Abstract
Chemokine stromal cell–derived factor-1 (SDF-1) is expressed by bone marrow (BM) stromal cells and plays key roles in BM cell migration. Modulation of its expression could affect the migratory capacity of cells trafficking the BM, such as hematopoietic progenitor and leukemic cells. Transforming growth factor-β1 (TGF-β1) is present in the BM environment and constitutes a pivotal molecule controlling BM cell proliferation and differentiation. We used the BM stromal cell line MS-5 as a model to investigate whether SDF-1 expression constitutes a target for TGF-β1 regulation and its functional consequences. We show here that TGF-β1 down-regulates SDF-1 expression, both at the mRNA level, involving a decrease in transcriptional efficiency, and at the protein level, as detected in lysates and supernatants from MS-5 cells. Reduction of SDF-1 in supernatants from TGF-β1–treated MS-5 cells correlated with decreased, SDF-1–dependent, chemotactic, and transendothelial migratory responses of the BM model cell lines NCI-H929 and Mo7e compared with their responses to supernatants from untreated MS-5 cells. In addition, supernatants from TGF-β1–exposed MS-5 cells had substantially lower efficiency in promoting integrin α4β1–mediated adhesion of NCI-H929 and Mo7e cells to soluble vascular cell adhesion molecule-1 (sVCAM-1) and CS-1/fibronectin than their untreated counterparts. Moreover, human cord blood CD34+ hematopoietic progenitor cells displayed SDF-1–dependent reduced responses in chemotaxis, transendothelial migration, and up-regulation of adhesion to sVCAM-1 when supernatants from TGF-β1–treated MS-5 cells were used compared with supernatants from untreated cells. These data indicate that TGF-β1–controlled reduction in SDF-1 expression influences BM cell migration and adhesion, which could affect the motility of cells trafficking the bone marrow.
Introduction
Stromal cell–derived factor-1 (SDF-1; CXCL12) is a CXC chemokine expressed by bone marrow stromal cells that potently attracts hematopoietic progenitor cells (HPCs) and leukemic cells1-9 and that exerts its chemoattractive and activating functions on binding to its G-protein–coupled receptor CXCR4.10,11 Studies on knock-out mice for SDF-1 and CXCR4 revealed that SDF-1/CXCR4 interaction is required for B lymphopoiesis and myelopoiesis in the bone marrow (BM), whereas fetal liver myelopoiesis is not affected.12-15
Cell migration triggered by SDF-1 is thought to play important roles in HPC motility within the BM and in homing to the BM of transplanted CD34+ HPCs. Reduction of bone marrow SDF-1 levels has been reported to mediate HPC emigration from the BM to the blood with the addition of stimuli that induce stem cell mobilization.16,17 The α4β1 integrin, one of the principal adhesion molecules involved in the interactions of HPC and leukemic cells with the BM microenvironment,18-23 constitutes a target of SDF-1 activation. Hence, cell migration triggered by SDF-1 involves transient increases in the adhesive activity of α4β1 on CD34+ cells.24,25 In addition, we have previously shown that SDF-1 is capable of modulating α4β1 integrin–dependent adhesion of myeloma cells,9 raising the possibility that this modulation could influence malignant plasma cell trafficking in BM during multiple myeloma.
Transforming growth factor-β1 (TGF-β1) is a multifunctional cytokine that regulates cell proliferation, differentiation, and migration, and it has important roles in tissue recycling and repair.26,27 TGF-β1 performs its biologic functions through binding to a heteromeric cell surface complex formed by types 1 and 2 TGF-β receptors, which are transmembrane serine/threonine kinases that are required for TGF-β signalling.26,28 Members of the Smad group of proteins mediate signaling from the receptors to the nuclei, acting as intermediates for transcriptional regulation and cell cycle arrest.27,29,30 TGF-β1 is present in the BM environment and constitutes a pivotal molecule controlling the quiescence of stem cells and the proliferation and differentiation of maturing hematopoietic progenitors.31 On the other hand, TGF-β1 is overproduced by BM stromal cells in several pathologic conditions in the BM, resulting in a failure of early hematopoietic progenitors.32,33 BM stromal cells express TGF-β receptor types 1 and 2,34 and we previously showed that TGF-β1 increased fibronectin expression on these cells, which was associated with a differential use of integrins α4β1 and α5β1 in subsequent cell adhesions.35
Given the important roles of SDF-1 in the migration and adhesion of BM cells during controlled and uncontrolled hematopoiesis, changes in its expression by BM stromal cells could affect several cell trafficking scenarios in the bone marrow, including cell motility within the BM and cell homing to the BM. In the present study we used the mouse BM stromal cell line MS-5, from which SDF-1 was originally purified,1 as a model to investigate whether SDF-1 expression constitutes a target of regulation by TGF-β1 and its functional consequences.
Materials and methods
Cells and antibodies
The multiple myeloma NCI-H929 and megakaryocytic leukemia-derived Mo7e human cell lines were cultured in RPMI 1640 medium (Gibco BRL, Paisley, Scotland) supplemented with 10% fetal bovine serum (FBS) (BioWhittaker, Verviers, Belgium), antibiotics, and 50 μM β-mercaptoethanol or 5 ng/mL recombinant human granulocyte macrophage–colony-stimulating factor (rhGM-CSF) (R&D Systems, London, England), respectively. Human umbilical cord blood samples from normal full-term deliveries were obtained from the Centro de Transfusión de la Comunidad de Madrid with signed consent from donors. After Ficoll-Hypaque density gradient centrifugation, CD34+ cells were enriched from mononuclear fractions using the CD34 Progenitor Cell Selection kit (Dynal A.S., Oslo, Norway), as indicated by the manufacturer, and the purity of the CD34+ cell population ranged between 85% and 90%. Murine bone marrow stromal cell lines MS-5 and NIH-3T3 were maintained in α-minimum essential medium (MEM; BioWhittaker) or Dulbecco modified Eagle medium (DMEM; Gibco BRL), respectively, supplemented with 10% FBS and antibiotics. Human umbilical vein endothelial cells (HUVECs) were obtained as described36 and were grown in Medium 199 (Gibco BRL) supplemented with 15% FBS, 5% heparin, and 50 μg/mL endothelial cell growth supplement. Monoclonal antibodies (mAbs) used in this study included anti-CXCR4 44.717.111 (R&D Systems), anti-α4 HP1/2 (a gift from Dr Francisco Sánchez-Madrid, Hospital de la Princesa, Madrid, Spain), anti-α5 (PharMingen, San Diego, CA), and anti–SDF-1 K15C37 (a gift from Dr Fernando Arenzana-Seisdedos, Institut Pasteur, Paris, France). Anti–TGF-β1 polyclonal antibodies were purchased from R&D Systems, and anti-CD34 was purchased from Serotec (Raleigh, NC). The small molecule T134, an antagonist of CXCR4,38 was from Dr Arenzana-Seisdedos.
Reverse transcription–polymerase chain reaction analysis and Western blotting
MS-5 or NIH-3T3 cells were grown to 80% to 90% confluence and were treated for different lengths of time with or without TGF-β1 (R&D Systems) added to the growth media. Cells were lysed in TriReagent (Sigma-Aldrich, St Louis, MO), and RNA was extracted and reverse transcribed using Superscript reverse transcriptase (Gibco BRL). Amplification of SDF-1 was performed by PCR using primers 5′-ACGCCAAGGTCGTCGCCGTGCTGG-3′ and 5′-GTTAGGGTAATACAATTCCTTAGA-3′ and Amplitaq DNA polymerase (Roche Applied Science, Indianapolis, IN). The PCR profile consisted of 2-minute initial denaturation at 94°C followed by 35 cycles of 1-minute denaturation at 94°C, 1-minute annealing at 60°C, and 1-minute polymerization at 72°C, and finally by 10-minute extension at 72°C. Aliquots of each sample were amplified using the same conditions with β-actin primers 5′-CCTTCCTTCTTGGGTATGG-3′ and 5′-AGCACTTGCGGTGCACGAT-3′ as the cDNA loading control. For Western blotting, supernatants recovered from MS-5 cells treated with or without TGF-β1, or their corresponding cell lysates, were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and were analyzed by Western blot with K15C mAb, as previously reported.37
Transient transfection and luciferase assays
pCMVLuc plasmid containing the cytomegalovirus (CMV) promoter upstream of the luciferase gene was created by subcloning the HindIII-XhoI fragment from pGEMLuc (Promega, Madison, WI) in pCDNA3.1 (Invitrogen, Paisley, Scotland). This plasmid was used as luciferase positive. A negative control plasmid, denominated pΔPROMLuc was created by deleting the SpeI-BamHI fragment that contains the CMV promoter sequence. A 1.1-kb fragment of the SDF-1 5′ flanking region and first exon was amplified from human genomic DNA and subcloned in pΔPROMLuc (C.G.-M. et al, manuscript submitted). MS-5 cells were transiently transfectd by electroporation (260 V, 1200 μF) with 4 μg luciferase reporter plasmids per 106 cells in 0.5 mL RPMI 1640 supplemented with 20% fetal calf serum. Transfected cells were cultured for 24 hours in RPMI 1640 with 10% fetal calf serum, trypsinized, and plated on 6-well plates at 0.5 × 106 per well. Cells were incubated with TGF-β1 several times, washed with phosphate-buffered saline (PBS), and processed according to the manufacturer's instructions for the luciferase assay system (Promega) and the Bradford protein assay (Bio-Rad, Hercules, CA). Luciferase activity was measured in triplicate using an automatic luminometer (Sirius V2; Berthold, Pforzheim, Germany). The relative luciferase units were calculated after normalization by microgram of protein.
Cell chemotaxis and transendothelial migration assays
NCI-H929 and Mo7e cells (2 × 105) or CD34+ cells (1.5 × 105) in 100 μL adhesion medium (RPMI 1640/0.5% bovine serum albumin [BSA]) were placed in the upper chamber of Transwells (5-μm pore size; Costar, Cambridge, MA). The lower chamber contained 600 μL adhesion medium, MS-5 supernatants diluted 1:2 in adhesion medium, or 100 ng/mL recombinant SDF-1α (R&D Systems). MS-5 supernatants were obtained from cells grown to 80% to 90% confluence and then were treated for 3 days with daily additions of 2 ng/mL TGF-β1. For transendothelial migrations, 5 × 104 HUVECs were plated on the upper chambers of Transwells 1 day before the assay was performed. In some experiments, the lower chamber contained an MS-5 monolayer that had been treated with or without TGF-β1 as described above for the obtainment of MS-5 supernatants. Chemotaxis and transendothelial migration were carried out for 3 hours at 37°C. Viable migrated cells were counted using a flow cytometer (Coulter Epics XL, Holliston, MA) by passing each sample in the same predetermined time and flow conditions.
Cell adhesion assays
The fibronectin fragment FN-H89, which contains the CS-1 site and lacks the Arg-Gly-Asp (RGD) central binding domain, was generated as previously described.39 Recombinant human sVCAM-1 was obtained from R&D Systems, and plasma fibronectin was obtained from Sigma. Cells were labeled for 20 minutes at 37°C with the fluorescent dye BCECF-AM (Molecular Probes, Leiden, The Netherlands), washed, resuspended in adhesion medium, and preincubated with or without antibodies or reagents. Cells were then treated for 1 minute at 37°C with 100 ng/mL SDF-1α or MS-5 supernatants diluted 1:2 and were added (5 × 104 for cell lines or 1 × 105 for CD34+ cells) in triplicate to 96-well dishes (High-binding; Costar) previously coated with the corresponding ligands, as described.35 Plates were spun for 15 seconds to place cells in contact with the ligand and were allowed to adhere for 2 minutes at 37°C for NCI-H929 and Mo7e cells or 4 minutes for CD34+ cells. Unbound cells were removed by 3 washes with RPMI 1640 medium, and adhered cells were quantified in a fluorescence analyser (POLARStar Galaxy; BMG Labtechnologies, Offenburg, Germany).
Statistical analysis
The results are expressed as the mean ± SD of duplicate or triplicate data obtained from 2 or more experiments. Statistical significance was determined using the 2-tailed Student t test.
Results
TGF-β1 down-regulates expression of SDF-1 on BM stromal cells
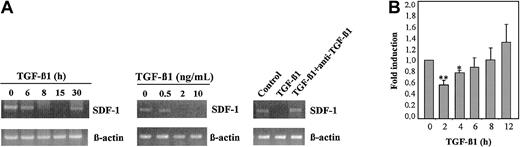
We used MS-5 BM stromal cells to investigate the role of TGF-β1 in SDF-1 expression. Reverse transcription–polymerase chain reaction (RT-PCR) time kinetics revealed that SDF-1 mRNA levels were substantially reduced after 8 to 15 hours of incubation with TGF-β1 (2 ng/mL) and recovered after 30 hours of incubation with the cytokine (Figure 1A, left panel). Dose-response experiments carried out using 8-hour incubation with TGF-β1 showed that the reduction in SDF-1 mRNA was best detected with concentrations of the cytokine of 2 ng/mL or higher (Figure 1A, middle panel). In addition, anti–TGF-β1 antibodies inhibited the decrease in SDF-1 mRNA levels triggered by TGF-β1 (Figure 1A, right panel). Reporter gene assays performed with MS-5 cells electroporated with a plasmid containing the luciferase gene under control of the SDF-1 5′-flanking region (C.G.-M. et al, manuscript submitted) indicated that TGF-β1 (4 ng/mL) caused a near 50% reduction in the activity of SDF-1 proximal promoter after 2-hour treatment (Figure 1B), suggesting that a TGF-β1–induced decrease in SDF-1 expression is at least partially mediated through transcriptional regulation.
TGF-β1 down-regulates SDF-1 mRNA expression. MS-5 stromal cell monolayers were incubated for the indicated times with 2 ng/mL TGF-β 1 (A, left) for 8 hours with different concentrations of TGF-β 1 (A, middle) or for 8 hours with 2 ng/mL TGF-β 1 in the presence or absence of anti–TGF-β 1 antibodies (A, right). RNA from cell lysates was reverse transcribed, and amplification of SDF-1 mRNA was performed by PCR using SDF-1–specific primers. Also shown is control PCR amplification of each sample using β -actin–specific primers. (B) Kinetics of induction of SDF-1 promoter activity by TGF-β 1 in MS-5 cells. Fold induction values indicate relative luciferase activity per microgram protein in TGF-β 1–treated cells divided by the values of untreated cells. Data represent the mean ± SD of triplicate samples from a representative result of 4 experiments. Reduction was significant (**P < .005; *P < .05) according to the Student 2-tailed t test.
TGF-β1 down-regulates SDF-1 mRNA expression. MS-5 stromal cell monolayers were incubated for the indicated times with 2 ng/mL TGF-β 1 (A, left) for 8 hours with different concentrations of TGF-β 1 (A, middle) or for 8 hours with 2 ng/mL TGF-β 1 in the presence or absence of anti–TGF-β 1 antibodies (A, right). RNA from cell lysates was reverse transcribed, and amplification of SDF-1 mRNA was performed by PCR using SDF-1–specific primers. Also shown is control PCR amplification of each sample using β -actin–specific primers. (B) Kinetics of induction of SDF-1 promoter activity by TGF-β 1 in MS-5 cells. Fold induction values indicate relative luciferase activity per microgram protein in TGF-β 1–treated cells divided by the values of untreated cells. Data represent the mean ± SD of triplicate samples from a representative result of 4 experiments. Reduction was significant (**P < .005; *P < .05) according to the Student 2-tailed t test.
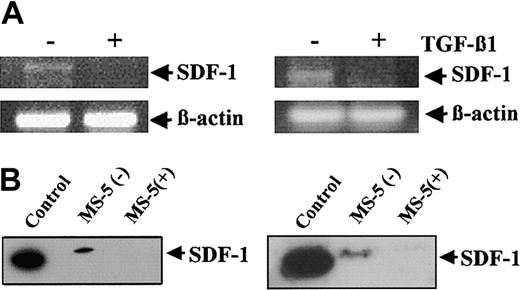
To study whether the reduction in SDF-1 mRNA expression in MS-5 cells treated with TGF-β1 correlated with a decrease in SDF-1 protein in the supernatants, we performed Western blot analysis using the anti-SDF–1α mAb K15C. However, no SDF-1 could be detected in supernatants from untreated MS-5 cells after 24-hour culture (not shown). Therefore, to increase the accumulation of SDF-1 protein, we decided to test whether daily additions of TGF-β1 (2 ng/mL) to MS-5 cells for several days could also result in a reduction of SDF-1 mRNA levels. RT-PCR analyses using these incubation conditions for 3 days revealed that SDF-1 mRNA levels in MS-5 cells were substantially decreased by TGF-β1 (Figure 2A, left panel). Similarly, incubation of NIH-3T3 embryonic BM fibroblasts with TGF-β1 for 3 days resulted in a large inhibition of SDF-1 mRNA expression compared with control untreated samples (Figure 2A, right panel). Trypan blue exclusion and cell cycle analysis by flow cytometry showed no significant differences in viability between MS-5 cells incubated for 3 days with or without TGF-β1 (not shown).
Effect of prolonged exposure of BM stromal cells to TGF-β 1 on SDF-1 expression. (A) MS-5 (left) or NIH-3T3 (right) cells were incubated for 3 days in the absence (–) or in the presence (+) of TGF-β 1, with the addition of this cytokine (2 ng/mL) every 24 hours. Cells were lysed and subjected to RT-PCR analysis using SDF-1 or β -actin primers, as described for Figure 1. (B) MS-5 cells were treated for 3 days as described in panel A, without (–) or with (+) TGF-β 1. Supernatants (left) and cell lysates (right) were subjected to Western blot using anti–SDF-1α antibodies. Recombinant SDF-1 (50 ng) was used as control.
Effect of prolonged exposure of BM stromal cells to TGF-β 1 on SDF-1 expression. (A) MS-5 (left) or NIH-3T3 (right) cells were incubated for 3 days in the absence (–) or in the presence (+) of TGF-β 1, with the addition of this cytokine (2 ng/mL) every 24 hours. Cells were lysed and subjected to RT-PCR analysis using SDF-1 or β -actin primers, as described for Figure 1. (B) MS-5 cells were treated for 3 days as described in panel A, without (–) or with (+) TGF-β 1. Supernatants (left) and cell lysates (right) were subjected to Western blot using anti–SDF-1α antibodies. Recombinant SDF-1 (50 ng) was used as control.
Western blot analysis of 3-day supernatants of MS-5 cells displayed SDF-1 protein, as detected with the K15C mAb (Figure 2A, left). In contrast, no SDF-1 could be detected in the supernatants from MS-5 cells incubated with TGF-β1. As expected, the K15C mAb did not detect SDF-1 protein in lysates from TGF-β1–treated MS-5 cells, in contrast to nontreated samples (Figure 2B, right). The slight difference in electrophoretic mobility observed between control recombinant SDF-1 and SDF-1 from MS-5 supernatants likely reflected posttranslational modifications taking place in these cells. Together, these data indicate that continuous exposure to TGF-β1 results in a large decrease in SDF-1 expression in MS-5 cells.
Effect of TGF-β1–triggered down-regulation of SDF-1 secretion on cell migration and adhesion
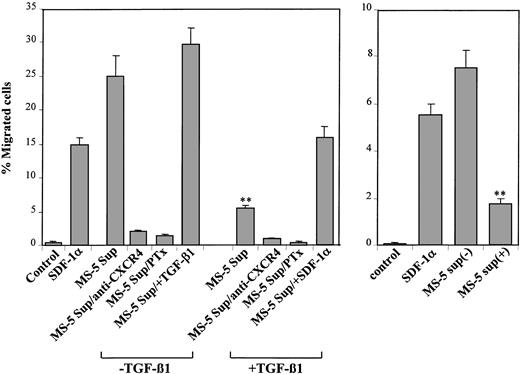
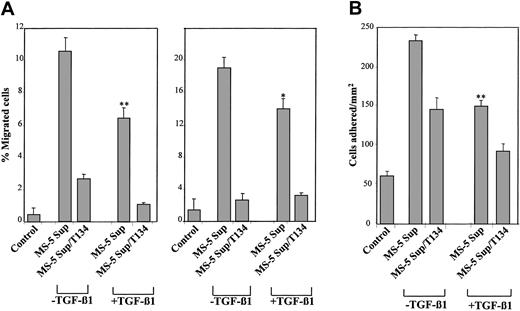
To determine whether the reduction in SDF-1 protein in the supernatants of MS-5 cells incubated with TGF-β1 had functional consequences in cell migration, we first tested the capability of 2 BM model cell lines, myeloma NCI-H929 and megakaryocytic leukemia-derived, GM-CSF–dependent Mo7e cells, both expressing CXCR4,9,25 to respond to the chemotactic activity present in those supernatants. NCI-H929 cells displayed a notable chemotactic response to supernatants from MS-5 cells, and this chemotaxis was inhibited by anti-CXCR4 antibodies and by pertussis toxin (Ptx) (Figure 3, left), indicating the presence of SDF-1 chemotactic activity in these supernatants. Addition of TGF-β1 (2 ng/mL) to these supernatants during the chemotaxis assay did not significantly influence the migratory response of these cells. When NCI-H929 cells were assayed in chemotaxis to supernatants from MS-5 cells exposed to TGF-β1 for 3 days, a notable reduction (60%-75%) in chemotactic efficiency was observed that was blocked by Ptx and anti-CXCR4 mAb. Furthermore, the addition of exogenous SDF-1 to these supernatants just before the chemotaxis assay resulted in a recovery of the chemotactic efficiency of NCI-H929 cells to levels close to those displayed toward their untreated counterparts (Figure 3, left). Likewise, the reduction in chemotaxis to MS-5 supernatants obtained from incubations with TGF-β1 was also clearly detected when we used Mo7e cells (Figure 3, right).
Cell chemotaxis to supernatants from untreated and TGF-β 1–treated MS-5 cells. (Left) NCI-H929 cells were preincubated with anti-CXCR4 antibodies, Ptx, or adhesion medium alone and were allowed to migrate to lower chambers containing supernatants from MS-5 cells treated with or without TGF-β 1 (+ TGF-β 1 and –TGF-β 1, respectively). To some conditions, TGF-β 1 or SDF-1α was added to the medium (MS-5 Sup/+ TGF-β 1 or MS-5 Sup/+ SDF-1α, respectively). (Right) Mo7e cells were subjected to chemotaxis to supernatants from MS-5 cells incubated in the absence (–) or in the presence (+) of TGF-β 1. Also shown is cell chemotaxis to recombinant SDF-1α (100 ng/mL) compared with the chemotaxis to adhesion medium (control). Data represent the means ± SDs of duplicate samples from a representative result of 4 (left) and 3 (right) experiments. Reduction in chemotaxis was significant (**P < .005), according to Student 2-tailed t test.
Cell chemotaxis to supernatants from untreated and TGF-β 1–treated MS-5 cells. (Left) NCI-H929 cells were preincubated with anti-CXCR4 antibodies, Ptx, or adhesion medium alone and were allowed to migrate to lower chambers containing supernatants from MS-5 cells treated with or without TGF-β 1 (+ TGF-β 1 and –TGF-β 1, respectively). To some conditions, TGF-β 1 or SDF-1α was added to the medium (MS-5 Sup/+ TGF-β 1 or MS-5 Sup/+ SDF-1α, respectively). (Right) Mo7e cells were subjected to chemotaxis to supernatants from MS-5 cells incubated in the absence (–) or in the presence (+) of TGF-β 1. Also shown is cell chemotaxis to recombinant SDF-1α (100 ng/mL) compared with the chemotaxis to adhesion medium (control). Data represent the means ± SDs of duplicate samples from a representative result of 4 (left) and 3 (right) experiments. Reduction in chemotaxis was significant (**P < .005), according to Student 2-tailed t test.
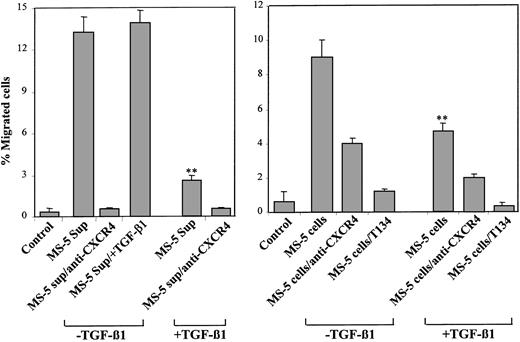
We next performed transendothelial migration assays using NCI-H929 cells, measuring their ability to migrate across HUVEC monolayers in response to chemotactic stimuli present in the supernatants of MS-5 cells. NCI-H929 cells exhibited a substantial migratory response through HUVEC to supernatants from MS-5 cells that was blocked by anti-CXCR4 antibodies (Figure 4, left). Addition of TGF-β1 (2 ng/mL) to these supernatants during transendothelial migration did not significantly influence the migratory response of NCI-H929 cells. As in the chemotaxis assays, NCI-H929 cells showed a notable decrease in transendothelial migration to supernatants from TGF-β1–treated MS-5 cells that was also abolished by anti-CXCR4 mAb. Moreover, when the transendothelial migration assays were carried out using bottom chambers containing cultures of MS-5 cells treated for 3 days with or without TGF-β1, the migration response of NCI-H929 cells was significantly lower to MS-5 cultures incubated with TGF-β1 than to MS-5 cultures incubated without this cytokine (Figure 4, right). These migrations were inhibited by anti-CXCR4 antibodies and by T134, a small molecule known to specifically block SDF-1/CXCR4 interactions.38
Transendothelial migration to supernatants from untreated and TGF-β 1–treated MS-5 cells. NCI-H929 cells were preincubated with anti-CXCR4 antibodies, T134, or adhesion medium alone and were subjected to migration through HUVEC monolayers to lower chambers containing isolated supernatants from MS-5 cells treated with or without TGF-β 1 (+ TGF-β 1 and –TGF-β 1, respectively) (left) or to MS-5 cell cultures in the lower chambers treated for 3 days with or without TGF-β 1 (+ TGF-β 1 and –TGF-β 1, respectively) (right). To some conditions, TGF-β 1 was added to the medium (MS-5 Sup/+ TGF-β 1). Data represent the means ± SDs of duplicate samples from a representative result of 4 (left) and 2 (right) experiments. Reduction in cell migration was significant (**P < .005), according to Student 2-tailed t test.
Transendothelial migration to supernatants from untreated and TGF-β 1–treated MS-5 cells. NCI-H929 cells were preincubated with anti-CXCR4 antibodies, T134, or adhesion medium alone and were subjected to migration through HUVEC monolayers to lower chambers containing isolated supernatants from MS-5 cells treated with or without TGF-β 1 (+ TGF-β 1 and –TGF-β 1, respectively) (left) or to MS-5 cell cultures in the lower chambers treated for 3 days with or without TGF-β 1 (+ TGF-β 1 and –TGF-β 1, respectively) (right). To some conditions, TGF-β 1 was added to the medium (MS-5 Sup/+ TGF-β 1). Data represent the means ± SDs of duplicate samples from a representative result of 4 (left) and 2 (right) experiments. Reduction in cell migration was significant (**P < .005), according to Student 2-tailed t test.
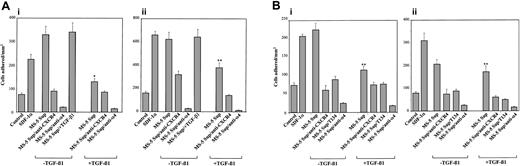
We previously reported that SDF-1 up-regulates integrin α4β1-dependent adhesion of NCI-H929 and Mo7e cells.9,25 To analyze whether the decrease in SDF-1 protein in the supernatants of MS-5 cells treated with TGF-β1 could influence the extent of enhancement of this adhesion, we used both cell lines to perform adhesion assays to sVCAM-1, a soluble form of VCAM-1, and to FN-H89, a CS-1–containing fragment of fibronectin. NCI-H929 and Mo7e adhesions to sVCAM-1 and FN-H89 were substantially up-regulated by supernatants from untreated MS-5 cells, and the increase in adhesion was inhibited by anti-CXCR4 mAb and T134 (Figure 5A-B). The increased adhesions were blocked by HP1/2 anti-α4 mAb, confirming the specificity of the up-regulated adhesion. When we performed the adhesion assays using supernatants from MS-5 cells exposed to TGF-β1, we obtained a considerably lower triggering of cell adhesion to both α4β1 ligands that was blocked to control medium levels by anti-CXCR4 antibodies and T134 (Figure 5A-B). The addition of TGF-β1 to the 1-minute NCI-H929 preincubation with MS-5 supernatants did not alter the subsequent up-regulation in α4β1-dependent adhesion.
Effect of TGF-β 1–triggered down-regulation of SDF-1 secretion in α 4β 1-dependent cell adhesion. BCECF-AM–labeled NCI-H929 (A) and Mo7e (B) cells were preincubated with anti-CXCR4 or anti-α 4 antibodies, T134, or adhesion medium alone and subsequently were exposed for 1 minute at 37°C to supernatants from MS-5 cells treated with or without TGF-β 1 (+ TGF-β 1 and –TGF-β 1, respectively). Cells were added to wells containing sVCAM-1 (i) or FN-H89 (ii) and were subjected to adhesion for 2 minutes after a short spin. To some adhesions, TGF-β 1 was added to the medium (MS-5 Sup/+ TGF-β 1). Nonbound cells were washed, and the extent of adhesion was measured in a fluorescence analyzer. Also shown is the triggering of cell adhesion by recombinant SDF-1α (100 ng/mL) compared with samples incubated with adhesion medium alone (control). Data represent the means ± SDs of triplicate samples from a representative result of 3 experiments for each cell type. Reduction in triggering of cell adhesion was significant (**P < .005; *P < .05), according to Student 2-tailed t test.
Effect of TGF-β 1–triggered down-regulation of SDF-1 secretion in α 4β 1-dependent cell adhesion. BCECF-AM–labeled NCI-H929 (A) and Mo7e (B) cells were preincubated with anti-CXCR4 or anti-α 4 antibodies, T134, or adhesion medium alone and subsequently were exposed for 1 minute at 37°C to supernatants from MS-5 cells treated with or without TGF-β 1 (+ TGF-β 1 and –TGF-β 1, respectively). Cells were added to wells containing sVCAM-1 (i) or FN-H89 (ii) and were subjected to adhesion for 2 minutes after a short spin. To some adhesions, TGF-β 1 was added to the medium (MS-5 Sup/+ TGF-β 1). Nonbound cells were washed, and the extent of adhesion was measured in a fluorescence analyzer. Also shown is the triggering of cell adhesion by recombinant SDF-1α (100 ng/mL) compared with samples incubated with adhesion medium alone (control). Data represent the means ± SDs of triplicate samples from a representative result of 3 experiments for each cell type. Reduction in triggering of cell adhesion was significant (**P < .005; *P < .05), according to Student 2-tailed t test.
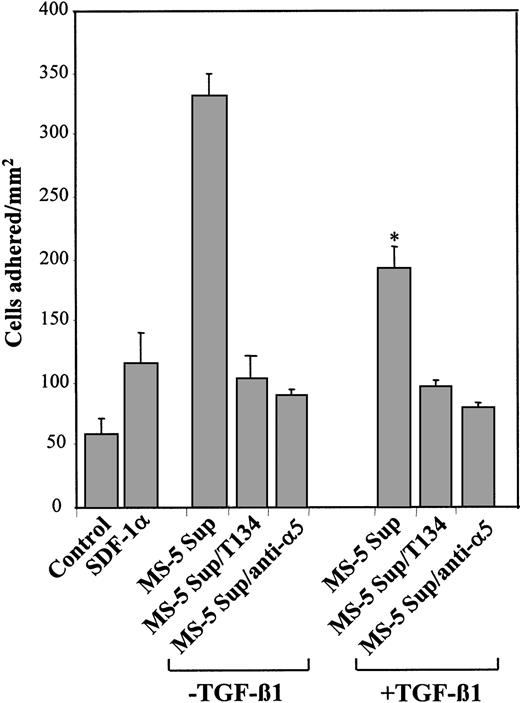
We also tested whether the stimulation of integrin α5β1–dependent cell adhesion to fibronectin by SDF-1 could be affected in supernatants from TGF-β1–treated MS-5 cells. Results showed that supernatants from untreated cells notably up-regulated α5β1-mediated adhesion of NCI-H929 to plasma fibronectin, which was substantially inhibited by T134 and anti-α5 mAb. As with α4β1, supernatants from TGF-β1–treated MS-5 cells exhibited significantly decreased efficiency in the up-regulation of this adhesion (Figure 6). Altogether, these data indicate that the decrease in SDF-1 protein levels in the supernatants of TGF-β1–treated MS-5 cells resulted in impairment of SDF-1–mediated NCI-H929 and Mo7e cell migration and adhesion-promoting activities.
Effect of TGF-β 1–triggered down-regulation of SDF-1 secretion inα 5β 1-dependent cell adhesion to fibronectin. BCECF-AM–labeled NCI-H929 cells were preincubated with T134 or anti-α 5 mAb and subsequently were exposed for 1 minute at 37°C to supernatants from MS-5 cells treated with or without TGF-β 1 (+ TGF-β 1 and –TGF-β 1, respectively). Cells were added to wells containing plasma fibronectin and were subjected to adhesion for 2 minutes after a short spin. Nonbound cells were washed, and the extent of adhesion was measured in a fluorescence analyzer. Also shown is the triggering of cell adhesion by recombinant SDF-1α (100 ng/mL) compared with samples incubated with adhesion medium alone (control). Data represent the means ± SDs of triplicate samples from a representative result of 3 experiments. Reduction in triggering of cell adhesion was significant (*P < .05), according to Student 2-tailed t test.
Effect of TGF-β 1–triggered down-regulation of SDF-1 secretion inα 5β 1-dependent cell adhesion to fibronectin. BCECF-AM–labeled NCI-H929 cells were preincubated with T134 or anti-α 5 mAb and subsequently were exposed for 1 minute at 37°C to supernatants from MS-5 cells treated with or without TGF-β 1 (+ TGF-β 1 and –TGF-β 1, respectively). Cells were added to wells containing plasma fibronectin and were subjected to adhesion for 2 minutes after a short spin. Nonbound cells were washed, and the extent of adhesion was measured in a fluorescence analyzer. Also shown is the triggering of cell adhesion by recombinant SDF-1α (100 ng/mL) compared with samples incubated with adhesion medium alone (control). Data represent the means ± SDs of triplicate samples from a representative result of 3 experiments. Reduction in triggering of cell adhesion was significant (*P < .05), according to Student 2-tailed t test.
To investigate whether the migration and adhesion of primary cells was affected by the reduction of SDF-1 protein levels as a result of TGF-β1 action on BM stromal cells, we used human cord blood CD34+ cells in chemotaxis, transendothelial migration, and adhesion assays in response to supernatants from untreated and TGF-β1–treated MS-5 cells. CD34+ cells displayed notable chemotactic and transendothelial migration responses to supernatants from untreated MS-5 cells that were inhibited by T134 (Figure 7A). When supernatants from TGF-β1–exposed MS-5 cells were used, significant SDF-1–dependent decreased chemotaxis and migration across HUVECs of CD34+ cells were detected, though the extent of reduced migration was lower than that observed with cell lines. Similarly, CD34+ cells showed decreased stimulation in adhesion to sVCAM-1 when supernatants from TGF-β1–exposed MS-5 cells were used compared with untreated ones (Figure 7B). The triggering of CD34+ cell adhesion to sVCAM-1 in response to MS-5 supernatants was inhibited by T134, and anti-α4 HP1/2 mAb blocked adhesion to sVCAM-1 (not shown). Therefore, these results indicate that the down-regulation in cell migration and adhesion as a consequence of TGF-β1–dependent reduction in SDF-1 protein expression levels in stromal MS-5 supernatants can also be detected with primary CD34+ cells.
Migration and adhesion of cord blood CD34+ cells to supernatants from untreated and TGF-β 1–treated MS-5 cells. CD34+ cells isolated from human cord blood samples were preincubated with T134 and subjected to chemotaxis (A, left) or transendothelial migration across HUVECs (A, right) to supernatants from MS-5 cells treated with or without TGF-β 1 (+ TGF-β 1 and –TGF-β 1, respectively). Data represent the mean ± SD of 6 (left) and 5 (right) experiments. Reduction in cell migration was significant (**P < .005; *P < .05), according to Student 2-tailed t test. (B) BCECF-AM–labeled CD34+ cells were preincubated with T134 and subsequently exposed for 1 minute at 37°C to supernatants from MS-5 cells incubated with or without TGF-β 1. Cells were added to wells containing sVCAM-1 and were subjected to adhesion for 4 minutes after a short spin. Nonbound cells were washed, and the extent of adhesion was measured in a fluorescence analyzer. Data represent the means ± SDs of triplicate samples from a representative result of 3 experiments. Reduction in triggering of cell adhesion was significant (**P < .005), according to Student 2-tailed t test.
Migration and adhesion of cord blood CD34+ cells to supernatants from untreated and TGF-β 1–treated MS-5 cells. CD34+ cells isolated from human cord blood samples were preincubated with T134 and subjected to chemotaxis (A, left) or transendothelial migration across HUVECs (A, right) to supernatants from MS-5 cells treated with or without TGF-β 1 (+ TGF-β 1 and –TGF-β 1, respectively). Data represent the mean ± SD of 6 (left) and 5 (right) experiments. Reduction in cell migration was significant (**P < .005; *P < .05), according to Student 2-tailed t test. (B) BCECF-AM–labeled CD34+ cells were preincubated with T134 and subsequently exposed for 1 minute at 37°C to supernatants from MS-5 cells incubated with or without TGF-β 1. Cells were added to wells containing sVCAM-1 and were subjected to adhesion for 4 minutes after a short spin. Nonbound cells were washed, and the extent of adhesion was measured in a fluorescence analyzer. Data represent the means ± SDs of triplicate samples from a representative result of 3 experiments. Reduction in triggering of cell adhesion was significant (**P < .005), according to Student 2-tailed t test.
Discussion
Cell motility inside the BM environment and migration to or from the BM are controlled by a close interplay between selective chemokines and adhesion molecules. Changes in the expression of these molecules could potentially affect the migration pattern of cells trafficking the bone marrow. The chemokine SDF-1 is expressed by BM stromal cells and plays key roles in the migration, adhesion, and activation of HPC and leukemic cells.2-9,40-42 TGF-β1 is also present in the BM environment and is a pivotal cytokine controlling cell proliferation and differentiation.31 In the present study we used the BM stromal cell line MS-5, which expresses SDF-1 and supports the differentiation of human HPCs,1,43 to investigate whether SDF-1 expression can be a target of regulation by TGB-β1. We show here that TGF-β1 down-regulates the expression of SDF-1 on MS-5 cells, both at the mRNA level involving at least a decrease in transcriptional efficiency and at the protein level, as detected in MS-5 cell lysates and supernatants. In addition to MS-5, a reduction in SDF-1 expression by TGB-β1 was also detected on NIH-3T3 BM stromal cells.
The functional relevance of these findings was characterized using the CXCR4+ cell lines myeloma NCI-H929 and CD34+ GM-CSF–dependent Mo7e cells. The reduction in SDF-1 protein levels in supernatants from MS-5 cells treated with TGF-β1 correlated with a decrease in the chemotaxis and transendothelial migration responses of NCI-H929 and Mo7e cells compared with their responses to supernatants from untreated MS-5 cells. Reduction in transendothelial migration was detected when we used isolated supernatants from TGF-β1–treated MS-5 cells in the bottom wells of chemotactic chambers and when we used confluent MS-5 monolayers cultured with TGF-β1 on those bottom wells. The recovery in cell chemotaxis with the addition of exogenous SDF-1 to supernatants from MS-5 cells treated with TGF-β1 further confirms the correlation between the decrease in SDF-1 protein levels with diminished chemotactic activity.
Cell migration involves a dynamic regulation of adhesion events. Cell adhesion mediated by integrin α4β1 constitutes a target of SDF-1 activation in HPCs and myeloma cells,9,24,25 and up-regulation in the adhesive activity of this integrin has been shown to mediate SDF-1–triggered CD34+ cell migration.44 Here we show that supernatants from TGF-β1–treated MS-5 cells have a substantially SDF-1–dependent, lowered efficiency in triggering α4β1-mediated adhesion of NCI-H929 and Mo7e cells to sVCAM-1 and to CS-1/FN compared with the up-regulation of adhesion obtained with supernatants from untreated MS-5 cells. Similarly, there was a significantly lower enhancement of α5β1-dependent NCI-H929 cell adhesion to plasma fibronectin with supernatants from MS-5 cells incubated with TGF-β1 compared with their untreated counterparts.
To test whether primary cells could also detect TGF-β1–controlled changes in SDF-1 protein levels and to show altered functional responses, we used human cord blood CD34+ hematopoietic progenitor cells. The results revealed that CD34+ cells had reduced responses in chemotaxis, transendothelial migration, and induction of adhesion to sVCAM-1 when supernatants from TGF-β1–treated MS-5 cells were used compared with untreated MS-5 counterparts, though the level of reduction in the response was smaller than that obtained with cell lines. Altogether these data indicate that the reduction in SDF-1 expression by TGF-β1 in BM stromal cells affects subsequent cell migration and that an impairment in the triggering of SDF-1–dependent, α4β1- or α5β1-mediated adhesion could contribute to the decrease in cell migration. Although in most migration and adhesion assays NCI-H929, Mo7e, and CD34+ cell preincubation with anti-CXCR4 antibodies or CXCR4 antagonist T134 abrogated SDF-1 activity from MS-5 supernatants, in a few instances inhibition was incomplete, raising the possibility that additional, but minor, chemotactic stimuli might be present in these supernatants.
It has previously been reported that several metalloproteinases (MMPs), including MMP-2 and MMP-9, are capable of cleaving and inactivating SDF-1.45 Zymography and Western blot analysis failed to detect increases in MMP-2 or MMP-9 in the supernatants of TGF-β1–treated MS-5 cells compared with their untreated counterparts (not shown), suggesting that at least the activity of these 2 MMPs was unlikely to contribute to reduced SDF-1 levels in the supernatants of TGF-β1–treated MS-5 cells. However, we cannot completely exclude the possibility that other soluble proteases activated by TGF-β1 might play some role in the reduction of SDF-1 levels.
The decrease in migration and adhesion achieved with the 2 BM model cell lines and with CD34+ cells as a result of TGF-β1–triggered reduction in SDF-1 levels suggests that it could possibly influence several cell trafficking scenarios in the bone marrow. SDF-1 chemotactic gradients between distinct niches inside the BM might be affected by TGF-β1 action on BM stromal cells, which in turn could influence hematopoietic progenitor cell migration, potentially involving integrin-dependent adhesion. Therefore, changes in these chemotactic gradients might control CD34+ cell localization and retention in the BM.
On the other hand, higher concentrations of TGF-β1 are found in BM samples from patients with leukemia, such as multiple myeloma and B-cell lymphocytic leukaemia.32,33 Because α4β1 constitutes one of the main adhesion molecules contributing to myeloma cell localization in the BM and its adhesive activity can be regulated by SDF-1,9,23 it is tempting to hypothesize that a reduction in SDF-1 production as a result of TGF-β1 action on multiple myeloma BM stromal cells might regulate myeloma cell trafficking in the bone marrow.
Moreover, SDF-1 expressed by BM stromal cells is exposed on BM endothelium,24 and down-regulation of SDF-1 expression in response to TGF-β1 might locally limit the amount of displayed SDF-1, which could also affect cell recruitment to the BM. Our data on reduced CD34+ and NCI-H929 cell migration across HUVEC monolayers to supernatants from TGF-β1–treated MS-5 cells, an in vitro model for cell migration to the BM, do suggest that a decrease in the amount of SDF-1 might restrict cell homing to the BM.
The control of SDF-1 expression by TGF-β1 might not be limited to the BM environment because both molecules are widely expressed. TGF-β1–deficient mice showed multifocal inflammatory disease in several tissues that was associated with an increase in the expression of proinflammatory mediators such as TNF-α and MIP-1α,46,47 suggesting that TGF-β1 controls the expression of these molecules. Although SDF-1 is not considered a proinflammatory chemokine, it can contribute to leukocyte extravasation because it can modulate α4 integrin–dependent lymphocyte adhesion48,49 ; therefore, down-regulation in the expression of SDF-1 by TGF-β1 could potentially influence leukocyte transendothelial migration.
In addition to its effect on SDF-1 expression reported here, TGF-β1 has also been shown to decrease the expression of MIP-1α,50 another chemokine present in the BM environment. On the other hand, it has recently been reported that G-CSF transiently increased SDF-1 expression on murine BM osteoblasts, but the general effect was a decrease in SDF-1 in the BM by a mechanism involving neutrophil elastase degradation.17 These data indicate that different mechanisms underlay the differential regulation of SDF-1 expression by TGF-β1 and G-CSF.
Collectively, the results from the present work indicate that, in addition to controlling the proliferation and differentiation of HPCs by down-regulating SDF-1 expression, TGF-β1 could influence the migration and integrin-dependent adhesion of CD34+ and leukemia cells in the bone marrow.
Prepublished online as Blood First Edition Paper, May 29, 2003; DOI 10.1182/blood-2002-10-3190.
Supported by grants from Ministerio de Ciencia y Tecnología (SAF99-0057, SAF02-00207) (J.T.), Ministerio de Sanidad y Consumo (01/1168) (J.T.), Ministerio de Educación y Ciencia (1999-0122) (A.C.), and Fundación para la Investigación y prevención del SIDA (3120/00) (A.C.).
N.W. and T.L.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Francisco Sánchez-Madrid, Fernando Arenzana-Seisdedos, Françoise Baleux, and Santiago Lamas for reagents and Dr Angel Corbí for helpful discussions. We also thank Dr Francisco Sanz-Rodríguez for his help in the initial parts of this work and Dr Pedro Lastres for flow cytometry.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal