Abstract
Cyclic neutropenia is a rare disease that occurs both in humans and gray collie dogs and is characterized by recurrent severe neutropenia leading to bacterial infections and shortened life expectancy. Daily injections of recombinant granulocyte colony-stimulating factor (rG-CSF) are effective in shortening the period of severe neutropenia and reducing infections. After demonstrating that rG-CSF induced elevated neutrophil production in an affected dog, cytokine administration was stopped and 109 infectious units (IUs) of a lentivirus pseudotyped with vesicular stomatitis virus G protein (VSV-G) encoding canine G-CSF cDNA was administered intramuscularly. Serial blood cell counts showed elevated neutrophil production for longer than 17 months. Although neutrophil counts continued to cycle, the range at nadirs was from 3710 to 5300 cells/μL, well above the nadirs before lentivirus administration. After the injection of lentivirus, mean neutrophil counts ± SD were 12 460 ± 4240 cells/μL, significantly increased over both pretreatment values of 3040 ± 2540 cells/μL (P < .0001) and neutrophil counts during G-CSF administration of 10 290 ± 4860 cells/μL(P < .007). The changes in blood counts from lentivirus injection were associated with absence of clinical signs of infection and fever. The gray collie continued to gain weight and was no longer housed in a pathogen-free environment. Genomic DNA from muscle at injection sites was positive for provirus, whereas gonad, lung, spleen, heart, liver, kidney, leukocytes, and noninjected muscle samples were all negative for provirus. Thus, intramuscular administration of lentivirus encoding G-CSF provided sustained therapeutic levels of neutrophils, suggesting this approach may be applied for long-term treatment of patients with cyclic and other neutropenias.
Introduction
Cyclic neutropenia is a rare disease that occurs both in man and gray collie dogs. In collie dogs, cyclic hematopoiesis is inherited as an autosomal recessive disease.1,2 The dogs have 12- to 14-day cyclic variations in blood neutrophils, monocytes, lymphocytes, eosinophils, reticulocytes, and platelets due to periodic fluctuations in blood cell production by the bone marrow.1,2 The recurrent severe neutropenia leads to bacterial infections and shortened life expectancy. The disorder can be cured by bone marrow transplantation from a normal dog to a gray collie as well as transferred from a gray collie to a normal littermate.3,4 This transplantability strongly supports the concept that this is a disease of defective regulation of hematopoietic stem cells.
Gray collie dogs with cyclic hematopoiesis have been successfully treated with granulocyte colony-stimulating factor (G-CSF).5,6 The severe recurrent neutropenia in gray collie dogs was not abrogated by in vivo interleukin 3 (IL-3) or granulocyte-macrophage colony-stimulating factor (GM-CSF) treatment.5 In contrast, G-CSF prevented the recurrent neutropenia and obliterated periodic fluctuation of monocyte, eosinophil, reticulocyte, and platelet counts.5,6 Furthermore, long-term daily administration of G-CSF to 3 gray collies for up to 24 months provided effective therapy without any loss of hematopoietic response.6,7 Recently, a genetic defect in neutrophil elastase has been identified as the cause of human cyclic neutropenia.8,9 It is not known if the same genetic defect occurs in gray collie dogs.
Human cyclic neutropenia is a rare, autosomal-dominant disease usually discovered in childhood.10 In almost all patients, recurrent severe neutropenia occurs every 19 to 21 days producing a typical clinical syndrome of fever, malaise, aphthous stomatitis, and cervical lymphadenopathy. Serious and even life-threatening infections may accompany the neutropenic periods and a number of deaths from bacterial infections have occurred.10 This disease has been shown to be transplantable.11 Human12 and canine (Boone T, Souza L, unpublished data, 1989) G-CSF cDNAs have been cloned and recombinant human G-CSF has been used to treat patients with severe neutropenias,13-16 including cyclic neutropenia.17 In humans, treatment of cyclic neutropenia with daily subcutaneous injections of recombinant human G-CSF is very effective in shortening the period of severe neutropenia (ie, to reduce the number of days per cycle with absolute neutrophil counts < 500 per μL) and reducing infections.13,17 On chronic treatment, even for several years, oscillations in blood neutrophils continue with a greater amplitude than before treatment and with a shorter period length (ie, a shift in cycle length from 21 to 14 days).13,14,17 Oscillations in other blood cells, if detectable before therapy, generally persist with recombinant G-CSF treatment.
Although the precise cause of canine cyclic hematopoiesis remains unknown, gray collies provide an ideal model system for the treatment of defective hematopoiesis by gene therapy. Delivery of canine G-CSF to gray collie dogs by lentivirus vectors has the potential to treat their recurrent neutropenia. The recently described lentivirus vectors have the advantage over murine leukemia viral vectors of enabling provirus integration into nondividing cells.18-23 Lentivirus vectors are commonly pseudotyped with envelope glycoprotein G from vesicular stomatitis virus (VSV-G).18-20,24-27 Two major benefits conferred by VSV-G pseudotyping are a broad tropism and a more robust virus that can be concentrated by centrifugation. The incorporation of a central polypurine tract (cPPT)28-30 and a posttranscriptional regulatory element (PRE)31,32 into lentivirus vectors has shown increased transduction efficiency and transgene expression.33 We generated lentivirus encoding canine G-CSF and incorporated these regulatory elements to investigate treatment of a collie dog with cyclic neutropenia.
Materials and methods
Construction and packaging of vectors
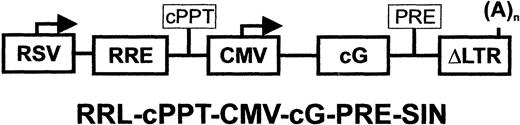
The expression plasmid pRRL-cPPT-CMV-cGCSF-PRE-SIN (Figure 1) was constructed by inserting the canine G-CSF cDNA34 into the multiple-cloning site (X) of pRRL-cPPT-CMV-X-PRE-SIN.33 The parent vector pRRL-CMV-GFP-SIN was a generous gift of Drs Zufferey and Trono (University of Torino, Italy). Lentivirus encoding enhanced green fluorescent protein (eGFP), pRRL-cPPT-CMV-eGFP-PRE-SIN, was used as a control.33 Lentivirus packaging was performed by transient transfection of 293T cells.33 The day prior to transfection, confluent 15-cm plates of 293T cells were split 1:5. The pRRL-based lentiviral vectors were generated by calcium phosphate cotransfection of the transfer vector, the HIV gag/pol packaging construct, the HIV rev expression plasmid, and the VSV-G expression plasmid35 into 293T cells as we have previously described.33,36 Briefly, for each 15-cm diameter dish, 23 μg transfer vector, 15 μg pMDL-g/p-RRE packaging plasmid, 11.5 μg pRSV-REV, and 8 μg pCMV–VSV-G envelope were mixed. The DNA was resuspended in 450 μL 0.1 × TE (1 mM Tris, pH 8.0; 0.1 mM EDTA [ethylenediaminetetraacetic acid]) and 50 μL 2.5 M CaCl2 was added and the mixture was incubated at room temperature for 10 minutes. The DNA/CaCl2 solution was added dropwise to 500 μL (2 ×) HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered saline under vigorous bubbling, and once slightly turbid, the solution was immediately added to the cells. All transfections proceeded for 16 hours followed by media replacement and virus collection 48 hours later. Viral supernatant from 500 plates was filtered through 0.2-μm–pore filters and stored at 4°C. Virus was concentrated by ultracentrifugation for 1.5 hours at 22 000 rpm in a Beckman SW 28.1 rotor (Beckman Coulter, Hialeah, FL). Batches of 6 × 16 mL were processed and the virus was pooled. Virus pellets were resuspended in 5 mL phosphate-buffered saline (PBS) and centrifuged for 2 hours at 35 000 rpm in a TL100 tabletop ultracentrifuge with a relative centrifugal force (RCF) of 55 000g. The final pellet was resuspended in Tris-buffered saline (TBS) and stored at –80°C.
Schematic representation of lentiviral vector. CMV indicates cytomegalovirus immediate early promoter; cG, canine granulocyte colony-stimulating factor cDNA; ΔLTR, long terminal repeat with majority of U3 region deleted; RSV, Rous Sarcoma Virus enhancer/promoter; RRE, rev responsive element; cPPT, HIV-1 central polypurine tract; and PRE, hepatitis B virus posttranscriptional regulatory element.
Schematic representation of lentiviral vector. CMV indicates cytomegalovirus immediate early promoter; cG, canine granulocyte colony-stimulating factor cDNA; ΔLTR, long terminal repeat with majority of U3 region deleted; RSV, Rous Sarcoma Virus enhancer/promoter; RRE, rev responsive element; cPPT, HIV-1 central polypurine tract; and PRE, hepatitis B virus posttranscriptional regulatory element.
Virus titer
Lentiviruses encoding canine G-CSF were assayed for virus p24 Gag content and expressed as infectious units per mL by comparison with the eGFP virus titer determined by flow cytometry. This method is based on the assumption that the frequency of functional viral particle production is essentially the same for all preparations.21,37,38 Virus titer for eGFP lentivirus was determined by infection of HeLa cells followed 3 days later by fluorescence activated cell sorting (FACS) analysis.33,36 Briefly, 5 × 104 HeLa cells were plated in 6-cm dishes and virus from pRRL-cPPT-CMV-eGFP-PRE-SIN vector was serially titrated on duplicate plates in Dulbecco modified Eagle medium (DMEM), 10% fetal calf serum (FCS), 2 mM glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin, in the presence of 10 μg/mL diethylaminoethyl (DEAE) dextran. After 16 hours, the media was replaced and the plates were incubated for a further 48 hours. Plates were then trypsinized and, after washing 3 times in PBS-5% FCS and fixing in 4% paraformaldehyde, samples were analyzed by flow cytometry using a Becton Dickinson FACS+ instrument (Becton Dickinson, Franklin Lakes, NJ). Quantification of transduction by eGFP-encoding virus was performed using untransduced cells to set the negative control gates for fluorescein isothiocyanate (FITC). Data were analyzed using Cell Quest software (Becton Dickinson). Viral p24 Gag protein was determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Beckman Coulter) and compared with titers of eGFP virus obtained by FACS analysis. This assay showed a functional titer of 2.5 × 106 infectious units (IUs) per mL for eGFP virus and this was equivalent to 1100 ng of p24 protein per mL.
Screening for replication competent virus
Virus preparations were screened for replication-competent virus using HeLa MAGI cells.33,39,40 These cells have a genomic β-galactosidase reporter gene under transcriptional regulation of the HIV-1 long terminal repeat (LTR) such that wild-type HIV Tat activity activates the reporter gene in cells infected by virus-encoding wild-type tat gene product.39 In brief, 5 × 104 HeLa MAGI cells were plated in 6-cm dishes one day prior to assay. On day 2, the medium was replaced and 1 mL, 100 μL, or 10 μL of supernatant from test samples was added to the plate in the presence of 10 μg/mL DEAE dextran. After 16 hours of incubation, the medium was replaced and the cells cultured for a further 24 hours. Cells were then fixed and stained for β-galactosidase using X-gal substrate (Sigma, St Louis, MO). Cells were scored positive if there was visible blue staining in the cytoplasm after 4 to 16 hours at 37°C compared with the staining of untransduced controls over the same time period.41 In addition, as an indicator of replication-competent virus, the supernate of serially passaged transduced cells was screened for p24 Gag protein using a specific ELISA.42
G-CSF production in vitro
For cell transduction, a viral equivalency of 2 ng of p24 Gag protein was used to infect 5 × 103 HeLa cells in the presence of 10 μg/mL DEAE dextran, giving a multiplicity of infection (MOI) of about 1. After infection for 16 hours, the medium was changed and cells were cultured for another 48 hours. Then, cells encoding eGFP were harvested for FACS analysis, and supernate was harvested from cells transduced to express G-CSF to measure cytokine secretion. Bioactivity of virally expressed canine G-CSF was monitored using a murine cell line, NFS-60, that proliferates in response to G-CSF.43 Recombinant canine G-CSF (Amgen, Thousand Oaks, CA) was used to construct a proliferation-response curve with murine NFS-60 cells.43,44 Proliferation was assayed by a commercial dye production assay (Cell Titer 96; Promega, Madison, WI). In brief, NFS-60 cells seeded at a concentration of 105 cells/well in 96-well microtiter plates were cultured for 24 hours at 37°C, 5% CO2. Limiting dilutions of standards, unknowns, and controls were added to the cells to a final volume of 200 μL and the cells were incubated for a further 72 hours. To measure the proliferation, a 15-μL dye solution was added, cells were incubated for further 4 hours, and then the reaction was stopped with 100 μL of stop solution. Proliferation was recorded as dye absorbance at 570 nm using a BioRad ELISA plate reader (Hercules, CA). G-CSF expression was 8 ng/24 hours/107cells from transduced smooth muscle cells. These assays indicated expression of a bioactive gene product from the lentivirus vector.
Dog care
Procedures involving animals were approved by the Animal Care and Use Committee of the University of Washington. Before lentivirus treatment, the dog was housed in a modified pathogen-free facility. However, after successful treatment with G-CSF lentivirus the dog was placed in regular housing. Lentivirus was administered to each hind leg by multiple injections to thigh muscle. The dog showed no ill effects to the virus injections (ie, lack of fever, stiffness, and soreness).
Genomic DNA isolation and detection of lentivirus vector
At 522 days after lentivirus administration the dog was killed by an overdose of barbiturate and tissues were harvested immediately and fixed in neutral buffered formalin for histologic examination or stored at –80°C for provirus assay. To reduce risk of contamination, fresh sterile instrument sets were used for each tissue, and collection started with gonads and finished with muscle injection sites. Genomic DNA was isolated from frozen solid tissue samples using the DNeasy Tissue kit (Qiagen, Valencia, CA) following the manufacturer's instructions. Harvested solid tissues were the injection site in the hind leg muscle, front leg muscle, spleen, kidney, liver, testes, heart, and lung. Genomic DNA from leukocytes was isolated from whole blood using the FlexiGene DNA kit (Qiagen) following the manufacturer's instructions. As a positive control, genomic DNA was isolated from HeLa cells that had been transduced with the same virus administered to the dog (MOI < 1) using the DNeasy Tissue kit.
In order to establish the integrity of DNA, the polymerase chain reaction (PCR) was used to amplify a 209-bp fragment from the canine β-actin gene. PCR was carried out with 250 ng of template DNA in a 50-μL reaction volume using HotStarTaq DNA polymerase (Qiagen) and the primers 5′-TTCAACACCCCAGCCATGTA-3′ (forward) and 5′-CGCTCCGTGAGGATCTTCAT-3′ (reverse). Reactions were incubated for 15 minutes at 95°C followed by 35 cycles of 94°C for 45 seconds, 59°C for 45 seconds, and 72°C for 1 minute. Amplification of the 1066-bp vector band was also performed with HotStarTaq DNA polymerase using a forward primer to the cPPT region (5′-GATAAGCTTGGGAGTTCCGCGTTACATA-3′) and a reverse primer that spans the junction of exons 3 and 4 of the canine G-CSF cDNA (5′-GATCTTCCATCTGCTGCCAGATGTTG-3′). Reaction volumes were 50 μL and were incubated for 15 minutes at 95°C followed by 35 cycles of 94°C for 45 seconds, 56°C for 30 seconds, and 72°C for 1 minute and 30 seconds. A competitive titration of vector was achieved by adding 10 to 0.01 fg of vector DNA to 500 ng of kidney genomic DNA that had been previously determined to be negative for integrated vector. For analysis of PCR reactions, 10 μL was run on a 1.2% Seakem LE (BioWhitaker Molecular Applications, Rockland, ME) agarose gel and stained with ethidium bromide.
Southern blot analysis of vector PCR reactions was performed using the 32P-labeled 1066-bp lentiviral vector PCR product as a probe. The blot was exposed to Biomax MS film (Eastman Kodak, Rochester, NY) for 1 hour at room temperature. The sensitivity of this blot was between 0.01 and 0.1 fg of vector DNA, which is 1 and 10 vector copies, respectively.
Histologic analysis
Tissues for histologic analysis were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Bone was decalcified prior to embedment. All major tissues were evaluated with special attention paid toward assessment of myelopoietic activity.
Lentivirus-mediated G-CSF expression in vivo
Results
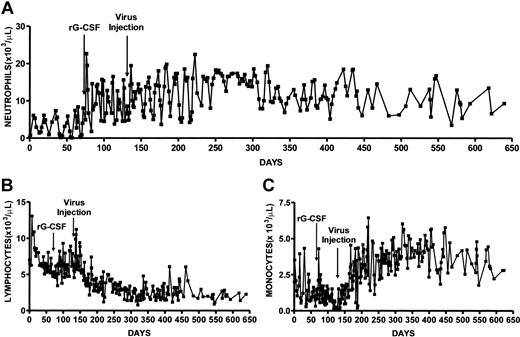
The affected male dog was recognized as a gray collie at birth by his silver-gray coat color and lack of growth in comparison to his littermates. When the dog was weaned at 7 weeks of age he was admitted to our institute and serial blood counts were monitored (Figure 2; Table 1). These data showed that the neutrophil counts were cycling with a periodicity of 13 to 14 days and showed minimum and maximum neutrophil counts of less than 100 and 8430/μL, respectively, and a mean ± SD of 3040 ± 2540/μL (Figure 2A; Table 1). The high SD is a reflection of the large fluctuations in neutrophil production over each cycle. The WBC count showed essentially the same periodicity and larger changes in amplitude than the neutrophils (not shown), and we observed minimum and maximum WBC count of 5900 and 19 800/μL, respectively, and a mean ± SD of 11 720 ± 3120 counts/μL (Table 1). During the period of no treatment, monocyte counts were cycling regularly with a periodicity of 13 to 14 days (Figure 2B). Monocyte numbers had a mean ± SD of 1440 ± 950/μL and minimum and maximum values of 280 and 4320/μL, respectively. Platelet values were 432 000 ± 88 000/μL and showed minimum and maximum counts of 241 000 and 652 000/μL, respectively. Lymphocyte counts also cycled with a periodicity of 13 to 14 days, trended downwards, and showed minimum and maximum counts of 4720 and 13 070/μL, respectively (Figure 2C; Table 1).
Serial blood cell counts of gray collie before and after treatment with rG-CSF and lentivirus. After confirming that neutrophil counts were cycling we administered recombinant canine G-CSF at 1.5 μg per kg per day (day 71). On day 131, G-CSF administration was stopped and 109 IUs of lentivirus encoding canine G-CSF was injected intramuscularly. Shown are absolute neutrophil counts (A), lymphocytes (B), and monocytes (C).
Serial blood cell counts of gray collie before and after treatment with rG-CSF and lentivirus. After confirming that neutrophil counts were cycling we administered recombinant canine G-CSF at 1.5 μg per kg per day (day 71). On day 131, G-CSF administration was stopped and 109 IUs of lentivirus encoding canine G-CSF was injected intramuscularly. Shown are absolute neutrophil counts (A), lymphocytes (B), and monocytes (C).
Analysis of blood cell counts before and after treatment with rG-CSF and G-CSF lentivirus
Treatment . | Neutrophils, 103/μL . | WBC, 103/μL . | Monocytes, 103/μL . | Lymphocytes, 103/μL . | Platelets, 103/μL . | Hematocrit, % . |
|---|---|---|---|---|---|---|
| None, n = 36 | 3.04 ± 2.54 | 11.72 ± 3.12 | 1.44 ± 0.95 | 6.88 ± 1.80 | 432 ± 88 | 31.7 ± 2.0 |
| rG-CSF, n = 38 | 10.29 ± 4.86* | 18.61 ± 5.53* | 1.06 ± 0.82† | 6.20 ± 1.43‡ | 325 ± 58* | 32.7 ± 1.8† |
| Lentivirus, n = 170 | 12.13 ± 4.26* | 19.20 ± 4.46* | 3.20 ± 1.37* | 3.42 ± 1.87* | 317 ± 48* | 39.6 ± 3.3* |
Treatment . | Neutrophils, 103/μL . | WBC, 103/μL . | Monocytes, 103/μL . | Lymphocytes, 103/μL . | Platelets, 103/μL . | Hematocrit, % . |
|---|---|---|---|---|---|---|
| None, n = 36 | 3.04 ± 2.54 | 11.72 ± 3.12 | 1.44 ± 0.95 | 6.88 ± 1.80 | 432 ± 88 | 31.7 ± 2.0 |
| rG-CSF, n = 38 | 10.29 ± 4.86* | 18.61 ± 5.53* | 1.06 ± 0.82† | 6.20 ± 1.43‡ | 325 ± 58* | 32.7 ± 1.8† |
| Lentivirus, n = 170 | 12.13 ± 4.26* | 19.20 ± 4.46* | 3.20 ± 1.37* | 3.42 ± 1.87* | 317 ± 48* | 39.6 ± 3.3* |
Data expressed as means ± SDs.
P < .0001.
P < .05.
P < .1.
After establishing that the neutrophil counts of the gray collie dog were cycling, recombinant canine G-CSF (rG-CSF; 1.5 μg/kg per weekday; initial weight 11.4 kg) was administered subcutaneously for 2 months. Serial blood counts showed significant increases in neutrophil numbers both at the nadirs and the peaks of cycles (Figure 2A). Although his neutrophil counts continued to cycle, the lowest number was 1820 cells/μL that occurred 6 days after starting rG-CSF therapy and subsequently the minimum counts were quite consistent and varied only from 4800 to 5000 cells/μL (Figure 2A). The maximum neutrophil numbers were 22 670 cells/μL. The mean neutrophil counts were 10 290 ± 4860 cells/μL and were significantly increased over pretreatment period (P < .0001; Table 1). WBCs had a mean ± SD of 18 610 ± 5530 cells/μL with minimum and maximum values of 7000 and 28 700 cells/μL. The mean WBC count was significantly elevated over values recorded during nontreatment (P < .0001; Table 1). Monocytes showed a decline from 1440 ± 950 to 1060 ± 820 cells/μL (P < .05; Table 1). During rG-CSF treatment, lymphocyte counts and platelet values decreased significantly (P < .1 and P < .05, respectively; Table 1). Hematocrit values increased from 31.7% ± 2.0% to 32.7% ± 1.79% (P < .05). During the period when rG-CSF was administered the gray collie dog was healthy and his weight increased to16.2 kg.
Following the demonstration that rG-CSF induced elevated neutrophil production in the affected dog, cytokine administration was stopped and we administered intramuscularly 109 IUs lentivirus particles encoding canine G-CSF cDNA at a dose of about 108 IU/kg. We calculated this lentivirus dose from data obtained from rats administered an analogous lentivirus encoding rat G-CSF intramuscularly (data not shown). Lentivirus was administered in a total volume of 6 mL of two 3-mL aliquots to each hind leg by multiple injections to muscle. Serial blood cell counts showed elevated neutrophil counts for over 17 months (Figure 2). However, neutrophil counts continued to cycle with a range at nadirs of 3710 to 5300 cells/μL and an average cell number at nadirs of 5000 cells/μL. The minimum and maximum neutrophil numbers were 3710 and 22 440 cells/μL and mean neutrophil counts were 12 130 ± 4260 cells/μL, significantly increased over no-treatment values (P < .0001). The mean neutrophil value is about 2-fold higher than normal dog neutrophil counts of 6000 ± 1800 cells/μL.45 Furthermore, the mean neutrophil counts following lentivirus administration were significantly increased over cell numbers recorded during rG-CSF administration (P < .007). The periodicity of neutrophil counts following lentivirus treatment averaged 13 days and was more regular than observed during rG-CSF administration (Figure 2A). After 8 months when the dog continued to be well, the frequency of blood cell monitoring was lowered and cell cycling was not readily evaluated.
Mean WBC counts were 19 200 ± 4460 cells/μL, significantly elevated over pretreatment (P < .0001) and showed minimum and maximum counts of 10 300 and 30 900 cells/μL. Normal dog WBC values are 9800 ± 2400 cells/μL, 2-fold lower than WBCs after lentivirus treatment. Monocyte counts were more than 2-fold elevated after lentivirus administration in comparison to pretreatment values (P < .0001) and in contrast to G-CSF treatment where we observed a decrease in monocytes (Table 1). The monocyte counts rose gradually over the first 70 days after virus administration and then were relatively constant (Figure 2B). The lentivirus-treated dog showed mean monocyte counts of 3200 ± 1370 cells/μL, a more than 5-fold increase over normal dog monocyte counts of 580 ± 210 cells/μL.45 There have been previous reports of much smaller monocyte increases after rG-CSF administration to both patients and normals.46,47 Lymphocyte numbers were significantly reduced after lentivirus administration in comparison to both pretreatment and G-CSF treatment, although the largest decrease followed lentivirus administration (Table 1). During the initial 40 days after virus administration there was a decline in lymphocyte numbers and then nearly constant lymphocyte counts throughout the remaining monitoring period (Figure 2C). However, the mean value of 3610 ± 1910 cells/μL was within the range of 2600 ± 800 cells/μL recorded for normal dogs.45
Treatment of the dog with G-CSF and with lentivirus decreased the platelet counts compared with the pretreatment level (Table 1), similar to the decline in humans with chronic neutropenia on treatment with G-CSF.13 The platelet values we observed after both forms of treatment were within normal limits of 367 000 ± 100 000 cells/μL described for normal dogs.45 The mean hematocrit value after lentivirus administration was 39.3% ± 3.3%. Although this value was significantly increased over the mean pretreatment hematocrit values, this change is probably attributable to the increasing maturity of the dog. Normal dog hematocrit is 35.4% ± 0.7% and this is between the values we observed for rG-CSF and lentivirus treatment of 32.7% and 39.3%, respectively. Also, the hematocrit values increased in a steady continuous process rather than a marked increase in response to lentivirus injection (data not shown).
The lentivirus injection was not associated with clinical signs of inflammation, infection, or fever and after treatment with lentivirus the dog had few problems with recurrent fevers or infections and had no need for antibiotic treatments. After lentivirus administration, the dog continued to grow and gain weight to 29 kg and was no longer housed in a pathogen-free environment. Despite this improvement, at about 17 months after lentivirus administration, the dog developed progressive renal failure with anorexia, weakness, and elevated blood urea nitrogen and creatinine. These abnormalities persisted and the decision was reached that it was best to kill the dog.
Histologic evaluation of tissues confirmed the presence of renal failure due to severe renal glomerular amyloidosis, the most frequent cause of death in the gray collies before and since the availability of canine G-CSF.48,49 At autopsy, other than mild, scattered, intestinal amyloidosis, tissues were within normal limits. Granulopoiesis, as evident by clusters of immature and mature polymorphonuclear cells, was present within marrow cavities of long bones and the skull and multifocally within the red pulp of the spleen (data not shown).
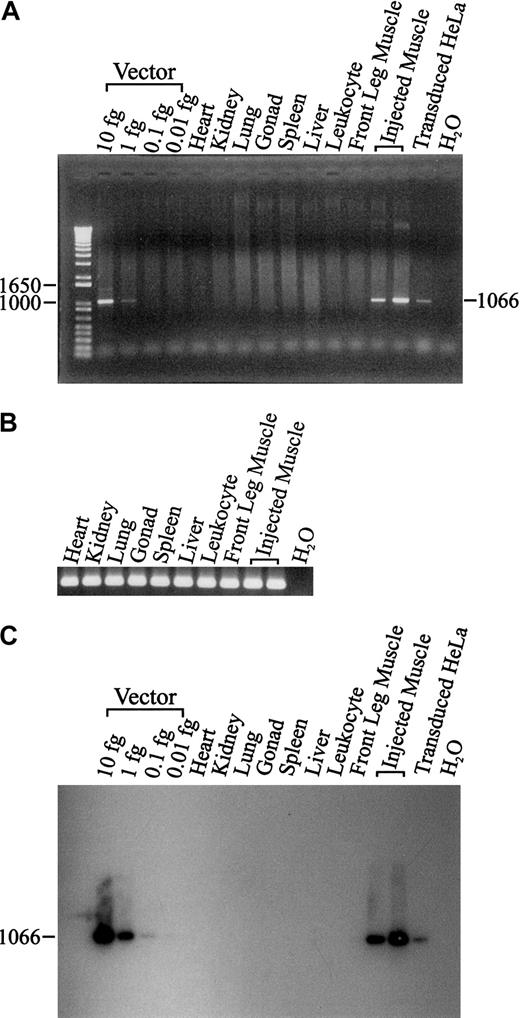
To determine provirus at the muscle injection sites and potential virus spread beyond these injection sites, sensitive PCR and Southern blots were performed on genomic DNA from a variety of tissues, including gonad (Figure 3). The only dog tissues positive for vector sequences were muscle obtained from the injection sites. The PCR assay of canine β actin showed high-quality tissue genomic DNA and equivalency of sample loading (Figure 3). The control DNA samples spiked with expression plasmid show that the Southern blot was about 10-fold more sensitive that the PCR protocol and was able to reveal 0.1 fg of vector per lane. We estimate the sensitivity of these assays to be about one provirus per 2.5 μg of genomic DNA. We were unable to detect provirus sequences in gonad tissue and this is important for potential clinical application.
PCR and Southern blot of lentiviral vector from dog tissues. PCR of 500 ng genomic DNA was used to detect the lentiviral vector from a variety of tissues in the treated dog (A). The integrity of tissue genomic DNA was confirmed by PCR amplification of the canine β-actin gene (B). After visualization of the ethidium bromide stained bands, the same agarose gel was used to perform Southern blot analysis (C).
PCR and Southern blot of lentiviral vector from dog tissues. PCR of 500 ng genomic DNA was used to detect the lentiviral vector from a variety of tissues in the treated dog (A). The integrity of tissue genomic DNA was confirmed by PCR amplification of the canine β-actin gene (B). After visualization of the ethidium bromide stained bands, the same agarose gel was used to perform Southern blot analysis (C).
Discussion
We have shown that intramuscular administration of a lentivirus vector expressing canine G-CSF permits sustained treatment of canine cyclic neutropenia. Before treatment, the neutrophil counts cycled with a periodicity of about 14 days, in agreement with the 12 to 14 days periodicity previously reported for gray collie dogs.5,6 During rG-CSF administration the neutrophil cycling did not display the same consistent regularity as shown in the pretreatment period. This may be a result of the single-dose administration and its interrupted delivery. After lentivirus administration, neutrophil cycling was quite regular with a periodicity of about 13 days, shorter than cycle length of the untreated dog. Reduction of the neutrophil cycle period has been previously reported for patients13,14,17 and dogs17 receiving rG-CSF. In this study, we administered a single dose of virus that provided neutrophil production probably greater than that required for therapy. In patients and dogs with cyclic neutropenia and patients with severe chronic neutropenia treated with rG-CSF, it has been estimated that provision of neutrophil counts in excess of 500 cells/μL would prevent severe recurrent infection.6,17,50 The treated collie dog received 108 IUs of lentivirus per kg and over an 11-month period showed minimum neutrophil counts of around 5000 cells/μL. This suggests a significant reduction in lentivirus dose would have provided a therapeutic level of neutrophil production. The mean neutrophil counts as percentages of mean WBCs changed from pretreatment value of 26% to 56% and 62% for rG-CSF and lentivirus administration, respectively, reflecting increased neutrophil production with either form of cytokine administration.
Monocyte counts showed a 25% decrease after rG-CSF administration and a 2-fold increase after lentivirus administration. Dose-related increases in monocyte counts have been reported in patients and healthy subjects receiving G-CSF.46,47 A 2-fold increase in monocytes was reported in subjects receiving high-dose G-CSF of 6 μg/kg administered subcutaneously twice daily.46 The lack of increase in monocytes after G-CSF administration may be related to the relatively low levels of cytokine we administered to the collie dog.
Significant decreases in platelet counts of 25% following G-CSF and 30% after lentivirus administration were shown, a change known to occur with chronic G-CSF therapy for various forms of severe chronic neutropenia.46 We have previously shown in rats receiving G-CSF delivery long-term from implants of transduced cells that production of platelets, lymphocytes, and red cells (hematocrit) were not different from pretreatment levels.44 Previous studies of G-CSF administration to mice have shown reduction in red cell number.51,52
Although we saw a slight decrease in lymphocyte counts over the period of G-CSF administration, there was a very significant 2-fold decrease in lymphocytes after lentivirus delivery (6880 cells/μL to 3610 cells/μL). However, we do not believe this is clinically significant as the lymphocyte counts after lentivirus administration are within the range of normal dog values of 2600 ± 800 cells/μL.45
Further evidence of sustained G-CSF delivery was the presence of elevated numbers of neutrophils and bands in spleen and bone marrow (data not shown). Therapeutic efficiency of lentivirus administration that mediates constitutive G-CSF delivery, in comparison to daily subcutaneous injection, may be estimated from our data as both forms of treatment gave similar neutrophil numbers (Table 1). We determined in vitro that lentivirus-transduced vascular smooth muscle cells would secrete 0.8 μg G-CSF per day/109 transduced cells. Assuming 20% transduction efficiency in vivo, we estimate secretion of 0.16 μg G-CSF per day. Recombinant G-CSF was administered at 17 μg per weekday. These data suggest that constitutive G-CSF expression is about 100-fold more efficient than daily subcutaneous G-CSF administration at inducing neutrophil production.
In a thorough search for provirus we were unable to detect evidence of lentivirus transduction in tissues from liver, spleen, lung, kidney, heart, lymphocytes, or gonads. Liver and spleen are the major sites of provirus integration when lentivirus vectors were administered intravenously to mice, with moderate to minimal transduction of heart, skeletal muscle, lung, brain, kidney, ovaries, and bone marrow.53,54 Thus, leakage of lentivirus from the muscle injection sites would be expected to preferentially transduce liver and spleen, and these tissues were negative for provirus. However, a recent study showed high-level transduction of bone marrow in mice administered VSV-G–pseudotyped lentivirus by the tail vein.55 We did not detect provirus in leukocytes, suggesting dog bone marrow stem cells were not transduced. We were only able to detect provirus in muscle at injection sites using a sensitive assay able to detect a single provirus copy per sample. In a study of intramuscular administration to rats of lentivirus expressing erythropoietin (EPO) we were unable to detect vector sequences in tissue other than muscle at the site of delivery.40 Together these data present strong evidence that virus administration intramuscularly is a relatively safe procedure that does not involve virus spread in particular to reproductive tissue.
The treated dog developed kidney failure showing amyloid accumulation in kidneys at 2 years of age, about 17 months after lentivirus administration. Kidney failure is a common cause of death in gray collie dogs and amyloidosis is particularly common in those over 30 weeks of age.48,49 Based on this prevalence, we believe the renal pathology was unrelated to lentivirus administration.
This is the first demonstration of successful long-term therapy of this disease employing a gene therapy approach. It demonstrates that G-CSF lentivirus delivered intramuscularly can provide sustained therapeutic levels of neutrophils for longer than 17 months and suggests this approach may be applied for long-term treatment of patients with cyclic and other neutropenias. It is unclear if any significance should be attached to the rise in monocytes and the slight decline in lymphocytes and platelets in this animal. Although we studied only one dog, further evidence for the suitability of this approach to gene therapy for humans will be possible with treatment of additional gray collies and normal dogs. At the time of lentivirus administration, the gray collie was 6 months old. As patients may not be juveniles it will be interesting to determine if there is an age-related efficiency of vector expression. Cyclic neutropenia in humans is a milder disease than in dogs, and therefore the ability to treat gray collie dogs is a significant step toward potential human application.
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2002-12-3722.
Supported by National Institutes of Health grants DK 43727 and DK 47754.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Linda Ward for donating the gray collie dog and her continued interest in this project, and the Department of Comparative Medicine for enthusiastic animal care.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal