Abstract
A hematopoietic cell transplantation (HCT) approach was developed for elderly or ill patients with hematologic malignancies that employed nonmyeloablative conditioning to avoid common regimen-related toxicities and relied on graft-versus-tumor effects for control of malignancy. Eighty-nine patients, median age 53 years, were given fludarabine (90 mg/m2) and 2 Gy total body irradiation. Marrow (n = 18) or granulocyte colony-stimulating factor (G-CSF)–stimulated peripheral blood mononuclear cells (G-PBMCs; n = 71) were transplanted from unrelated donors matched for human leukocyte antigen A (HLA-A), -B, -C antigens and -DRB1 and -DQB1 alleles. Postgrafting immunosuppression included mycophenolate mofetil and cyclosporine. Donor T-cell chimerism was higher for G-PBMCs compared with marrow recipients. Durable engraftment was observed in 85% of G-PBMCs and 56% of marrow recipients. Cumulative probabilities of grade II, III, and IV acute graft-versus-host disease (GVHD) were 42%, 8%, and 2%, respectively. Nonrelapse mortality at day 100 and at 1 year was 11% and 16%, respectively. One-year overall survivals and progression-free survivals were 52% and 38%, respectively. G-PBMC recipients had improved survival (57% vs 33%) and progression-free survival (44% vs 17%) compared with marrow recipients. HLA-matched unrelated donor HCT after nonmyeloablative conditioning is feasible in patients ineligible for conventional HCT. G-PBMCs conferred higher donor T-cell chimerism, greater durable engraftment, and better progression-free and overall survivals compared with marrow.
Introduction
Hematopoietic cell transplantation (HCT) from unrelated donors has been successfully used to treat patients with hematologic malignancies.1-4 Results for younger patients have begun approaching those seen with human leukocyte antigen (HLA)–identical sibling transplantation. A major limitation to unrelated HCT has been toxicities related to the high-dose conditioning regimens that have largely restricted unrelated HCT to patients 50 years of age or younger who are medically fit.1,5-8 This restriction has excluded most patients with chronic myelogenous leukemia (CML), acute myeloid leukemia (AML), chronic lymphoid leukemia (CLL), multiple myeloma (MM), myelodysplastic syndromes (MDS), myeloproliferative syndromes (MPS), and non-Hodgkin lymphoma (NHL) from conventional unrelated HCT, since median ages at diagnoses of these diseases are 65 to 70 years.9 To get around this restriction, reduced-intensity conditioning regimens have been developed that depend largely or solely on graft-versus-tumor (GVT) effects rather than high-dose therapy to eliminate malignant cells.10-13
Based on experimental animal studies14 and studies in recipients of HLA-matched sibling grafts,13,15 a nonmyeloablative HCT regimen was developed that employs pretransplantation host immunosuppression with 2 Gy total body irradiation (TBI) and 3 doses of fludarabine and posttransplantation immunosuppression, affecting both residual host and donor T cells, with a combination of mycophenolate mofetil (MMF) and cyclosporine (CSP).15 Early results with HCT from HLA-matched related donors have encouraged us to carry out a pilot study with this regimen in 52 patients given grafts from HLA-matched and mismatched unrelated donors.16 The preliminary results were encouraging and showed that such grafts were feasible, even when donors and recipients were not fully HLA compatible.
Here, we evaluated the use of the nonmyeloablative conditioning regimen on a protocol that required matching of patients and their unrelated donors for HLA-A, -B, and -C antigens and -DRB1 and -DQB1 alleles. The study addressed engraftment, nonrelapse mortality and, secondarily, graft-versus-host disease (GVHD) and GVT effects. Also, because some donor centers provided granulocyte colony-stimulating factor–“mobilized” peripheral blood mononuclear cells (G-PBMCs) and others provided marrow, a comparison of these 2 HCT products could be made. Here, we report on the 89 consecutive patients enrolled in this study. All 89 patients had hematologic malignancies and were not considered candidates for conventional high-dose HCT because of age and/or other known risk factors (eg, having already failed preceding high-dose HCT). Also, pharmacokinetic studies of mycophenolic acid, the biologically active metabolite of MMF, were conducted.
Patients, materials, and methods
Eligibility criteria
This multi-institutional trial included patients who received transplants at 9 centers including the Fred Hutchinson Cancer Research Center (FHCRC), University of Washington, Seattle Children's Regional Medical Center, University of Leipzig (Germany), Stanford University, University of Colorado, University of Utah, Seattle Veterans Administration Medical Center, and Baylor University, with the FHCRC acting as the coordinating center. The protocol was approved by the Institutional Review Board (IRB) at the FHCRC and at each of the collaborating sites. All patients signed consent forms approved by the local IRB. Patients were eligible for the study if they were older than 50 years of age. Younger patients were included if they had comorbid conditions that excluded them from conventional allogeneic HCT. Forty-three patients 50 years of age or younger met this criterion either because of failed previous high-dose autologous (n = 23), allogeneic (2 unrelated donor and 1 after HLA mismatched sibling; n = 3), or syngeneic (n = 1) HCT; active or recent invasive fungal disease (n = 4); diabetes mellitus and multiple infections (n = 1); liver dysfunction (n = 2); central nervous system arterio-venous malformations (AVM) (n = 1); Schwachman-Diamond syndrome (n = 2); Wolfe Parkinson White syndrome (n = 1); cardiomyopathy (n = 2); cardiomyopathy with atrial myxoma (n = 1); chronic pain syndrome and leg ulcerations from reflex sympathetic dystrophy (n = 1); and patient refusal of conventional HCT (n = 1). Specifically, the 41-year-old patient had been diagnosed with his MM 2.75 years earlier. He had been unsuccessfully treated with 3 different chemotherapy regimens, had failed G-PBMC mobilization, and developed marrow failure requiring continuous erythropoietin and granulocyte colony-stimulating factor (G-CSF) support. Given the patient's refusal of a conventional HCT, his case was discussed at the FHCRC's patient care conference and the faculty concurred that he was eligible for the protocol, in particular since the faculty shared the patient's concern about regimen-related toxicities given his history.
Patients were not eligible for the current protocol if they were pregnant or had rapidly progressive B- or T-cell malignancies, decompensated liver disease, corrected pulmonary diffusion capacity less than 35%, cardiac ejection fraction of less than 30%, Karnofsky performance status less than 50, or serologic evidence of infection with the human immunodeficiency virus. No exclusions were made for renal insufficiency or active bacterial or fungal infections.
HLA typing and matching
Patients had unrelated donors who were matched for HLA-A, -B, and -C by intermediate resolution DNA typing to a level at least as sensitive as serology and for -DRB1 and -DQB1 by high-resolution techniques.17 Retrospective molecular analyses of HLA-A, -B, and -C loci using direct automated fluorescence sequencing methods have been completed in 85 of the 89 donor-recipient pairs.17 Sixty-nine pairs were completely matched at the allele level for all 10 loci and 16 pairs had allele-level mismatches at 1 (n = 15) or 3 (n = 1) HLA class I loci.
Mycophenolic acid pharmacokinetics
Mycophenolic acid, the active metabolite of MMF, was assayed from serum samples obtained at 2, 4, 6, and 8 hours after the morning MMF doses on days 7 and 14. Pharmacokinetic measurements of serum mycophenolic acid included area under the curve (AUC), maximal concentrations (Cmax), minimal concentrations (Cmin), and half-life (t1/2).
Patient characteristics
Ninety sequential patients were enrolled on this trial between January 26, 2000, and July 21, 2001. Eighty-nine patients survived to the day of HCT while one patient died 3 days before TBI and HCT from progressive central nervous system AML. Only data from the 89 patients who survived to the day of HCT are presented. Their characteristics are shown in Table 1. Of the 89 patients, 32 were females and 57 were males. Their median age was 53 years (range, 5-69 years). The patients' diagnoses included MDS (n = 21), AML (n = 12), ALL (n = 5), CML (n = 14), NHL (n = 13), MPS (n = 7), CLL (n = 5), HD (n = 4), MM (n = 7), and Waldenström macroglobulinemia (n = 1). Twenty-three patients (26%) had previous autologous, 3 (3%) previous allogeneic, and 1 (1%) previous syngeneic HCT. Patients were classified as being at low-risk or high-risk of nonrelapse mortality based upon the following. Low-risk: 20 patients (22%) had AML or ALL in first complete remission (CR), MDS refractory anemia (RA), or CML in chronic phase (CP). High-risk: 69 patients (78%) had advanced-stage AML, ALL, MDS, or CML in accelerated phase (AP) or blast phase (BP) or B-cell malignancies. The median time from patient diagnosis to transplantation was 19.1 months (range, 3.5-187.6 months). Early data on the first 22 patients treated under this protocol have been reported as part of a previous publication.16 Here, we update the results of those 22 patients and report data on 67 new patients.
Patient and disease characteristics
. | Marrow . | G-PBMC . | Overall . |
|---|---|---|---|
| Patient no. | 18 | 71 | 89 |
| Median age, y | 52.5 | 53 | 53 |
| Median no. of preceding therapies (range) | 4 (0-12) | 4 (0-11) | 4 (0-12) |
| Median follow-up after HCT, d | 425 | 390 | 390 |
| Preceding auto/allo HCT | 4/2 | 19/2 | 23/4 |
| Median no. CD34 cells × 106/kg (range) | 2.1 (0.21-4.1) | 7.0 (1.26-28) | 5.72 (0.21-28) |
| Median no. CD3 × 108/kg | 0.3 | 2.6 | 2.33 |
| Donor HLA match, no. of patients | |||
| 10 allele match | 15 | 54 | 69 |
| 1 class l allele mismatch | 3 | 13* | 16 |
| Diagnosis and status at HCT, no. of patients | |||
| AML/ALL, total | 4 | 13 | 17 |
| CR1 | 2 | 6 | 8 |
| CR2 | 2 | 2 | 4 |
| More than CR3 | 0 | 4 | 4 |
| Rel/ref/IF | 0 | 1 | 1 |
| MDS, total | 4 | 17 | 21 |
| RA | 1 | 3 | 4 |
| RAEB | 1 | 2 | 3 |
| RAEB-T | 0 | 3 | 3 |
| AML CR1/CR2 | 0/0 | 2/1 | 3 |
| AML Rel/ref | 0/2 | 2/1 | 2/3 |
| CMML PR/ref/IF | 0/0/0 | 1/1/1 | 1/1/1 |
| CML, total | 4 | 10 | 14 |
| CP/AP/BP | 2/1/1 | 6/1/0 | 8/2/1 |
| CP-2 | 0 | 3 | 3 |
| MPS, total | 2 | 5 | 7 |
| ET | 0 | 2 | 2 |
| AMM-transformation | 2 | 1 | 3 |
| NOS | 0 | 2 | 2 |
| Indolent/high-grade NHL, total | 3 | 10 | 13 |
| CR 3 | 0 | 2 | 2 |
| PR | 1 | 2 | 3 |
| Ref/rel | 0/2 | 4/2 | 4/4 |
| CLL, total | 0 | 5 | 5 |
| CR | 0 | 1 | 1 |
| FLU refractory | 0 | 4 | 4 |
| HD, total | 0 | 4 | 4 |
| PR | 0 | 2 | 2 |
| Rel | 0 | 2 | 2 |
| MM, total | 0 | 7 | 7 |
| PR/ref | 0 | 4/3 | 4/3 |
| Waldenstroms, total | 1 | 0 | 1 |
. | Marrow . | G-PBMC . | Overall . |
|---|---|---|---|
| Patient no. | 18 | 71 | 89 |
| Median age, y | 52.5 | 53 | 53 |
| Median no. of preceding therapies (range) | 4 (0-12) | 4 (0-11) | 4 (0-12) |
| Median follow-up after HCT, d | 425 | 390 | 390 |
| Preceding auto/allo HCT | 4/2 | 19/2 | 23/4 |
| Median no. CD34 cells × 106/kg (range) | 2.1 (0.21-4.1) | 7.0 (1.26-28) | 5.72 (0.21-28) |
| Median no. CD3 × 108/kg | 0.3 | 2.6 | 2.33 |
| Donor HLA match, no. of patients | |||
| 10 allele match | 15 | 54 | 69 |
| 1 class l allele mismatch | 3 | 13* | 16 |
| Diagnosis and status at HCT, no. of patients | |||
| AML/ALL, total | 4 | 13 | 17 |
| CR1 | 2 | 6 | 8 |
| CR2 | 2 | 2 | 4 |
| More than CR3 | 0 | 4 | 4 |
| Rel/ref/IF | 0 | 1 | 1 |
| MDS, total | 4 | 17 | 21 |
| RA | 1 | 3 | 4 |
| RAEB | 1 | 2 | 3 |
| RAEB-T | 0 | 3 | 3 |
| AML CR1/CR2 | 0/0 | 2/1 | 3 |
| AML Rel/ref | 0/2 | 2/1 | 2/3 |
| CMML PR/ref/IF | 0/0/0 | 1/1/1 | 1/1/1 |
| CML, total | 4 | 10 | 14 |
| CP/AP/BP | 2/1/1 | 6/1/0 | 8/2/1 |
| CP-2 | 0 | 3 | 3 |
| MPS, total | 2 | 5 | 7 |
| ET | 0 | 2 | 2 |
| AMM-transformation | 2 | 1 | 3 |
| NOS | 0 | 2 | 2 |
| Indolent/high-grade NHL, total | 3 | 10 | 13 |
| CR 3 | 0 | 2 | 2 |
| PR | 1 | 2 | 3 |
| Ref/rel | 0/2 | 4/2 | 4/4 |
| CLL, total | 0 | 5 | 5 |
| CR | 0 | 1 | 1 |
| FLU refractory | 0 | 4 | 4 |
| HD, total | 0 | 4 | 4 |
| PR | 0 | 2 | 2 |
| Rel | 0 | 2 | 2 |
| MM, total | 0 | 7 | 7 |
| PR/ref | 0 | 4/3 | 4/3 |
| Waldenstroms, total | 1 | 0 | 1 |
G-PBMC indicates granulocyte colony-stimulating factor—mobilized peripheral blood mononuclear cells; HCT, hematopoietic cell transplantation; auto, autologous; allo, allogeneic; AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; CR, complete remission; rel, relapsed; ref, refractory; IF, induction failure; MDS, myelodysplastic syndromes; RA, refractory anemia; RAEB, refractory anemia with excess blasts; RAEB-T, refractory anemia with excess blasts in transformation; CMML, chronic myelomonocytic leukemia; PR, partial remission; CML, chronic myelogenous leukemia; CP, chronic phase; AP, accelerated phase; BP, blast phase; MPS, myeloproliferative syndromes; ET, essential thrombocytosis; AMM transformation, agnogeneic myeloid metaplasia in transformation; NOS, not otherwise specified; NHL, non-Hodgkin lymphoma; CLL, chronic lymphoid leukemia; FLU, fludarabine; HD, Hodgkin disease; and MM, multiple myeloma.
One patient mismatched at 3 class l alleles.
Conditioning regimen and postgrafting immunosuppression
Patients received fludarabine (30 mg/m2/d) on days –4, –3, and –2 before HCT and 2 Gy of TBI at a rate of 0.07 Gy/minute from a linear accelerator on day 0. Immunosuppressive therapy with oral CSP (6.25 mg/kg twice a day) was started on day –3 and MMF (15 mg/kg twice a day) was started 4 to 6 hours after HCT on day 0. Intravenous formulations of CSP and MMF were administered if patients were not able to tolerate oral medications. In patients without GVHD, MMF was tapered at day 40 over 56 days and CSP at day 100 over 80 days.
Collection of hematopoietic cells
All hematopoietic cell collections were coordinated through unrelated donor registries. Eighteen donors had marrow harvested by multiple aspirations from the posterior iliac crests on day 0 while under general or local anesthesia. The median CD34 and CD3 cell doses of harvested marrow were 2.1 × 106 (range, 0.21-4.1 × 106) and 0.3 × 108 (range, 0.03-0.46 × 108) per kilogram recipient body weight, respectively. The collection of G-PBMCs was per unrelated donor registry protocol. The National Marrow Donor Program donors received G-CSF (10 μg/kg/d) subcutaneously on days –5 through –1. Leukaphereses of 12 L of blood volume were performed on days –1 and 0. Both G-PBMC collections were combined, transported in a cold pack to the transplantation center, and transfused on day 0. Patients with high titers of antidonor isohemagglutinins received red blood cell (RBC)–depleted HCT. The median CD34 and CD3 cell doses of G-PBMC were 6.99 × 106 (range, 1.26-16.4 × 106) and 2.61 × 108 (range, 0.8-37.7 × 108) per kilogram recipient body weight, respectively. In patients given G-PBMC grafts, a portion of the G-PBMC product containing 1 × 107 CD3+ cells/kilogram was cryopreserved in anticipation for use as donor lymphocyte infusions.
Supportive care
At most collaborating institutions, patients were seen in the ambulatory care setting except during the scheduled infusion of the hematopoietic cells and whenever transplantation complications mandated closer medical supervision and/or therapy. All patients at the University of Leipzig were treated for at least one month on a hematology ward per institutional practice. Antimicrobial prophylaxis was administered per local standard practice guidelines of the respective institutions. This measure always included antibiotics for prophylaxis of Pneumocystis pneumonia, Candida albicans, and herpes zoster/simplex. Standard broad-spectrum bacterial antibiotic prophylaxis with a third-generation cephalosporin or quinolone was commonly given to patients who became neutropenic. All blood products except for the graft were irradiated with 25 Gy before transfusion. The thresholds of RBC and platelet transfusions, in general, were a hematocrit of less than 25% and platelet count of less than 10 × 109/L. Cytomegalovirus (CMV)–seronegative patients were given CMV-seronegative or leukocyte-depleted blood products. CMV-seropositive patients were monitored and treated according to their respective institution's standard practice guidelines. Most patients had weekly blood monitoring for CMV reactivation using measurements of CMV pp65 antigen or other institutional standards. Preemptive therapy with ganciclovir (5 mg/kg twice daily × 2 weeks then once daily to day 100) was given when more than 1 antigen-positive cell per slide was observed.
GVHD grading and therapy
The local investigators performed GVHD grading using standard criteria.18 Treatment of acute GVHD was conducted per each institution's standard practice guidelines. Usually, first-line therapies consisted of 2 mg methyl-prednisolone/kilogram/day or oral beclamethasone with or without systemic corticosteroids in cases of isolated gut GVHD, but some patients had resumption of full doses of CSP and/or MMF.
Follow-up
Patients were examined by a physician at least 3 times a week for the first month and then weekly or more frequently, depending on the patient's clinical status. Disease-dependent restaging evaluations after HCT occurred monthly for the first 3 months and then at 6 months, 1 year, and then yearly thereafter. Evaluations consisted of marrow aspirates for pathology, cytogenetic, flow cytometry, and molecular studies, and, when indicated, biochemical and radiographic studies. Patients were discharged to the care of their referring physicians approximately 100 days after HCT.
Treatment of persistent/progressive or relapsed malignancies
Persistent, progressive, or relapsed malignancies in the absence of severe manifestations of acute or chronic GVHD were treated by rapid discontinuation of systemic immunosuppression in order to initiate GVT effects. In addition, one patient received a donor lymphocyte infusion.
Chimerism analyses
For chimerism analyses, nucleated cells were isolated from the marrow and T cells and granulocytes from the peripheral blood using flow cytometry on days 28, 56, 84, 180, 365, and then yearly after HCT. Percentages of donor-host chimerism for recipients of sex-mismatched HCT were evaluated by fluorescent in situ hybridization (FISH) for X and Y chromosomes,19 while those for recipients of sex-matched HCT were based upon polymerase chain reaction (PCR)–based amplification of variable-number tandem repeat (VNTR) sequences unique to donors and hosts.20 All patients treated at Stanford University had donor-host chimerism assessed by VNTR. Quantitation of chimerism using VNTR was by visual inspection of silver-stained agarose gels with a standard error of 1% to 5%.
Study end points
Data were analyzed as of June 1, 2002. The major study end points were sustained allogeneic engraftment and nonrelapse mortality. Secondary outcomes included the incidence and severity of GVHD, GVT responses, survival, and progression-free survival. Patients were considered to have died of nonrelapse mortality if there was no evidence of disease relapse or progression. Patients with persistent disease were considered at risk for nonrelapse mortality. Disease responses and progression were defined by standard criteria. Neutrophil recovery was defined as the first of 3 consecutive days with neutrophil counts more than 0.5 × 109/L. Platelet recovery was defined as the first of 3 consecutive days with platelet counts more than 20 × 109/L without transfusion support.
Statistical methods
Chimerism levels in G-PBMCs and marrow recipients were compared using the Wilcoxon rank-sum test. The percentages of patients engrafting (based on detection of > 5% donor chimerism) were compared by the chi-square test. Survival and progression-free survival were estimated by the Kaplan-Meier method. Cumulative incidence estimates were calculated for graft rejection, acute GVHD, relapse, and nonrelapse mortality. Hazard ratios were estimated from Cox regression models. Deaths were treated as competing events in analyses of graft rejection, GVHD, and disease progression. Rejection was treated as a competing event in analyses of GVHD. Progression and nonrelapse mortality were the components of progression-free survival and were treated as competing events. Multivariate models were constructed in a stepwise fashion using a threshold significance level of 0.05 for inclusion in the model. Multivariate P values for a variable were based on adjustment for all other variables in the model. All P values were derived from likelihood ratio statistics and are 2-sided. The evaluation of the relationship of acute and chronic GVHD with disease relapse and progression-free survivals was made using the tested factors as time-dependent covariates.
Results
Engraftment
Recoveries of neutrophil counts to more than 0.5 × 109/L for at least 3 consecutive days were observed in 83 of 89 patients (93%). The median time to neutrophil recovery was 15 days (range, 0-55 days), and 24 of the 89 patients (27%) did not develop neutropenia (< 0.5 × 109/L). The median time to platelet recovery more than 20 × 109/L was 4 days (range, 0-53 days), and 49 of the 89 patients (55%) did not develop thrombocytopenia (< 20 × 109/L). Transfusions of platelets and RBCs were not required in 19 (21%) and 13 (15%) of the 89 patients, respectively. Six patients did not have recoveries of neutrophil counts more than 0.5 × 109/L and were classified as primary graft failures. Five of the 6 patients were later found to have experienced primary graft rejection and 1 had progressive MM. Of the 6 patients with marrow failure, 2 died after progression or relapse of AML, 1 died of respiratory failure after a second intermediate-dose unrelated donor HCT, and 3 died of bleeding complications.
Donor engraftment at day 28 (defined as > 5% donor T-cell chimerism) was observed in 77 of the 89 patients (87%) and was sustained in 70 of 89 (79%) patients. The sustained engraftment rate was higher for recipients of G-PBMC (60 of 71 patients or 85%) than those given marrow (10 of 18 patients or 56%; P = .007). Overall, 19 patients experienced graft rejection. Graft rejection typically resulted in prolonged declines of platelet and neutrophil counts, with 14 of 19 patients having eventual autologous recovery of blood counts. Multivariate statistical analysis identified significantly increased risks of graft rejection for patients who received marrow instead of G-PBMCs (P = .003) and those without preceding chemotherapy (P = .003; Table 2). Preceding chemotherapy was defined as all cytotoxic chemotherapy excluding chlorambucil, hydroxyurea, imatinib mesylate, and immunomodulators.
Multivariate analysis of risk factors for rejection
. | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Hematopoietic cell source | ||
| G-PBMC, n = 71 | 1.0 | .003 |
| Marrow, n = 18 | 4.7 (1.8-12) | |
| Preceding chemotherapy | ||
| No, n = 24 | 1.0 | .003 |
| Yes, n = 65 | 0.2 (0.1-0.6) |
. | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Hematopoietic cell source | ||
| G-PBMC, n = 71 | 1.0 | .003 |
| Marrow, n = 18 | 4.7 (1.8-12) | |
| Preceding chemotherapy | ||
| No, n = 24 | 1.0 | .003 |
| Yes, n = 65 | 0.2 (0.1-0.6) |
CI indicates confidence interval. All other abbreviations are explained in Table 1. Factors considered included diagnosis, hematopoietic cell source, disease type, CD34 dose, CD3 dose, patient sex, donor sex, sex mismatch, HLA class I mismatch, preceding transfusion, preceding chemotherapy, and prior autologous transplant.
Factors considered included diagnosis, hematopoietic cell source, disease type, CD34 dose, CD3 dose, patient sex, donor sex, sex mismatch, HLA class I mismatch, preceding transfusion, preceding chemotherapy, and prior autologous transplantation.
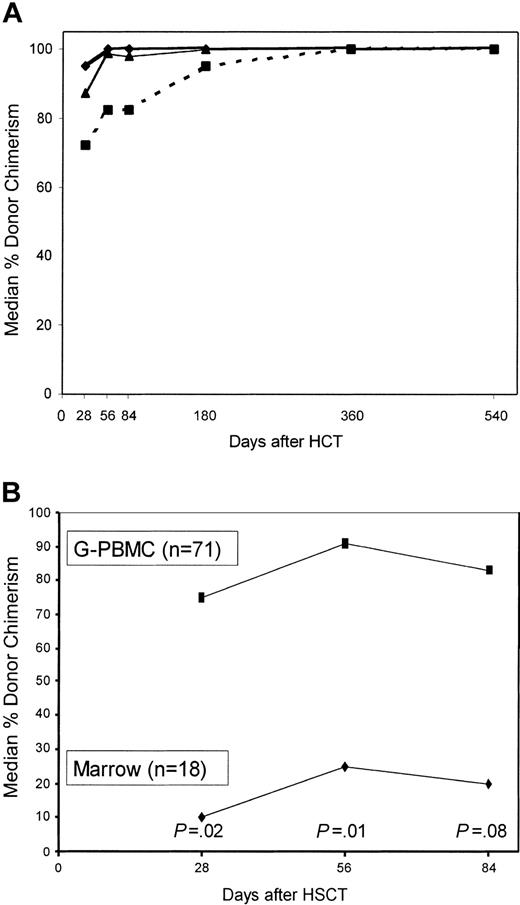
The median percentages of donor chimerism among marrow mononuclear cells and peripheral blood granulocytes and T cells over the first 540 days are shown in Figure 1A. Generally, no significant differences in the degrees of donor chimerism among marrow cells and granulocytes were seen between G-PBMC and marrow recipients at any time except for higher donor granulocyte chimerism on day 56 among G-PBMC recipients (P = .03; data not shown). However, levels of donor T-cell chimerism were much higher for recipients of G-PBMC compared with marrow recipients at days 28 (P = .02), 56 (P = .01), and 84 (P = .08; Figure 1B). Donor T-cell chimerism less than 50% at day 28 was associated with eventual graft rejection (P = .001), higher risk of relapse (P = .05), and a trend toward worse progression free-survival (P = .10) but had no effects on survival (P = .89) and nonrelapse mortality (P = .98). In 7 patients, loss of donor T-cell chimerism (rejection) was observed within the first 6 months. Specifically, these events were observed in 3 patients at day 56, in 2 patients at day 84, and in 2 patients at day 180. Two additional patients have shown progressive decreases in donor T-cell, granulocyte, and marrow chimerisms over periods of 360 and 540 days, respectively, which occurred in one case in the context of progressive CML and in the other during the development of secondary MDS of host cell origin.
Median percentage donor chimerism among nucleated marrow cells and peripheral blood granulocytes and T cells among evaluable patients. There were 77 patients studied at day 28, 68 on day 56, 60 on day 84, 44 on day 180, 29 on day 365, and 15 on day 540 (A). ♦ indicates granulocytes; ▴, marrow mononuclear cells; and ▪, T cells (CD3+). Median percentage donor chimerism among peripheral blood T cells in recipients given G-PBMCs versus those given marrow (B).
Median percentage donor chimerism among nucleated marrow cells and peripheral blood granulocytes and T cells among evaluable patients. There were 77 patients studied at day 28, 68 on day 56, 60 on day 84, 44 on day 180, 29 on day 365, and 15 on day 540 (A). ♦ indicates granulocytes; ▴, marrow mononuclear cells; and ▪, T cells (CD3+). Median percentage donor chimerism among peripheral blood T cells in recipients given G-PBMCs versus those given marrow (B).
Toxicities and nonrelapse mortality
New onset alopecia, mucositis, or veno-occlusive disease of the liver was not seen in any patient. Most patients (91%) required hospitalization at some point after HCT, while 8 patients were hospitalized only overnight for the infusion of the unrelated donor grafts. The median time of hospitalization over the first 2 months was 8.5 days (range, 0-60 days). The frequencies of grade III and IV organ toxicities are listed in Table 3. Cardiac complications were the most frequent organ toxicity with 26% of patients having grade III or IV events. The cardiac events were primarily related to CSP-related hypertension (13%) or atrial fibrillation (11%). Other grades III to IV toxicities included pulmonary (19.8%), hepatic (17.6%), neurologic (9.9%), gastrointestinal (7.8%), renal (5.5%), and hemorrhagic (5.5%). Forty-six (51%) patients experienced 84 grades III to IV toxicity events with 2 patients having 5 events, 3 patients having 4 events, 2 patients having 3 events, 17 patients having 2 events, and 22 patients having 1 event, each. Overall, 15 patients (16.9%) suffered grade IV events, the most common of which were pulmonary (n = 11), hepatic (n = 5), and renal (n = 4).
Incidence of grade III and IV toxicities (n = 89)
. | Grade, no. episodes (% of patients) . | . | |
|---|---|---|---|
. | III . | IV . | |
| Cardiac | 23 (26) | 1 (1) | |
| Pulmonary | 7 (8) | 11 (12) | |
| Hepatic | 11 (12) | 5 (6) | |
| Neurologic | 7 (8) | 2 (2) | |
| Gastrointestinal | 6 (7) | 1 (1) | |
| Renal | 1 (1) | 4 (4) | |
| Hemorrhage | 4 (4) | 1 (1) | |
. | Grade, no. episodes (% of patients) . | . | |
|---|---|---|---|
. | III . | IV . | |
| Cardiac | 23 (26) | 1 (1) | |
| Pulmonary | 7 (8) | 11 (12) | |
| Hepatic | 11 (12) | 5 (6) | |
| Neurologic | 7 (8) | 2 (2) | |
| Gastrointestinal | 6 (7) | 1 (1) | |
| Renal | 1 (1) | 4 (4) | |
| Hemorrhage | 4 (4) | 1 (1) | |
There were 7 episodes of grade III and 2 episodes of grade IV neurologic toxicities, respectively. The grade III episodes consisted of seizures (n = 2) and ataxia (n = 1), suspected to be related to CSP, and mental status changes, thought to be due to transient metabolic derangements (uremia, n = 2) and dehydration (n = 1). One grade III episode resulted from an adjustment disorder with depressed mood that improved with antidepressants. The grade IV episodes were caused by seizures from human herpesvirus 6 (HHV-6) encephalitis (n = 1) and confusion possibly related to CSP toxicity (n = 1). Imaging studies were normal in 3 cases, while the patient with grade IV confusion had increased parietal occipital white matter changes, and 3 patients with suspected CSP toxicity showed bilateral cortical and subcortical white matter lesions with T2 hyperintensity signals. All episodes of neurologic toxicity were transient with the exception of ataxia. The patient with grade IV confusion died with rapidly progressive central nervous system (CNS) decompensation one year after transplantation, and the patient with HHV-6 encephalitis and seizures died with a CNS hemorrhage after discontinuation of immunosuppression for rapidly progressive MM.
No patient experienced veno-occlusive disease of the liver. However, grade III hyperbilirubinemia occurred in 11 patients and was attributed to hemolysis (n = 3), CSP toxicity (n = 5), biliary sludge (n = 1), cholecystitis (n = 1), and the use of ambisone (n = 1). The 5 grade IV hepatic events occurred in the context of cholangitis lenta (n = 1), hemolysis combined with CSP toxicity in a patient with a history of hepatitis (n = 1), GVHD (n = 1), decompensated chronic liver disease (n = 1), and multiorgan failure from progressive pre-existing portal vein thrombosis (n = 1).
Fifteen of the 89 patients (16.9%) died from nonrelapse causes. The cumulative probabilities of nonrelapse mortality were 11% at day 100 and 16% at one year, respectively. There was no difference in nonrelapse mortality between G-PBMC and marrow recipients. Causes of death included grade IV GVHD (n = 2), infections or sepsis syndrome with GVHD (n = 2), infections or sepsis without GVHD (n = 2), pulmonary bleeding or failure after graft rejections (n = 3), bleeding from duodenal Dieulafoy21 vascular abnormality (n = 1), cardiopulmonary arrest after variceal bleed (n = 1), congestive heart failure (myocardial fibrosis noted on autopsy; n = 1), hepatic failure (cirrhosis noted on autopsy; n = 1), suicide (n = 1), and complications of induction chemotherapy for de novo AML of host cell origin in a patient who originally received a transplant for Waldenström's macroglobulinemia (n = 1).
Graft-versus-host disease
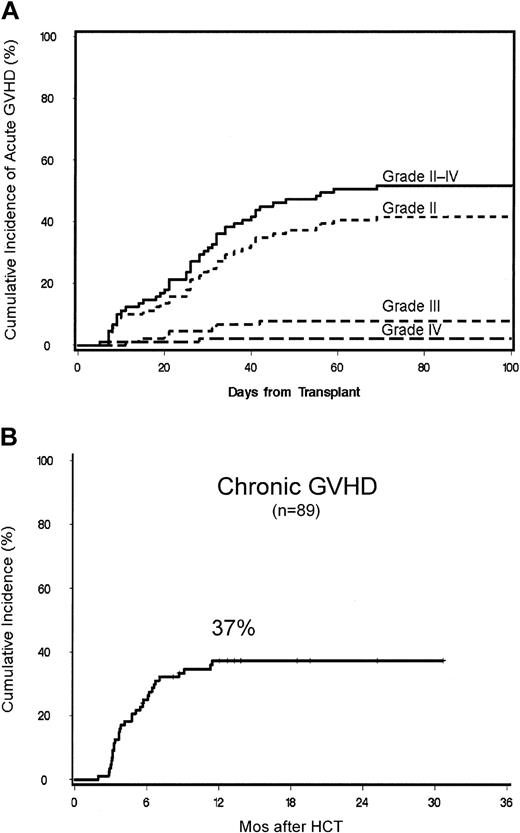
The cumulative incidence of grades II to IV acute GVHD was 52% (Figure 2A). Of this, 42% was grade II, 8% grade III, and 2% grade IV acute GVHD. There was no difference in the cumulative probabilities of grades II to IV acute GVHD among G-PBMC and marrow recipients, though the probability of grades III to IV acute GVHD was higher among G-PBMC recipients (11% vs 0%, P = .05). Chronic GVHD requiring therapy occurred in 40 patients. The cumulative probability of chronic extensive GVHD at 1 year for all 89 patients was 37% (Figure 2B). Four patients without relapse of their disease died from complications arising from either acute or chronic GVHD (4%). The only significant risk factor for chronic GVHD identified by univariate analysis was acute grades II to IV GVHD (P = .04).
Cumulative probabilities. Cumulative probabilities of grade II, III, and IV acute GVHD (A) and chronic extensive GVHD (B) are shown.
Cumulative probabilities. Cumulative probabilities of grade II, III, and IV acute GVHD (A) and chronic extensive GVHD (B) are shown.
Survival
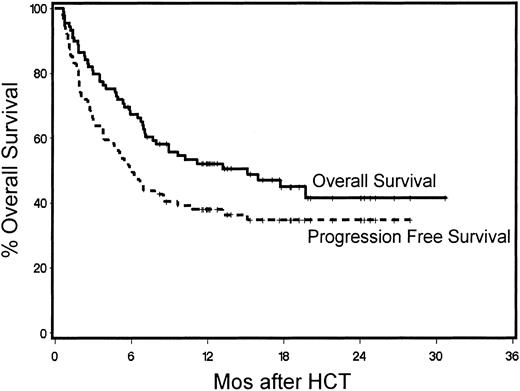
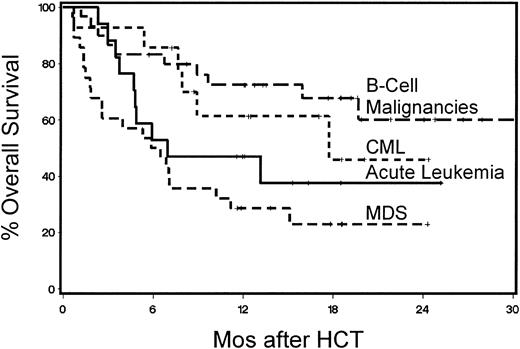
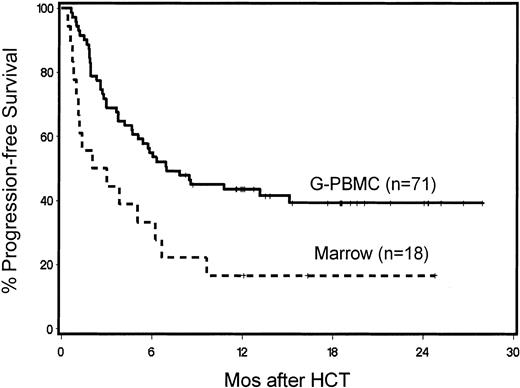
Forty-two patients were surviving between 5.7 and 28.4 months (median, 13 months) after HCT. The probability of 1-year survival was 52% (Figure 3). The recipients of G-PBMCs had a trend toward better overall survival compared with marrow recipients (57% vs 33%, P = .13). The Kaplan-Meier probability of 1-year overall survival for patients with B-cell malignancies was 73%, for those with acute leukemias 47%, for those with CML 61%, and for those with MDS/MPS 29% (Figure 4). Multivariate models were constructed to evaluate pretransplantation risk factors that affected survival or progression-free survival. Risk factors favorably associated with improved survival included 5 blast cells or less in the marrow before HCT (P = .002), low-risk disease category (P = .04), transplantation from a female donor (P = .01), not receiving previous transfusions (P = .003), and CD3+ cell dose greater than or equal to the median (P = .0006; Table 4). However, if marrow recipients were excluded from this analysis, the CD3+ cell dose was no longer significant.
Kaplan-Meier product estimates of survival and progression-free survival for all 89 patients.
Kaplan-Meier product estimates of survival and progression-free survival for all 89 patients.
Kaplan-Meier product estimates of survival for patients with B-cell malignancies, chronic myeloid leukemia, acute leukemia, or myelodysplastic/myeloproliferative syndromes.
Kaplan-Meier product estimates of survival for patients with B-cell malignancies, chronic myeloid leukemia, acute leukemia, or myelodysplastic/myeloproliferative syndromes.
Multivariate analysis of risk factors risk factors for survival (n = 87)*
. | Hazard ratio (95% CI) . | P . |
|---|---|---|
| CD3 dose | ||
| More than or equal to median, n = 37 | 1.0 | .0006 |
| Less than median, n = 37 | 2.8 (1.4-5.5) | |
| Unknown, n = 12† | 5.6 (2.1-15) | |
| Risk group | ||
| Low, n = 18 | 1.0 | .04 |
| High, n = 69 | 2.8 (1.0-8.1) | |
| Donor sex | ||
| Female, n = 54 | 1.0 | .01 |
| Male, n = 33 | 2.1 (1.0-4.4) | |
| Preceding transfusion | ||
| No, n = 16 | 1.0 | .003 |
| Yes, n = 70 | 3.4 (1.0-12) | |
| Marrow blasts at HCT | ||
| Less than or equal to 5%, n = 70 | 1.0 | .002 |
| More than 5%, n = 16 | 3.3 (1.6-6.6) |
. | Hazard ratio (95% CI) . | P . |
|---|---|---|
| CD3 dose | ||
| More than or equal to median, n = 37 | 1.0 | .0006 |
| Less than median, n = 37 | 2.8 (1.4-5.5) | |
| Unknown, n = 12† | 5.6 (2.1-15) | |
| Risk group | ||
| Low, n = 18 | 1.0 | .04 |
| High, n = 69 | 2.8 (1.0-8.1) | |
| Donor sex | ||
| Female, n = 54 | 1.0 | .01 |
| Male, n = 33 | 2.1 (1.0-4.4) | |
| Preceding transfusion | ||
| No, n = 16 | 1.0 | .003 |
| Yes, n = 70 | 3.4 (1.0-12) | |
| Marrow blasts at HCT | ||
| Less than or equal to 5%, n = 70 | 1.0 | .002 |
| More than 5%, n = 16 | 3.3 (1.6-6.6) |
Abbreviations are explained in Table 1.
Patients who died prior to day 28 were excluded.
CD3 cell enumeration did not take place.
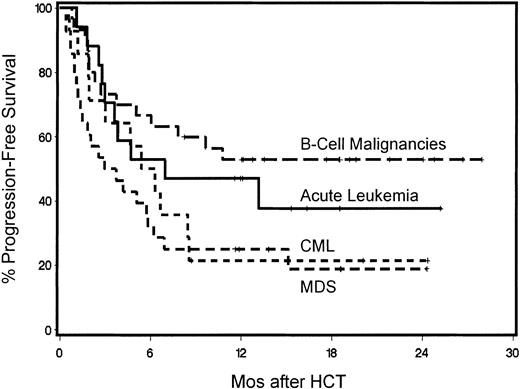
Status of underlying disease and progression-free survival
One-year overall progression-free survival for the 89 patients was 38% (Figure 3). Those values were 44% for G-PBMC and 17% for marrow recipients, respectively (P = .02). Multivariate analysis identified the use of G-PBMCs (P = .006; Figure 5) and the finding of 5% marrow blasts or less at the time of HCT resulting in significantly better progression-free survival (P < .0001; Table 5). Disease responses were seen among all disease categories. Forty percent of patients with measurable disease at the time of HCT had CR and 4% PR after HCT. Progression-free 1-year survival for patients with B-cell malignancies was 53%, for those with acute leukemias 47%, for those with CML 21%, and those with MDS/MPS 25% (Figure 6). Specifically, of the 5 patients with ALL, 2 have remained in CR by morphology and flow cytometry at 7 and 12 months, respectively, while one patient died of transplantation-related causes, 1 committed suicide, and 1 died from leukemic relapse. Of the 12 patients with AML, 5 were alive in CR by morphology and flow cytometry between 11.5 and 25.6 months after HCT, while 2 died of nonrelapse causes, 1 died of refractory progressive disease, and 4 died of leukemic relapse. Of the 5 patients with CLL, 3 were alive at 12 to 22 months including 2 in CR by morphology, flow cytometry, and computed tomography (CT) scan and 1 in PR, while 1 had died with progressive disease and 1 from nonrelapse causes. Of the 14 patients with CML, 8 patients were alive, 3 in morphologic and molecular CRs by PCR at 11 to 20 months and 4 in relapse (after graft rejection) with 1 alive with stable disease; 6 patients had died, 5 from disease progression and 1 from nonrelapse causes. Of the 4 patients with HD, 3 were alive at 6 to 8.5 months, 1 in CR by CT scan, 1 with progression, and 1 in relapse, while 1 patient died of relapse. Of the 20 patients with myelodysplasia, 4 were in CR by morphology and flow cytometry at 6.6 to 19 months while 16 had died, 9 from relapse and 7 from nonrelapse causes. Of the 8 patients with MPS, 3 were alive in CR by morphology and flow cytometry at 17 to 24 months while 5 had died, 4 from disease progression or relapse and 1 from nonrelapse causes. Of the 7 patients with MM, 5 were alive at 12 to 21 months; 1 in CR by morphology, serum protein electrophoresis, and urine protein electrophoresis and 4 in PRs, while 2 had died with progressive disease. Of the 13 patients with NHL, 9 were alive at 12 to 25 months after HCT, 7 were in CR by CT scan (including 5 patients with mantle cell NHL, 1 with high-grade B-cell and one with high-grade T-cell NHL) and 2 with disease progression (1 each with relapse of mantle cell and progression of small-cell NHL). Of the 4 NHL patients who died, 3 died with disease progression and 1 died of nonrelapse causes. The patient with Waldenström's macroglobulinemia died in remission of his primary disease (by morphology and negative serum protein electrophoresis) during induction therapy for de novo AML of host origin.
Kaplan-Meier product estimates of progression-free survival of patients given G-PBMCs (n = 71) or marrow grafts (n = 18).
Kaplan-Meier product estimates of progression-free survival of patients given G-PBMCs (n = 71) or marrow grafts (n = 18).
Multivariate analysis of risk factors for progression-free survival (n = 89)
. | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Blasts at HCT | <.0001 | |
| Less than or equal to 5% blast cells, n = 73 | 1.0 | |
| More than 5%, n = 16 | 4.5 (2.4-8.4) | |
| Hematopoietic cell source | ||
| G-PBMC, n = 71 | 1.0 | .006 |
| Marrow, n = 18 | 2.5 (1.4-4.7) |
. | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Blasts at HCT | <.0001 | |
| Less than or equal to 5% blast cells, n = 73 | 1.0 | |
| More than 5%, n = 16 | 4.5 (2.4-8.4) | |
| Hematopoietic cell source | ||
| G-PBMC, n = 71 | 1.0 | .006 |
| Marrow, n = 18 | 2.5 (1.4-4.7) |
Abbreviations are explained in Table 1.
Kaplan-Meier progression-free survival for patients with B-cell malignancies, chronic myeloid leukemia, acute leukemia, or myelodysplastic/myeloproliferative syndromes.
Kaplan-Meier progression-free survival for patients with B-cell malignancies, chronic myeloid leukemia, acute leukemia, or myelodysplastic/myeloproliferative syndromes.
Relationships between acute and chronic GVHD with progression-free survival and relapse were examined as time-dependent covariates. Grades II to IV acute GVHD was associated with a decreased risk of relapse (P = .02). Chronic GVHD was associated with a trend toward better progression-free survival (P = .14) and a decreased risk of relapse (P = .04).
Donor lymphocyte infusions
One patient with progressing HD received a single dose of 1 × 106 donor CD3 cells/kilogram but failed to achieve a disease response. Among the remaining patients with disease progression, donor lymphocyte infusion (DLI) was contraindicated in some due to graft rejection (n = 14) or because of acute GVHD preceding (n = 9) or occurring soon after (n = 1) disease progression. Further, DLI was not offered to 12 patients with florid relapse and 2 patients with CML and small cell NHL, respectively, who had complete responses after withdrawal of immunosuppression. Of the 2 remaining patients, 1 refused DLI for progressive HD, and 1 patient with mantle cell NHL refused further therapy or follow-up.
Mycophenolic acid pharmacokinetics
Pharmacokinetic data were obtained by testing sera from 19 patients at day 7 and from 12 patients at day 14. Results on both time points were comparable. The median mycophenolic acid area under the curve concentration on day 7 was 20.3 μg × mL/hour. The median maximal (Cmax) and minimal (Cmin) concentrations of mycophenolic acid at day 7 were 5.3 μg/mL and 0.5 μg/mL, respectively. The median serum half-life of mycophenolic acid was 3.0 hours.
HLA class I allele-level mismatching
Using high-resolution typing, 16 patients had HLA class I allele-level mismatches, of whom 15 had single-allele mismatches and 1 had mismatches for 3 alleles (one A and 2 C alleles). Five of the 15 single HLA class I allele-mismatched recipients rejected their donor grafts (2 of 3 marrow recipients and 3 of 12 G-PBMC recipients) and the 1 multiple HLA class I allele-mismatched recipient rejected a G-PBMC graft. HLA class I allele-level mismatch (16 of 85 pairs) was associated with a trend toward increased risk of graft rejection (P = .13) but was not associated with an increased risk of acute GVHD or changes in survival or progression-free survival.
Performance status in surviving patients
The median Karnofsky performance score in the 42 surviving patients was 90, with a range of 50 to 100.
Discussion
This study was undertaken to evaluate engraftment, GVHD, and GVT effects in patients with advanced hematologic malignancies who received HCT using transplants from unrelated donors matched for HLA-A, -B, -C, -DRB1, and -DQB1 using a nonmyeloablative regimen. Further, while most donor centers provided the requested G-PBMCs, some procured exclusively marrow for transplantation and this circumstance allowed us to compare the relative merits of the 2 sources of hematopoietic cells in a retrospective manner. Study entry required patients to be ineligible for conventional HCT after myeloablative conditioning. The study was designed to include 90 consecutive patients. Unfortunately, one patient died of rapidly progressive central nervous system leukemia 3 days before HCT and data from this patient were not included in the final analysis. Also, retrospective high-resolution typing of HLA-A, -B, and -C antigens in 85 of the 89 patients showed one HLA class I allele-level mismatch in 15 patient/donor pairs and 3 allele-level mismatches in one. Within the limitations of the study design and the patient numbers, no significant adverse effects related to the single-allele mismatches could be observed, while the patient with the 3 allele-level mismatches rejected the graft.
Several observations have been made. First and foremost, G-PBMCs appear to be a superior source of hematopoietic donor cells in this setting compared with marrow. Use of G-PBMCs led to a higher donor T-cell chimerism at 4 weeks, a greater engraftment rate, better GVT effects as manifested by better progression-free survival, and a comparable overall rate of GVHD, though perhaps a modest increase in grades III to IV acute GVHD. We hypothesize, based on the multivariate analysis of risk factors for survival, that both the benefit and the adverse effects (slightly more severe GVHD) are related to the greater content of CD3+ T lymphocytes in the G-PBMC product.22 Because better engraftment and progression-free survival were seen with G-PBMCs, this will be the preferred HCT product in future studies with this nonmyeloablative regimen.
Second, independent of the source of hematopoietic cells, patients with the diagnoses of CML, MDS, or MPS who had not received preceding chemotherapy were at higher risk of graft rejection. A similar finding has been reported for patients with CML who received HCT using transplants from conventional unrelated donors,23 but not for those with MDS,7 and is likely related to intact host immune responses that may require slightly more intensive immunosuppression before and/or after HCT for engraftment to be more uniformly successful. In this context, the observation of the relatively short half-life of mycophenolic acid of less than 4 hours may be important. Given that mycophenolic acid binds reversibly to inosine monophosphate dehydrogenase (IMPDH), the de novo purine pathway that is critical for lymphocyte proliferation may only be transiently blocked by twice-a-day dosing of MMF. Indeed, the inhibition of IMPDH has been correlated to the serum concentration of mycophenolic acid.24 It is possible that thrice-a-day dosing of MMF may resolve the engraftment problem among patients who received HCT using transplants from HLA-matched unrelated donors after myeloablative conditioning.
Another observation was that, while GVT effects were seen among all disease categories studied, these were least pronounced in patients who had 5% marrow blasts or more at the time of HCT. More than 5% marrow blasts were primarily seen in patients with advanced myeloid malignancies (AML, CML, MDS, and MPS). Relative lack of effectiveness of GVT reactions have been previously described in patients who had relapsed with blasts after conventional HCT and were given donor lymphocyte transfusions.25 The reasons for the relative “insensitivity” of blast cells to the GVT are not clear but could be due to the fact that rapidly proliferating blast cells outpace the tempo of cell kill through donor lymphocytes. Because of that, and also as a result of a high incidence of graft rejection among patients with MDS and CML, progression-free 1-year survival for such patients was no better than 25%. The 47% progression-free survival for patients with acute leukemias was likely related to the facts that most of them were in remissions at the time of HCT and that they had a relatively low frequency of rejection. The best progression-free survival (55%) was seen in patients with B-cell malignancies (NHL, CLL, HD, and MM), most of whom had active and, in some cases, large, disease burdens at HCT. These patients also had a very low incidence of graft rejection. The improved progression-free survival in these patients might have been due to the slower growth characteristics of B-cell tumors and to better antigen expression and presentation by tumor cells, with resultant increased sensitivity to immune-mediated responses. Nevertheless, even among the “responsive” disease categories, there were individual patients whose disease progressed in the presence of fully established grafts and GVHD. In turn, other patients entered into complete remissions even in the absence of GVHD.
The fourth observation concerned GVHD. The cumulative incidence of grades II to IV acute GVHD was 52% and that of grade III and IV GVHD 10%. Grades III to IV acute GVHD were less than the 33% to 47% rate reported after conventional conditioning1-3 and slightly higher than the 6% incidence reported by Chakraverty et al26 using in vivo T-cell depletion with alemtuzumab after reduced-intensity conditioning. The relatively low incidence of severe acute GVHD may be related to a number of factors. One may be the low degree of tissue damage with the nonmyeloablative regimen, which may reduce the release of inflammatory cytokines especially from the gut.27 Another reason may be more effective suppression of GVHD with the combination of MMF and CSP compared with CSP ± methotrexate. Another possibility is that the initial mixed donor-host chimerism resulted in the generation of mutual host-donor tolerance reducing alloreactivity as has been shown in animal models.28,29 The cumulative incidence of chronic GVHD was lower than the 48% to 70% incidence in conventional HCT studies using unrelated grafts4,30,31 but higher than the 0% to 20% incidence reported after the use of reduced-intensity regimens with peritransplantation T-cell depletion.10,11,32 Acute GVHD was found to be a risk factor for chronic GVHD but patient age and the use of G-PBMC was not. This analysis should be viewed with caution given the relatively small numbers of evaluable patients. Since a substantial portion of the GVT effect is likely associated with acute and chronic GVHD, the optimal treatment of this complication, while maintaining GVT, remains an area of active investigation.
Fifth, nonrelapse mortality was 11% at 100 days and 16% at 1 year. This result is remarkable given the large number of patients at high risk for nonrelapse mortality from extensive previous chemotherapy including preceding high-dose HCT, organ dysfunctions, advanced-staged malignancies, and older age. Despite the higher rejection rate in the marrow compared with the G-PBMC recipients, no significant differences in nonrelapse mortality were observed. The incidence of nonrelapse mortality observed was less than the 38% to 54% incidence reported after myeloablative3,4,7,30 and comparable to the 14.9% to 37.4% incidences after reduced-intensity conditioning regimens10-12,32 for unrelated donor grafts in patients with hematologic malignancies. The relatively low nonrelapse mortality was likely related to the lower intensity of the regimen, which resulted in less myelosuppression and regimen related toxicity compared with more intensive regimens. The attribution of causes of death after nonmyeloablative conditioning can present unique challenges using traditional definitions for nonrelapse mortality and relapse mortality. The challenge stems from both the minimal antitumor activity and mortality of the nonmyeloablative regimen and the fact that GVT effects take time to develop. Therefore, most patients who survive the conditioning regimen are at risk for disease relapse or progression during the early months after transplantation. Engrafted patients who have relapsed or whose disease progressed are at risk of dying both from complications of their underlying malignancies and the morbidity and mortality from GVHD that may have been induced by interventions such as discontinuation of immunosuppression, DLI, or chemotherapy. Since the proximate causes of death after relapse or with severe GVHD can be similar (eg, infectious and/or bleeding complications), the attribution of ultimate causes of death can be difficult. Therefore, we have chosen to strictly adhere to the definitions of relapse and nonrelapse mortality that have been used in conventional HCT studies.
The rates of reported organ toxicities as measured using Common Toxicity Criteria were also relatively mild by transplantation standards. The most common organ toxicities were CSP-related hypertension and hyperbilirubinemia and were readily reversible. Other toxicities were relatively infrequent and were less than 10% for most organ systems and similar to other published studies.10-12,32 However, severe life-threatening toxicities still occurred and generally were clustered in patients with pretransplantation comorbid medical conditions and/or advanced disease.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2003-02-0482.
Supported in part by grants CA78902, K23 CA92058, CA18029, CA18221, CA15704, CA49605, HL36444, and DK56465 from the National Institutes of Health, Department of Health and Human Services (DHHS), Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank the data coordinator Debbie Bassuk and the study nurses Steve Minor, Mary Hinds, and Kathryn Keegan for their invaluable help in making the study possible. The authors also wish to thank Helen Crawford and Bonnie Larson for manuscript preparation, and all physicians, nurses, and support personnel for their care of patients on this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal