Abstract
Chemokines control inflammatory leukocyte recruitment. The propensity of chemokines such as CC chemokine ligand 5 (CCL5)/RANTES (regulated on activation, normal T cell expressed and secreted) to bind to glycosaminoglycans and to form higher order oligomers has been shown to be essential for its in vivo activity. However, the specific functional relevance of RANTES oligomerization for distinct steps of leukocyte recruitment on inflamed endothelium mediated by the RANTES receptors CC chemokine receptor 1 (CCR1) and CCR5 remains undefined. We studied RANTES mutants with deficient oligomerization in an assay in which recruitment of monocytes and CD45RO+ CD4+ T cells is triggered by RANTES immobilized on activated endothelium under flow conditions. Notably, the formation of higher order RANTES oligomers was crucial for CCR1-mediated arrest but not for CCR5-mediated spreading/transmigration in flow or transendothelial chemotaxis of leukocytes. Efficient leukocyte arrest in flow but not transmigration may thus require the presentation of RANTES oligomers to bridge surface-bound RANTES and CCR1.
Introduction
Chemokines control the selective migration of leukocytes in immune surveillance, inflammation, and atherogenesis.1,2 This involves not only the directed migration of leukocytes along chemokine gradients within the extracellular matrix but also the arrest of circulating leukocytes on activated endothelium under flow. The immobilization of chemokines to endothelium via binding to glycosaminoglycans (GAGs) has been implicated in triggering leukocyte arrest, as well as transmigration (or chemorheotaxis) in flow.3-7 Moreover, binding of chemokines to GAGs present on leukocytes may influence chemotactic responses.8,9 The BBXB motif, in which B represents a basic amino acid, has been implicated as the principal heparin or heparan-sulfate binding site on several proteins.10 This motif is important for the chemokine-heparin interaction by mutagenesis studies (eg, 44AANA47-RANTES).8,11-15 Single residue substitutions in this motif reduced GAG-binding and chemotaxis across endothelial monolayers, but not bare transwells, while retaining receptor affinity.16 The oligomerization of RANTES (regulated on activation, normal T cell expressed and secreted) may also influence its functional properties, for example, dimeric or tetrameric mutants retained G-protein–coupled receptor-mediated activities, but prolonged effects via protein tyrosine kinase activation were attenuated.17-19 While GAGs mediate cell surface oligomerization of chemokines,17 the state of oligomerization may conversely modulate the capacity for GAG-binding depending on the chemokine.20,21
We have recently found that both GAG-binding and oligomerization of chemokines represent essential structural features for their ability to recruit leukocytes in vivo.21 However, the contribution of oligomerization to distinct steps of leukocyte recruitment has not been studied. RANTES immobilized on activated endothelium can trigger leukocyte arrest and transmigration, which are mediated by specialized roles of CC chemokine receptor 1 (CCR1) and CCR5, respectively.6 Here, we have investigated the functional effects of RANTES mutants with defects in oligomerization in physiologic assays of RANTES-triggered leukocyte recruitment on endothelium. The results demonstrate differential requirements for oligomerization of RANTES in CCR1-mediated arrest and CCR5-mediated transmigration.
Study design
Cells and reagents
Human microvascular endothelial cells (HMVECs; PromoCell, Heidelberg, Germany) and monocytic Mono Mac 6 cells were cultured as described.5,6 HMVECs were stimulated with interleukin-1β (IL-1β; 10 ng/mL) for 24 hours. Monocytes and peripheral blood mononuclear cells (PBMCs) were isolated by NycoPrep 1.068 (Nycomed, Oslo, Norway) or Ficoll hypaque density gradient centrifugation, respectively.5,6 CD45RO+CD4+ T lymphocytes were purified by negative selection using magnetic separation (Miltenyi Biotec, Bergisch Gladbach, Germany). Human IL-1β and RANTES were from PeproTech (Rocky Hill, NJ). The E26A, E66A, 44AANA47-RANTES mutants, and chemically modified Nme-Thr7-RANTES were produced as described.13,21
Leukocyte recruitment under flow conditions, adhesion, and transendothelial chemotaxis assays
Monocyte adhesion experiments on purified intercellular adhesion molecule-1 (ICAM-1) were performed as described.22 Laminar flow assays and chemotaxis were performed as described.5,6,23 Confluent HMVECs activated with IL-1β (10 ng/mL) overnight were preincubated with RANTES or mutants at 20 nM for 30 minutes at 37°C. Surface enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN) was used to determine the heparan sulfate–specific immobilization of RANTES or mutants on HMVECs treated with or without heparitinase I (0.5 U/mL; Sigma, St Louis, MO) for 2 hours at 37°C. Leukocytes (5 × 105/mL) pretreated with the small molecule antagonists BX471 (provided by Dr R. Horuk, Berlex Biosciences), TAK-779 (provided by Takeda Pharmaceuticals) at 1 μg/mL, or vehicle for 15 minutes6 in assay buffer were perfused at 1.5 dyne/cm2. Arrest, spreading, and/or transmigration was analyzed in multiple high-power fields recorded by video microscopy.5,6,23 Unstimulated HMVECs were grown on Transwell-filter inserts (5-μm pore size; Co-star, Cambridge, MA). PBMCs in RPMI-1640/0.5% bovine serum albumin were allowed to transmigrate across bare or HMVEC-coated filters toward RANTES, mutants, or buffer control in the lower chamber for 90 minutes at 37°C. Transmigrated monocytes were counted by flow cytometry. The ratio of RANTES-induced versus spontaneous migration was defined as chemotactic index.
Results and discussion
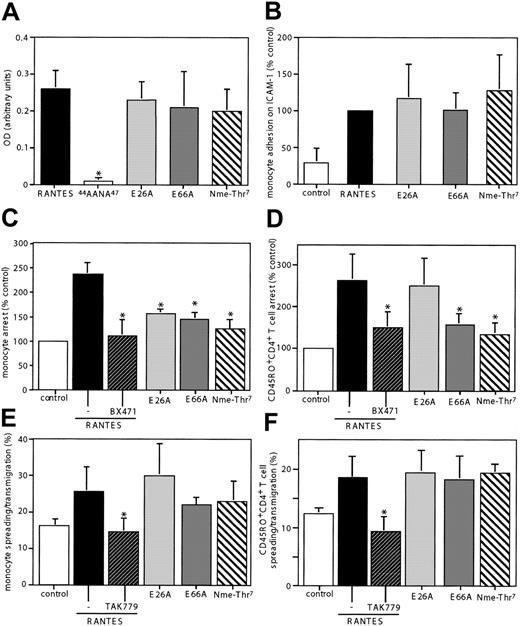
RANTES immobilized on cytokine-activated endothelium triggers leukocyte recruitment in flow.6 We used this model to investigate the effect of RANTES mutants with impaired ability to oligomerize on leukocyte arrest. The structural characteristics of these mutants are summarized in Table 1. While the tetrameric E26A and the dimeric E66A have been described,19,20 Nme-Thr7-RANTES is unable to form hydrogen bonds across the β-sheet dimer interface and was predicted to be monomeric by analogy to an IL-8 variant.24 This property was confirmed by analytical ultracentrifugation, the inability of Nme-Thr7-RANTES to oligomerize on immobilized GAG, and a marginally reduced capacity to bind heparin, reflecting oligomerization on heparin at high concentrations of chemokine in this assay.21 Surface ELISA on activated HMVECs revealed an unaltered heparan sulfate–dependent immobilization of the oligomerization mutants compared with wild-type RANTES (Figure 1A), while that of the GAG-binding mutant 44AANA47-RANTES was abolished. In line with preserved chemotactic activities,20 the ability of all oligomerization mutants to trigger adhesion on purified ICAM-1 in stasis was equivalent to wild-type RANTES, excluding an impairment in intrinsic receptor-binding or signaling properties (Figure 1B).
Biochemical properties of RANTES mutants
Mutation . | Function/structure . |
|---|---|
| 44AANA47 | Principal GAG binding site |
| E46A | Tetrameric |
| E66A | Dimeric |
| Nme-Thr7 | Monomeric |
Mutation . | Function/structure . |
|---|---|
| 44AANA47 | Principal GAG binding site |
| E46A | Tetrameric |
| E66A | Dimeric |
| Nme-Thr7 | Monomeric |
Role of RANTES oligomerization in RANTES-triggered leukocyte arrest and spreading in shear flow. Activated HMVECs were preincubated with wild-type RANTES or RANTES mutants, as indicated. The heparan sulfate–specific immobilization of RANTES or mutants on HMVECs was determined by subtracting the optical density after treatment of HMVECs with heparitinase I from that without heparitinase I (A). Monocytes stimulated with RANTES or mutants at 10 nM at 37°C were subjected to static adhesion on purified ICAM-1 for 15 minutes, and adhesion is given as percentage of input cells (B). Monocytes (C,E) or CD45RO+CD4+ T cells (D,F) pretreated with the CCR1 antagonist BX471, the CCR5 antagonist TAK-779, or dimethyl sulfoxide (0.1%) were perfused across activated HMVECs in a flow chamber at 1.5 dyne/cm2. After 5 minutes, firmly adherent cells per square millimeter were counted and expressed as percentage of control (without RANTES), and the percentage of cells undergoing spreading and/or transmigration among adherent cells was determined. Data represent mean ± SD of 6 separate experiments. *P < .05 versus wild-type RANTES (nonparametric signed-rank tests).
Role of RANTES oligomerization in RANTES-triggered leukocyte arrest and spreading in shear flow. Activated HMVECs were preincubated with wild-type RANTES or RANTES mutants, as indicated. The heparan sulfate–specific immobilization of RANTES or mutants on HMVECs was determined by subtracting the optical density after treatment of HMVECs with heparitinase I from that without heparitinase I (A). Monocytes stimulated with RANTES or mutants at 10 nM at 37°C were subjected to static adhesion on purified ICAM-1 for 15 minutes, and adhesion is given as percentage of input cells (B). Monocytes (C,E) or CD45RO+CD4+ T cells (D,F) pretreated with the CCR1 antagonist BX471, the CCR5 antagonist TAK-779, or dimethyl sulfoxide (0.1%) were perfused across activated HMVECs in a flow chamber at 1.5 dyne/cm2. After 5 minutes, firmly adherent cells per square millimeter were counted and expressed as percentage of control (without RANTES), and the percentage of cells undergoing spreading and/or transmigration among adherent cells was determined. Data represent mean ± SD of 6 separate experiments. *P < .05 versus wild-type RANTES (nonparametric signed-rank tests).
In support of published data,6 the immobilization of wild-type RANTES on activated HMVECs induced substantial arrest of isolated blood monocytes and CD45RO+CD4+ T cells in flow (Figure 1C-D). As shown by inhibition with the CCR1 antagonist BX471,6 RANTES-triggered arrest was mediated by CCR1 (Figure 1C-D). While all oligomerization mutants showed significantly reduced ability to induce shear-resistant monocyte arrest, the most marked defect was found with the monomeric form (Figure 1C). Similar results were obtained with CD45RO+CD4+ T cells for the dimeric E66A and monomeric Nme-Thr7-RANTES, while the tetrameric E26A was equivalent to wild-type RANTES (Figure 1D). The ability of E26A to trigger arrest of T cells but not monocytes may reflect lower levels of CCR1 on T cells,6 which may be sufficiently engaged by tetrameric RANTES. This is in agreement with results in an in vivo model of chemotaxis, in which RANTES displayed a structural requirement of a tetramer to recruit cells to the peritoneal cavity.21 These data infer that oligomerization of immobilized RANTES on HMVECs plays a critical role for triggering the arrest component of leukocyte recruitment in flow.
As opposed to arrest, RANTES-triggered leukocyte spreading and transmigration in flow, a phenomenon termed chemorheotaxis, is mediated by CCR5 rather than CCR1.6,7 Inhibition with the CCR5 antagonist TAK-779 revealed that spreading/transmigration of monocytes and CD45RO+CD4+ T cells on activated endothelium in flow were dependent on CCR5 (Figure 1E-F). Notably, the oligomerization mutants retained the ability to trigger CCR5-mediated spreading/transmigration (Figure 1E-F). This demonstrates a dichotomy in the structural features of RANTES required for distinct functional properties in triggering arrest versus migration.
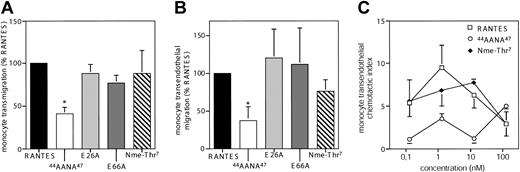
While GAG-binding mutants variably affect chemotaxis across bare filters or endothelium,13,16,17 the ability of oligomerization mutants to induce transendothelial chemotaxis has not been investigated. In extension to recent data,21 the oligomerization mutants showed no significant alteration in their ability to induce monocyte chemotaxis across bare filters or endothelial monolayers, which was preferentially mediated by CCR5 but also by CCR1 (Figure 2B). In accordance with previous studies,13,16 44AANA47-RANTES showed a significantly reduced potential to trigger monocyte chemotaxis across bare filters or endothelium (Figure 2A-B). While monomeric Nme-Thr7-RANTES is fully active in filter assays,21 its optimal concentration differed by 10-fold from that of wild-type RANTES for the induction of transendothelial migration.
Role of RANTES oligomerization in RANTES-induced transendothelial migration of monocytes. (A-B) Monocytes were subjected to chemotaxis assays across bare filters (A) or across resting HMVECs (B) for 90 minutes with RANTES or indicated RANTES mutants (1 nM) in the lower chamber. Transmigrated cells were counted by flow cytometry and expressed as chemotactic index (percent of control). Transendothelial chemotaxis of monocytes was reduced by pretreatment with the CCR5 antagonist TAK-779 or with the CCR1 antagonist BX471 to 34% ± 28% and 45% ± 19% of control, respectively. (C) Dose dependence of monocyte transendothelial chemotaxis in response to RANTES or mutants at indicated concentrations. Transmigrated cells were counted by flow cytometry and expressed as chemotactic index. Data represent mean ± SD of 4 to 6 separate experiments. *P < .05 versus wild-type RANTES (nonparametric signed-rank tests).
Role of RANTES oligomerization in RANTES-induced transendothelial migration of monocytes. (A-B) Monocytes were subjected to chemotaxis assays across bare filters (A) or across resting HMVECs (B) for 90 minutes with RANTES or indicated RANTES mutants (1 nM) in the lower chamber. Transmigrated cells were counted by flow cytometry and expressed as chemotactic index (percent of control). Transendothelial chemotaxis of monocytes was reduced by pretreatment with the CCR5 antagonist TAK-779 or with the CCR1 antagonist BX471 to 34% ± 28% and 45% ± 19% of control, respectively. (C) Dose dependence of monocyte transendothelial chemotaxis in response to RANTES or mutants at indicated concentrations. Transmigrated cells were counted by flow cytometry and expressed as chemotactic index. Data represent mean ± SD of 4 to 6 separate experiments. *P < .05 versus wild-type RANTES (nonparametric signed-rank tests).
A further reduction in potency was observed for 44AANA47-RANTES, which induced transendothelial chemotaxis only at a concentration 100-fold higher than that for wild-type RANTES, without reaching maximal efficacy (Figure 2C). Although the 44AANA47 mutation interferes with both GAG-binding and CCR1 receptor affinity,13 the ability of 44AANA47-RANTES to trigger largely CCR5-dependent spreading/transmigration of leukocytes in flow was also abolished (Weber et al6 and data not shown), indicating a relevance of GAG-binding. Conversely, the slightly reduced potency of Nme-Thr7-RANTES may be due to marginal effects on GAG-binding.21
Our data provide the first molecular evidence that the degree of chemokine oligomerization differentially contributes to the efficacy of distinct steps of leukocyte recruitment, by establishing a functional dichotomy for the requirement of RANTES self-oligomerization in CCR1-mediated arrest but not CCR5-mediated spreading and transmigration. Since the motifs for CCR1 and GAG-binding overlap,13 shear-resistant arrest may require the immobilization and correct presentation of RANTES oligomers via GAGs for bridging between immobilized RANTES and CCR1 on rolling leukocytes. Our data corroborate that RANTES monomers suffice for chemotaxis, while GAG-binding is indispensable for transendothelial chemotaxis and for chemorheotaxis (ie, spreading/transmigration triggered in flow).7,16 This may be due to interactions with GAGs on leukocytes8,9 but may also support the formation of a haptotactic gradient for transmigration. Defining structural prerequisites for RANTES-triggered leukocyte recruitment harbors important implications for the design of anti-inflammatory compounds25 precisely targeting the functionally relevant region.
Prepublished online as Blood First Edition Paper, May 22, 2003; DOI 10.1182/blood-2003-04-1175.
Supported by the Deutsche Forschungsgemeinschaft (grants WE-1913/2-3 and WE-1913/5-1 to C.W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr R. Rossaint for continuous support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal