNS | Neg | Neg | |||||||
| 03001 | NT | Neg | Neg | Neg | Neg | NA | NA | Neg | |||||||
| 05003 | NT | Neg | Neg | NA | Neg | Neg | Neg | Neg | |||||||
| 03002 | NT | Neg* | NA | NA | Neg | Neg | Neg | Neg | |||||||
| 02002 | NT | Neg | Neg† | Neg | Wk 13: Neg | Wk 20: Neg | Neg | Neg | |||||||
| 05004 | NT | Neg | Neg | Neg | Neg* | Neg | Neg | Neg | |||||||
| 03003 | NT | NA | NA | NA | NA | NA | Off study (2) | NA | |||||||
| 05005 | NT | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||||
| 05006 | NT | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||||
| 01001 | NT | Neg | Neg | PosTP | Wk 12: 13, 15: Neg | Neg | Neg | Neg | |||||||
| 05007 | NT | Neg | Neg | NA | Neg | Neg | Neg | Neg | |||||||
. | Week . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject ID no. . | 0 . | 2 . | 6 . | 9 . | 11 . | 17 . | 29 . | 53 . | |||||||
| 02001 | NT | Neg | NA, x2 | Neg | NA | Neg | Neg | NA | |||||||
| 05001 | NT | Neg | Neg | Neg | Neg | Neg | QNS | Off study (1) | |||||||
| 05002 | NT | Neg | QNS, x2 | Neg | QNS | QNS | Neg | Neg | |||||||
| 03001 | NT | Neg | Neg | Neg | Neg | NA | NA | Neg | |||||||
| 05003 | NT | Neg | Neg | NA | Neg | Neg | Neg | Neg | |||||||
| 03002 | NT | Neg* | NA | NA | Neg | Neg | Neg | Neg | |||||||
| 02002 | NT | Neg | Neg† | Neg | Wk 13: Neg | Wk 20: Neg | Neg | Neg | |||||||
| 05004 | NT | Neg | Neg | Neg | Neg* | Neg | Neg | Neg | |||||||
| 03003 | NT | NA | NA | NA | NA | NA | Off study (2) | NA | |||||||
| 05005 | NT | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||||
| 05006 | NT | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||||
| 01001 | NT | Neg | Neg | PosTP | Wk 12: 13, 15: Neg | Neg | Neg | Neg | |||||||
| 05007 | NT | Neg | Neg | NA | Neg | Neg | Neg | Neg | |||||||
ID indicates identification; NT, not tested; pretreatment samples were tested only if provector sequences were detected in posttreatment samples; Neg, negative, NA, no sample available; subject unable or refused to obtain specimen; QNS, quantity of sample obtained was not sufficient for testing; (1), subject discontinued study for personal reasons; (2), subject was noncompliant, refused to provide samples, and withdrew from study; and PosTP, classified as a “transient positive” due to subsequent negative tests. This sample result of 1 positive of 10 replicates tested translates to a transduction frequency estimate of 1 in 3 million cells.
Reduced limit of target sequence detection.
One primer site showed one tenth positive, but second primer confirmed negative.
Persistence of vector sequences
Results of studies to detect the presence of vector in peripheral blood mononuclear cells (PBMCs) of subjects are summarized in Table 3. These studies were performed as allowed by specimen availability, as they were not primary study end points. Through week 29, more than 90% of sample assays showed vector detectable in PBMCs. Of 16 assays on week-53 samples, 12 (75%) were positive for vector. The week-53 samples tested were from 4 subjects who received the 2 lowest doses of vector, including 1 subject at the lowest dose with positive assay results. Persistence of vector sequences even in one subject who received the lowest dose infused supports the conclusion that vector sequences are likely to be present in PBMCs at one year in subjects who received higher doses of the vector construct, hFVIII(V).
Detection of FVIII(V) vector in PBMCs
. | . | Week . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Dose group . | Dose, TU/kg . | 4 . | 13 . | 29 . | 53 . | |||
| 1 | 2.8 × 107 | 12/12 | 12/12 | 10/12 | 4/8 | |||
| 2 | 9.2 × 107 | 4/4 | 7/8 | 11/12 | 8/8 | |||
| 3 | 2.2 × 108 | ND | 8/8 | 12/12 | ND | |||
| 4 | 4.4 × 108 | 4/4 | 12/12 | 4/4 | ND | |||
| 5 | 8.8 × 108 | 4/4 | 4/4 | ND | ND | |||
. | . | Week . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Dose group . | Dose, TU/kg . | 4 . | 13 . | 29 . | 53 . | |||
| 1 | 2.8 × 107 | 12/12 | 12/12 | 10/12 | 4/8 | |||
| 2 | 9.2 × 107 | 4/4 | 7/8 | 11/12 | 8/8 | |||
| 3 | 2.2 × 108 | ND | 8/8 | 12/12 | ND | |||
| 4 | 4.4 × 108 | 4/4 | 12/12 | 4/4 | ND | |||
| 5 | 8.8 × 108 | 4/4 | 4/4 | ND | ND | |||
Numbers represent the number of PCR assays positive over the number of assays performed. When tested, each subject's sample was tested with 4 replicate assays. Thus at week 13 for dose group 1, for example, there were 12 replicate assays, 4 from each of 3 subjects in the group.
PBMC indicates peripheral blood mononuclear cell; ND, assay not performed.
Pharmacokinetic studies
The results for comparison of the baseline and the second 36-hour pharmacokinetic analyses at week 13 after infusion of hFVIII(V) are shown in Table 4.
Comparison of FVIII pharmacokinetic analyses at baseline and at end of phase 1 (week 13 after infusion of vector hFVIII(V))
. | Dose, TU . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | 2.8 × 107, n = 3 . | 9.2 × 107, n = 3 . | 2.2 × 108, n = 2* . | 4.4 × 108, n = 3 . | 8.8 × 108, n = 1 . | Overall, n = 12 . | ||||
| AUC† (% FVIII/h)/(IU/kg) baseline | 39 ± 21 | 40 ± 7 | 59 | 44 ± 9 | 24.7 | 43 ± 14 | ||||
| AUC, wk 13 | 47 ± 26 | 53 ± 3 | 63 | 39 ± 21 | 21.0 | 49 ± 17 | ||||
| % change from baseline‡ | 21.1 | 32.4 | 7.7 | 7.4 | -14.9 | 15.4 (P = .04)§ | ||||
| T1/2, h, baseline | 13.8 ± 3.3 | 16.9 ± 1.5 | 18.5 | 15.9 ± 2.3 | 9.3 | 15.5 ± 3.1 | ||||
| T1/2, h, wk 13 | 16.8 ± 5.2 | 23.5 ± 2.9 | 20.2 | 15.9 ± 0.6 | 11.7 | 18.4 ± 4.7 | ||||
| % change from baseline‡ | 21.9 | 38.9 | 9.3 | -0.3 | 25.8 | 18.6 (P = .02)§ | ||||
| Cmax†, % FVIII/(IU/kg) baseline | 2.1 ± 0.4 | 2.1 ± 0.3 | 2.3 ± 0.2 | 2.4 ± 0.6 | 2.3 | 2.2 ± 0.4 | ||||
| Cmax, wk 13 | 2.2 ± 0.5 | 2.5 ± 0.4 | 2.3 ± 0.3 | 2.6 ± 0.4 | 1.8 | 2.4 ± 0.4 | ||||
| % change from baseline‡ | 4.7 | 21.0 | 0.1 | 10.7 | -20.9 | 7.1 (P = .18)§ | ||||
. | Dose, TU . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | 2.8 × 107, n = 3 . | 9.2 × 107, n = 3 . | 2.2 × 108, n = 2* . | 4.4 × 108, n = 3 . | 8.8 × 108, n = 1 . | Overall, n = 12 . | ||||
| AUC† (% FVIII/h)/(IU/kg) baseline | 39 ± 21 | 40 ± 7 | 59 | 44 ± 9 | 24.7 | 43 ± 14 | ||||
| AUC, wk 13 | 47 ± 26 | 53 ± 3 | 63 | 39 ± 21 | 21.0 | 49 ± 17 | ||||
| % change from baseline‡ | 21.1 | 32.4 | 7.7 | 7.4 | -14.9 | 15.4 (P = .04)§ | ||||
| T1/2, h, baseline | 13.8 ± 3.3 | 16.9 ± 1.5 | 18.5 | 15.9 ± 2.3 | 9.3 | 15.5 ± 3.1 | ||||
| T1/2, h, wk 13 | 16.8 ± 5.2 | 23.5 ± 2.9 | 20.2 | 15.9 ± 0.6 | 11.7 | 18.4 ± 4.7 | ||||
| % change from baseline‡ | 21.9 | 38.9 | 9.3 | -0.3 | 25.8 | 18.6 (P = .02)§ | ||||
| Cmax†, % FVIII/(IU/kg) baseline | 2.1 ± 0.4 | 2.1 ± 0.3 | 2.3 ± 0.2 | 2.4 ± 0.6 | 2.3 | 2.2 ± 0.4 | ||||
| Cmax, wk 13 | 2.2 ± 0.5 | 2.5 ± 0.4 | 2.3 ± 0.3 | 2.6 ± 0.4 | 1.8 | 2.4 ± 0.4 | ||||
| % change from baseline‡ | 4.7 | 21.0 | 0.1 | 10.7 | -20.9 | 7.1 (P = .18)§ | ||||
FVIII value at one hour for subject no. 02002 was treated as an outlier.
Cmax and AUC were normalized for dose of FVIII administered in international units (IU) per kilogram (kg) of body weight.
Calculated as a percent increase from baseline. Calculations were performed prior to rounding off.
P value (2-tail) calculated using a 2-sided paired t test.
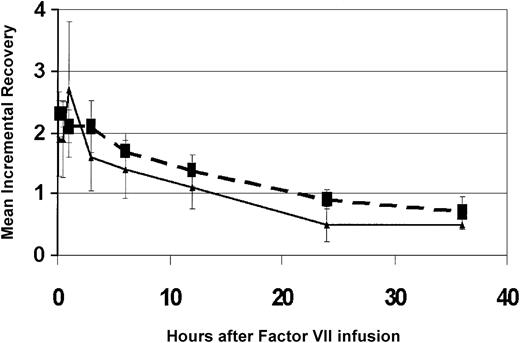
For the 13 subjects as a group, the area under the curve (AUC) of FVIII normalized for dose of FVIII administered (49 ± 17 vs 43 ± 14 [%FVIII/h]/[IU/kg]) and half-life (T1/2) (18.4 ± 4.7 vs 15.5 ± 3.1 hours) was significantly greater for the pharmacokinetic study at week 13 following treatment compared with the baseline pharmacokinetic study. The percent change from baseline in the maximum concentration (Cmax), normalized for dose of FVIII administered for the pharmacokinetic study, showed a trend toward higher levels at week 13 following treatment with hFVIII(V). The FVIII recoveries for the pharmacokinetic analyses for the 3 subjects who received the second dose are shown in Figure 1.
Mean FVIII recovery at week 13 is increased in subjects compared with mean FVIII recovery before hFVIII(V). Data shown are from the 3 subjects who received hFVIII(V) dose no. 2 (9.2 × 107 TU). Dashed line indicates FVIII recovery at week 13. Solid line indicates FVIII recovery at baseline pharmacokinetic study. FVIII incremental recovery is calculated as the percentage FVIII measured in plasma per unit of FVIII concentrate infused per kilogram body weight.
Mean FVIII recovery at week 13 is increased in subjects compared with mean FVIII recovery before hFVIII(V). Data shown are from the 3 subjects who received hFVIII(V) dose no. 2 (9.2 × 107 TU). Dashed line indicates FVIII recovery at week 13. Solid line indicates FVIII recovery at baseline pharmacokinetic study. FVIII incremental recovery is calculated as the percentage FVIII measured in plasma per unit of FVIII concentrate infused per kilogram body weight.
Clinical effects
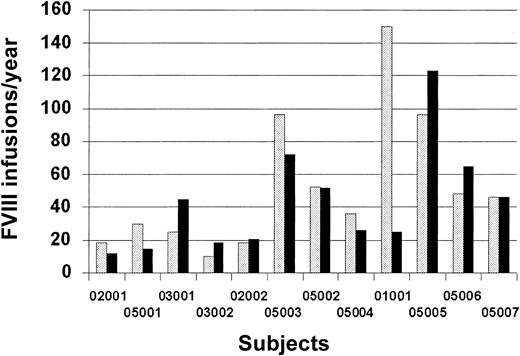
A summary of the treatment FVIII infusions per year before and after hFVIII(V) is represented in Figure 2. The prestudy number of infusions per year was obtained by history and/or clinic treatment records at the time of study enrollment, and the number after hFVIII(V) infusion was obtained prospectively from home diary records of treatments and bleeding episodes. There were 5 subjects who were treated with fewer infusions in the year after receiving the vector infusion than during the study. Subject no. 9 had been treated on prophylaxis 3 times weekly prior to enrollment to prevent recurrent bleeding in his knee. He agreed to forego prophylaxis when he enrolled in the study and bled infrequently after receiving the vector. Overall, however, no significant change in bleeding frequency was seen.
FVIII infusions per year before and after administration of hFVIII(V). The prestudy number of FVIII infusions per year was obtained by history at enrollment; the number after vector infusion was obtained prospectively from home diary records. No data are available for one subject. Hatched bar indicates before hFVIII(V); solid bar, after hFVIII(V).
FVIII infusions per year before and after administration of hFVIII(V). The prestudy number of FVIII infusions per year was obtained by history at enrollment; the number after vector infusion was obtained prospectively from home diary records. No data are available for one subject. Hatched bar indicates before hFVIII(V); solid bar, after hFVIII(V).
Small increases in basal concentrations of factor VIII were observed in some subjects. Of 12 subjects, 8 (66.7%) had detectable FVIII concentrations (> 1%) on 2 or more occasions (at least 5 days following an FVIII infusion) during the 53 weeks after treatment with hFVIII(V) (“responders”) (Table 5). While 5 individuals showed repeated elevations, the others showed only 1 to 3 elevations. The first detectable FVIII activity responses occurred as early as 8 to 10 days after administration of hFVIII(V) and as late as more than 300 days. Chromogenic FVIII assays showed similar levels to the coagulant assays. FVIII antigen levels were similar to activity levels, indicating that the FVIII protein was functional.
FVIII activity responses after hFVIII(V) administration
Dose and subject ID no. . | No. of FVIII values 1% or higher/no. of observations . | Study days . | FVIII activity, %* . | Infusions after hFVIII(V)† . |
|---|---|---|---|---|
| Dose 1 | ||||
| 02001 | 2/23 | 113, 315 | 1.0, 1.8 | Fewer |
| 05001 | 1/8 | 93 | 6.6 | Fewer |
| 05002 | 10/22 | 10-297 | 1.0-3.0 | No change |
| Dose 2 | ||||
| 03001 | 1/35 | 32 | 1.7 | More |
| 03002 | 14/38 | 19-159 | 1.0-1.3 | More |
| 05003 | 5/26 | 9-237 | 1.0-6.2 | Fewer |
| Dose 3 | ||||
| 02002 | 2/6 | 302, 342 | 19, 1.1 | More |
| 03003 | NA‡ | - | ||
| 05004 | 5/15 | 8-211 | 1.1-2.6 | Fewer |
| Dose 4 | ||||
| 01001 | 0/15 | - | - | Fewer |
| 05005 | 2/13 | 33, 44 | 1.0, 1.4 | More |
| 05006 | 4/11 | 40-317 | 1.1-4.4 | More |
| Dose 5 | ||||
| 05007 | 2/37 | 11, 56 | 1.4, 2.1 | No change |
Dose and subject ID no. . | No. of FVIII values 1% or higher/no. of observations . | Study days . | FVIII activity, %* . | Infusions after hFVIII(V)† . |
|---|---|---|---|---|
| Dose 1 | ||||
| 02001 | 2/23 | 113, 315 | 1.0, 1.8 | Fewer |
| 05001 | 1/8 | 93 | 6.6 | Fewer |
| 05002 | 10/22 | 10-297 | 1.0-3.0 | No change |
| Dose 2 | ||||
| 03001 | 1/35 | 32 | 1.7 | More |
| 03002 | 14/38 | 19-159 | 1.0-1.3 | More |
| 05003 | 5/26 | 9-237 | 1.0-6.2 | Fewer |
| Dose 3 | ||||
| 02002 | 2/6 | 302, 342 | 19, 1.1 | More |
| 03003 | NA‡ | - | ||
| 05004 | 5/15 | 8-211 | 1.1-2.6 | Fewer |
| Dose 4 | ||||
| 01001 | 0/15 | - | - | Fewer |
| 05005 | 2/13 | 33, 44 | 1.0, 1.4 | More |
| 05006 | 4/11 | 40-317 | 1.1-4.4 | More |
| Dose 5 | ||||
| 05007 | 2/37 | 11, 56 | 1.4, 2.1 | No change |
NA indicates not available.
Reproducibility in the central laboratory, performed repeatedly on plasma samples from severe hemophilia A subjects, was X = 0.40, SD = 0.008, and CV = 1.99%.
Comparison of number of treatments with factor VIII during the year prior to administration of hFVIII(V) with the number of treatments during the year after administration. Subject no. 05004 was receiving prophylaxis 3 times per week prior to entry on study.
Subject dropped out of the study for personal reasons and was noncompliant with FVIII assays and treatment records.
There was no correlation between time to first detectable FVIII activity response and dose of hFVIII(V) administered. In addition, no correlation was seen between FVIII activity responses and the individual pharmacokinetic results for incremental recovery of factor VIII activity, AUC, or the half-life of infused factor VIII. During the year following administration of hFVIII(V), 5 subjects reported decreased use of factor VIII infusions. This apparent clinical response of less need for treatment did not correlate with administered dose of hFVIII(V) or with improved pharmacokinetic parameters. None of the doses tested resulted in FVIII concentrations higher than 7% in at least 75% of blood samples over a 12-week period in any of the subjects, one of the original goals of the trial based on human FVIII protein levels achieved in rabbits.
Discussion
This phase 1, multicenter, dose-escalation, FVIII gene therapy trial in adult hemophilia A patients used for the first time intravenous peripheral vein infusion of a retroviral-based vector construct. The results of this study demonstrate the clinical and biologic safety for this approach using hFVIII(V), a Moloney murine leukemia virus–based vector containing the gene for B-domain–deleted human factor VIII. The findings confirm the safety profiles seen in the animal studies using this vector construct. The vector was well tolerated and no adverse effects were detected after a year's follow-up. There were small clinical benefits, in some subjects, suggesting that further testing of this vector using alternative dose schedules to optimize FVIII protein expression is feasible and warranted.
Integration of hFVIII(V) into PBMCs was demonstrated by PCR in the majority (> 80%) of all specimens tested prior to week 53 and in 50% of the specimens tested at 53 weeks. Although vector could be detected in the majority of PBMC specimens, FVIII activity unrelated to exogenous treatment was low and transient. We suspect that, while the amount of protein was insufficient to cause a sustained measurable increase in the peripheral blood, enough vector-generated FVIII protein was produced to result in these borderline, intermittently measurable levels. Previous studies using retroviral vector transduction of autologous fibroblasts in large animals have reported long-term persistence of the vector sequences but decreased expression of the transgene product.29-31 The mechanisms involved in loss of transgene expression despite persistence of vector sequences in those studies, as well as in the current human trial, have not been elucidated.
Of 12 subjects who were on study for at least 3 months, 9 had detectable FVIII activity (> 1%) on 2 or more occasions (range of maximum levels, 1.1%-19%). These FVIII levels were all measured at least 5 days after the last infusion of exogenous FVIII, according to infusion records from home diaries. The home diaries were collected weekly during the first 3 months of study and monthly throughout the first year in order to encourage timely and accurate reporting of home treatment. Nonetheless, it is possible that treatment records were not always completely accurate and that at least some FVIII levels measured could have been the result of residual exogenous FVIII from an unreported infusion. Although the FVIII levels were typically low and often transient, some responders reported fewer bleeding episodes, fewer courses of treatment for bleeding, and fewer units of FVIII product treatment while on-study compared with the prior year's treatment. However, none of these apparent trends for responders was statistically significant compared with nonresponders.
Alteration of the vector to optimize transcription of the integrated gene may increase the yield of FVIII protein in future studies. Another possible approach would be to administer repeated hFVIII(V) doses separated by longer intervals, in order to increase the likelihood of exposure to additional replicating cells, since retroviral (nonlentivirus) vectors preferentially enter the cell nucleus during mitotic cell division.32,33 Repeated doses over time may result in a higher number of cells being transduced, if more or different cell populations are in cell cycle at the time of vector infusion, but this hypothesis requires further study for confirmation.
There was no evidence for a dose-response relationship between dose of vector administered and FVIII levels subsequently, suggesting that increasing the amount of hFVIII(V) used in a single dose would not be particularly useful, although the lack of a dose response may simply indicate that the current doses are near a therapeutic threshold. At least 2 potential avenues for further study may be promising. Since retroviral constructs preferentially transduce dividing cells, several laboratories are studying the role for growth factors to stimulate transiently the number of cells in cell cycle.34,35 Alternatively, more frequent administration of hFVIII(V) (eg, one day each 2-3 weeks) might also allow additional newly cycling cells to be transduced.
Although clinical efficacy was not a primary objective in this phase 1 trial, the historical data on past bleeding frequency from hemophilia center records were compared with frequency measured prospectively after vector administration. Methods of past record keeping varied among sites and individual subjects. More uniform and reliable data could be collected in future studies by incorporating a run-in period prior to vector administration to collect baseline bleeding frequency. However, even with the best record keeping, treatment frequency in adults is not an ideal efficacy parameter because individuals with pre-existing musculoskeletal damage are highly susceptible to bleeding and would be unlikely to show significant decreases in frequency due to low levels of circulating FVIII. In contrast, young children or other individuals with normal joints and muscles have shown reduced bleeding with FVIII levels as low as 1% to 3% and would be the ideal population for a later phase gene transfer trial intended to demonstrate efficacy.36 For current early phase trials such as this study, however, FVIII activity levels in the blood remain the most useful efficacy surrogate.
One possible risk associated with gene transfer is that presentation of a normal transgene product through major histocompatibility complex (MHC) class I mechanisms might lead to the formation of antibodies to the transgene product.6,31 No FVIII inhibitory activity was demonstrable in this study by either direct measurement or by pharmacokinetics of administered FVIII product. In fact, availability of FVIII product at the end of phase 1 of the study (week 13) was greater than at baseline. This finding may be explained by a saturation of FVIII binding sites37,38 due to low-level FVIII protein production due to the vector.
There were no adverse effects related to administration of hFVIII(V), other than mild infusion-related symptoms. It is likely that the single, transient, very low-level positive signal in one semen specimen at week 29 in this study was a false-positive result. All preceding and subsequent samples in the subject were negative, and, in rabbit studies there was no evidence for vector transmission to the germ line with hFVIII(V).11 There appeared to be no adverse effects of hFVIII(V) infusion on the clinical course of HIV or HCV infection.
This was the first human study in which a retroviral vector was administered into a peripheral vein. The benign safety profile seen in this study is consistent with past experience in more than 1000 subjects who have received gene transfer mediated by retroviral vectors with no adverse consequences related to random integration. Nonetheless, the need for continued safety monitoring of these patients is underscored by a recent report to regulatory agencies of a leukemic syndrome developing in a child who had received ex vivo retroviral gene transfer in a French clinical trial.39 The etiology of this event is being evaluated, and a full published report is not yet available. However, it is believed that the leukemia is linked to the integration of the therapeutic vector near a cellular gene that was then overexpressed. Recently, a second child also experienced a similar adverse event.40 Although both the French clinical trial and this trial used vectors based on retroviral constructs, there are major differences between the vectors, the methods of delivery, and the subjects. In particular, the French trial used ex vivo transduction of highly enriched hematopoietic stem cells with very high vector to cell ratios, and the subjects were young children with severe immunodeficiency.41 For the current trial with FVIII(V), a follow-up protocol has been in place for annual visits to the clinic by each subject to provide annual reports on long-term safety to appropriate regulatory agencies. The follow-up protocol is being amended, per recent FDA request, to biannual visits for the first 5 years after vector administration.
Much remains to be learned. As with other human gene transfer trials for hemophilia using different techniques and approaches,42,43 it appears that animal studies may not accurately predict the doses of vector preparations required to achieve therapeutic expression of FVIII or FIX in humans.
In summary, this phase 1 trial using intravenous infusion of hFVIII(V) demonstrates that hFVIII(V) is safe at the doses and route of administration used, persists in PBMCs as long as one year, is associated with measurable factor VIII concentrations in some individuals, and with increased available plasma factor VIII after infusion of exogenous factor VIII without the development of inhibitors. This excellent safety profile and the potential clinical benefits suggest that further testing of this vector is warranted.
Prepublished online as Blood First Edition Paper, May 22, 2003; DOI 10.1182/blood-2003-01-0167.
Supported by a grant from Chiron. The work at UNC and UP was supported in part by grants (RR00046, RR00056) from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health.
Several of the authors (T.E.M., V.C., S.R.-G., H.R., N.S., D.J.J., D.H.) were employed by a company (Chiron Corporation) whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to acknowledge the invaluable assistance of the study coordinators at the sites: Janet A. Harrison, Kristin Jaworski, Aime Grimsley, and Brenda Nielsen. Many individuals at Chiron and the Chiron Center for Gene Therapy participated in the development, manufacturing, preclinical testing, and other preparation and support for this clinical trial, including: Lin Fei, Donald Gay, Judy Greengard, Carlos Ibanez, Martha Leibbrandt, Biao Liu, Paula Stemler, Edgar Kamantigue, Dale Johnson, and Biff Owen.
Patients were enrolled and treated at the following sites: University of California at Davis, Sacramento, CA; University of Pittsburgh, General Clinical Research Center, PA; University of North Carolina, Chapel Hill; and Wayne State University, Detroit, MI.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal