Abstract
DNA fragmentation is a hallmark of cells undergoing apoptosis and is mediated mainly by the caspase-activated DNase (CAD or DNA-fragmentation factor 40 [DFF40]), which is activated when released from its inhibitor protein (ICAD or DFF45) upon apoptosis signals. Here we analyzed the effect of heat shock protein 70 (Hsp70) on CAD activity in T-cell receptor (TCR)–induced apoptosis using a T-cell line (TAg-Jurkat). Overexpression of Hsp70 significantly augmented the apoptotic cell death as well as DNA fragmentation in CD3/CD28- or staurosporine-stimulated cells. Following stimulation of cells with CD3/CD28 or staurosporine, Hsp70 was coprecipitated with free CAD, but not with CAD associated with ICAD. Furthermore, the purified Hsp70 dose-dependently augmented DNA-fragmentation activity of caspase-3–activated CAD in a cell-free system. Peptide-binding domain–deleted Hsp70 could neither bind nor augment its activity, while adenosine triphosphate (ATP)–binding domain–deleted Hsp70 or the peptide-binding domain itself bound CAD and augmented its activity. These results indicate that the the binding of Hsp70 to the activated CAD via the peptide-binding domain augments its activity. Although CAD lost its activity in an hour after being released from ICAD in vitro, its activity was retained after an hour of incubation in the presence of Hsp70, suggesting that Hsp70 may be involved in stabilization of CAD activity. Finally, CAD that had been coprecipitated with Hsp70 from the cell lysate of staurosporine-activated 293T cells induced chromatin DNA fragmentation and its activity was not inhibited by ICAD. These results suggest that Hsp70 binds free CAD in TCR-stimulated T cells to stabilize and augment its activity.
Introduction
Apoptosis or programmed cell death is the innate mechanism by which the organism eliminates unwanted cells. It is the most common form of cell death and occurs during development, tissue remodeling, cell homeostasis, defense processes, and immune responses.1,2 Apoptosis-inducing signals such as Fas ligand or tumor necrosis factor (TNF) initiate apoptosis signal pathway through death receptors to activate pro–caspase-8.3 Activated casapase-8 then cleaves and activates effector caspases such as caspase-3, -6, and -7.4 Many apoptotic stimuli also activate the mitochondrion to release cytochrome c into the cytosol, and the released cytochrome c, together with deoxy adenosine triphosphate (dATP), binds to the apoptotic protease-activating factor 1 (Apaf-1) and activates pro–caspase-9.5 Activated caspase-9 also processes downstream effector caspases.6 The latter cleave various cellular proteins that lead to various cellular changes characteristic of apoptosis, such as chromatin condensation, DNA fragmentation, and membrane blebbing.7 These molecular pathways that mediate apoptosis are tightly regulated by a series of positive and negative signals, and their balances determine whether cells commit suicide.1
Chromatin DNA fragmentation, a hallmark of cells undergoing apoptosis, is mediated largely by caspase-activated DNase (CAD)/DNA-fragmentation factor 40 (DFF40)8,9 and may be also influenced by other apoptotic nucleases, including endonuclease G.10-12 CAD/DFF40 exists as an inactive complex with its inhibitor protein ICAD/DFF45 in normal cells. When active caspase-3 or caspase-7 cleaves ICAD/DFF45, CAD/DFF40 is released from the complex and degrades chromatin DNA into fragments of around 180 bp.13 Regarding the role of CAD/DFF40 in DNA fragmentation in apoptosis, Sakahira et al demonstrated that expression of ICAD/DFF45 or a noncleavable form of ICAD prevented DNA fragmentation in apoptotic Jurkat and HeLa cells induced by staurosporine.14 Furthermore, inactivation of ICAD/DFF45 by gene targeting in mice significantly inhibited DNA fragmentation in apoptotic thymocytes.15
In the immune system, apoptosis is a fundamental process regulating lymphocyte maturation, receptor repertoire selection, and homeostasis. The T-cell repertoire is shaped in the thymus by apoptosis and survival signals. Thymocytes carrying self-reactive T-cell receptors are executed by apoptosis (negative selection), and those whose receptors do not recognize self major histocompatibility antigen also die by apoptosis (default cell death).2 Thus, 97% to 98% of thymocytes initially generated will die by apoptosis before leaving the thymus. In periphery, apoptosis also occurs at the peak or the down phase of the immune response to down-regulate the number of reactive cells and to terminate the immune response. This process is called activation-induced cell death (AICD).16
During cell death execution of TCR-stimulated thymocytes or T cells, active CAD participates in the induction of chromosomal DNA degradation.15,17
Exposure of cells to conditions of environmental stress including heat shock, oxidative stress, heavy metals, or anticancer drugs induces the expression of heat shock proteins (Hsps).18 Hsps have been classified in 4 families according to their molecular size: Hsp90, Hsp70, Hsp60, and small Hsps. They function mainly as molecular chaperones and modulate the assembly, transport, and folding of other proteins.19,20 Recently, it has been demonstrated that Hsps also play important roles in the apoptosis process through physical interaction with key components of the apoptotic machinery. It was reported that increased Hsp90 expression led to increased TNF-α–induced apoptosis in the monoblastoid cell line.21 On the contrary, Hsp90 negatively regulated cytochrome c–mediated oligomerization of Apaf-1 and activation of pro–caspase-9.22 Hsp60 was found to accelerate the maturation of pro–caspase-3 by upstream activator proteases in a T-cell line,23 while Hsp70 was reported to inhibit apoptosis by binding to the Apaf-1, c-Jun N-terminal kinase (JNK), or apoptosis-inducing factor.24-26 In the immune system, it was demonstrated that Hsp70 enhanced the TCR/CD3- and Fas/Apo-1/CD95–mediated apoptotic cell death in Jurkat T cells.27 However, the molecular mechanism of Hsp70-induced enhancement of apoptosis in T cells remains to be determined. In this study, we tried to clarify the molecular mechanism of the influence of Hsp70 on apoptosis in CD3/CD28- or staurosporine-stimulated Jurkat T cells. Our results demonstrate that Hsp70 interacts specifically with active CAD released from ICAD and augments CAD-induced DNA fragmentation by stabilizing CAD activity.
Materials and methods
Antibodies and plasmid DNA
Rabbit polyclonal anti-ICAD/DFF45 antibody (Ab), goat polyclonal anti-Hsp70 Ab, and goat polyclonal anti-Ran Ab were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-CAD/DFF40 Ab, monoclonal anti–hemagglutinin (HA) and anti-Flag Abs, and monoclonal anti-CD3 and anti-CD28 Abs were purchased from Imgenex (San Diego, CA), Sigma (St Louis, MO), and Nichirei (Tokyo, Japan), respectively.
Human Hsp70, Hsc70, or Hsp40 cDNA was cloned into the XbaI and EcoRI sites of pcDNA3-Flag28 to produce expression vector encoding Flag-tagged Hsp70 (Flag-Hsp70), Flag-Hsc70, or Flag-Hsp40. In order to construct deletion mutants of Hsp70, a BglII-BglII fragment was deleted from the cDNA to produce the ATP-binding domain–deleted mutant (Flag-Hsp70ΔABD), or a SmaI-SmaI fragment was deleted to produce the peptide-binding domain–deleted mutant (Flag-Hsp70ΔPBD). To produce the peptide-binding domain fragment of Hsp70 (Flag-Hsp70PBD), the DNA fragment encoding the peptide-binding domain (amino acid residues 393-640) was obtained by polymerase chain reaction (PCR) and cloned into the XbaI and EcoRI sites of pcDNA3-Flag vector. An Hsp70 cDNA-containing retrovirus vector (pMX-Hsp70-IRES-GFP) or the vector for its mutants was constructed by insertion of Hsp70 or its mutant cDNA into a retrovirus vector, pMX-IRES-GFP, which was kindly provided by Dr T. Kitamura (Tokyo University, Tokyo, Japan). GST-Hsp40 expression vector was prepared by inserting Hsp40 cDNA into EcoRI and XhoI sites of pGEX-4T2 vector (Amersham Biosciences, Piscataway, NJ). Flag-Hsp90 expression vector was provided by Dr T. Matsuda (Hokkaido University, Hokkaido, Japan). Mouse ICAD cDNA that was generously presented by Dr S. Nagata (Osaka University, Osaka, Japan) was cloned into the XbaI and ApaI sites of pcDNA3.1 to produce expression vector encoding ICAD. pcDNA3-HA-CAD and pcDNA3-Flag-ICAD vectors were generous gifts from Dr G. Núñez (University of Michigan, Ann Arbor, MI).
Cells and transfection
TAg-Jurkat cells, a Jurkat T-cell line stably transfected with simian virus 40 (SV40) large T antigen (provided by Dr G. Crabtree, Stanford University, Stanford, CA),29 were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2 atmosphere. A human embryonic kidney cell line, 293T, and a retrovirus packaging cell line, Phoenix Ampho (a kind gift from Dr G. Nolan, Stanford University), were grown in Dulbecco modified Eagle medium containing 10% FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C and 5% CO2. Normal human T cells were isolated from peripheral blood using pan–T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instruction.
For transfection using recombinant retroviruses, production of retrovirus carrying the green fluorescent protein (GFP) cDNA alone, both GFP and Hsp70 cDNA, or both GFP and Hsp70 mutant cDNA as well as infection of the recombinant virus were performed as described previously,30 with some modifications. Briefly, the recombinant retroviruses were prepared by transfecting Phoenix Ampho cells with pMX-IRES-GFP vector containing Hsp70 cDNA or its mutants by the calcium-phosphate method as previously reported.31 Then the supernatant containing the recombinant virus was used for infection into TAg-Jurkat cells. At 48 hours after infection, GFP-expressing cells were sorted by EPICS ELITE (Beckman Coulter, Hialeah, FL) and cloned by the limiting dilution method.
For the coimmunoprecipitation experiment, 5 × 107 TAg-Jurkat cells were cotransfected with 16.7 μg each of Flag-Hsp70, Flag-Hsc70, or Flag-Hsp90 expression vectors, together with vector for HA-CAD and ICAD by diethylamino ethyl–dextran method as described previously.31 Where indicated, Flag-Hsp40 vector was also cotransfected. At 24 hours after transfection, the dead cells were removed with Ficoll-Paque (Pharmacia, Uppsala, Sweden), and the living cells (1 mL per well of 24-well plate at 5 × 105 cells/mL) were cultured in the absence or presence of 1 μg/mL staurosporine for 2 hours. For CD3/CD28 stimulation, the wells were precoated with or without 5 μg/0.5 mL per well of anti-CD3/CD28 Abs for 2 hours at room temperature; then the living cells were cultured for 24 hours as above. After culture, the cells were harvested and used for the immunoprecipitation experiment.
Induction and detection of apoptosis
For the induction of apoptosis in TAg-Jurkat cell transfectants, 1 × 106 cells were cultured (1 × 105 cells/0.2 mL per well of 96-well plate) in the absence or presence of various doses of staurosporine for 7 hours. Where indicated, the cells were cultured for 22 to 24 hours in the wells precoated with various doses of anti-CD3/CD28 Abs. After the stimulation, cells were harvested, washed with phosphate-buffered saline (PBS), and fixed by resuspending cells in 2 mL of 80% ice-cold ethanol while vortexing. After fixation for 15 hours, cells were washed twice with 0.8 mL PBS and resuspended in 0.5 mL PBS. Then, 0.5 mL DNA extraction buffer (192 mL of 0.2 M Na2HPO4 and 8 mL of 0.1 M citric acid; pH 7.8) was added to the cell suspension and the cells were incubated for 5 minutes at room temperature to facilitate the release of small fragmented DNA from the cells. Then the cells were resuspended in 1 mL propidium iodide solution (0.1% [wt/vol] sodium citrate, 50 μg/mL propidium iodide in PBS). Next, 50 μL of 10 mg/mL RNase A was added to the cells, and they were incubated for 30 minutes at room temperature in the dark. The stained cells were analyzed with FACSCalibur (Becton Dickinson, San Jose, CA) using the CELLQUEST program (Becton Dickinson). Apoptotic cells were assessed by calculating the subdiploid population.
Where indicated, DNA fragmentation was analyzed by extracting total DNA from 5 × 105 cells by overnight incubation of cells at 4°C in 0.3 mL DNA extraction buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 7.5, 10 mM EDTA [ethylenediaminetetraacetic acid], 200 mM NaCl, 1% nonidet P-40 [NP-40], and 50 μg/mL freshly added proteinase K). DNA was recovered by phenol-chloroform extraction and ethanol precipitation. DNA pellets were resuspended in 20 μg/mL RNase in 10 mM Tris-HCl, pH 8.0, and 1 mM EDTA and incubated at 37°C for 30 minutes. Samples were analyzed in 1.5% agarose gel containing 0.5 μg/mL ethidium bromide.
Immunoprecipitation, pull-down, and Western blotting
Cells were harvested and washed twice with PBS. The cell pellet was resuspended in the lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, and 1 mM phenylmethylsulfonyl fluoride) and incubated at 4°C for 2 hours. After centrifuging at 12 000g for 30 minutes, the cell lysate was harvested and incubated with either anti-Flag or anti-HA Ab-coated protein A beads at 4°C for 2 hours. The precipitates were recovered and resolved in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). After eletrophoresis, the separated proteins were transferred to polyvinylidene difluoride membrane (polyscreen; Perkin Elmer Life Sciences, Boston, MA). The membrane was blocked with 5% nonfat milk in 0.1% Tween-20, 25 mM Tris-HCl, pH 7.5, and 150 mM NaCl (TBS-T) for 3 hours. The blotted proteins were probed with indicated Abs for 1.5 hours, followed by goat Ab to mouse immunoglobulin G (IgG) or rabbit IgG conjugated with horseradish peroxidase (EY Laboratories, San Mateo, CA). The membrane was developed with chemiluminescence (ECL Plus Western blotting detection reagents; Amersham Pharmacia Biotech, Uppsala, Sweden) and exposed on RX-U film (Fuji Film, Tokyo, Japan).
For the pull-down experiment, caspase-3–activated CAD was prepared as described previously.17 Briefly, the HA-CAD/Flag-ICAD complex was purified from the cell lysate of 5 × 107 293T cells transfected with HA-CAD and Flag-ICAD expression vectors with anti-Flag Ab-coated protein A beads. The beads containing immunoprecipitates were washed, resuspended in the reaction buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]/KOH, pH 7.5, 50 mM KCl, 5 mM MgCl2, 1 mM β-mercaptoethanol, 2 mM EDTA, and 0.1 mg/mL bovine serum albumin [BSA]), and then incubated with 3 units of caspase-3 (Chemicon, Temecula, CA) at room temperature for 2 hours. The supernatant was harvested and used as an active CAD preparation. Recombinant His-Hsp70 (2 μg; BioDynamics Laboratory, Tokyo, Japan) and glutathione-S–transferase (GST) protein (2 μg) were immobilized on His-Mag agarose beads (Merck, Darmstadt, Germany) and glutathione-agarose beads (Amersham Pharmacia Biotech), respectively. Of the active CAD, 3 μL was incubated with these beads at 4°C for 2 hours with occasional agitation in reaction buffer. Bead-bound proteins were extensively washed, eluted, and analyzed by Western blot with anti-CAD antibody.
Preparation of chromatin DNA
Chromatin DNA was prepared according to the method described by Frenster et al with some modifications.32 All steps were performed at 4°C and centrifugation was performed at 600g for 7 minutes except where indicated. In brief, thymocytes were isolated from one gram of thymus of 4-week-old ICR mice (Sankyo Labo, Tokyo, Japan) in 10 mL PBS. After centrifugation, the cell pellet was washed twice with 10 mL of 0.25 M sucrose and 3.3 mM CaCl2 to break cells and remove the membrane fraction. The resultant nuclear pellet was washed twice to remove the cytoplasmic fraction and nuclear membrane with 0.1875 M sucrose, 3.3 mM CaCl2, 0.02 M glucose, 0.025 M Tris-HCl, pH 7.1, and 0.0128 M NaCl. The washed nuclear pellet was extracted 3 times with 10 mL of 0.01 M Tris-HCl, pH 7.1, and 3.3 mM CaCl2 to remove nuclear ribosomes and neutral proteins. The residual extracted nuclei were resuspended in 10 mL of cation-free 0.25 M sucrose, allowed to stand for 10 minutes to permit swelling of the nuclei, and then were sonicated with Ultrasonic Disruptor for 5 seconds at output 8 (Tomy Seiko, Tokyo, Japan). The suspension was centrifuged at 600g for 5 minutes, with the supernatant filtrated with flannelette to remove any aggregated materials. The filtrate was centrifuged at 3000g for 10 minutes, and the pellet was collected, resuspended in 0.3 mL of 0.25 M sucrose, and used as chromatin DNA.
Cell-free system for analyzing augmentation of CAD activity
Flag-Hsp70, its mutants, or Flag-Hsp90 was purified from the cell lysate of 5 × 107 293T cells transfected with either wild-type Flag-Hsp70, its mutant, or Flag-Hsp90 expression vector with anti-Flag Ab-bound protein A beads. As a negative control, the cell lysate from 5 × 107 control vector–transfected 293T cells was incubated with anti-Flag Ab-coated protein A beads. Then the beads containing immunoprecipitates were recovered, washed, and resuspended in the reaction buffer. The GST-ICAD and GST-Hsp40 were prepared as described previously17 from GST-ICAD– or GST-Hsp40–expressing Escerichia coli.
To analyze the effect of Hsp70 on CAD activity, 2 μL recombinant CAD, which was prepared as described above, was incubated in the absence or presence of Hsp70 beads or control beads in a final volume of 30 μLat 4°C for 2 hours with occasional agitation. Then, 2 μL chromatin DNA was added to the reaction mixtures and incubated at 37°C for a further 2 hours. After incubation, the DNA fragmentation was examined as described above.
Results
Enhancement of staurosporine- and CD3/CD28-induced apoptosis in TAg-Jurkat cells by ectopic expression of Hsp70
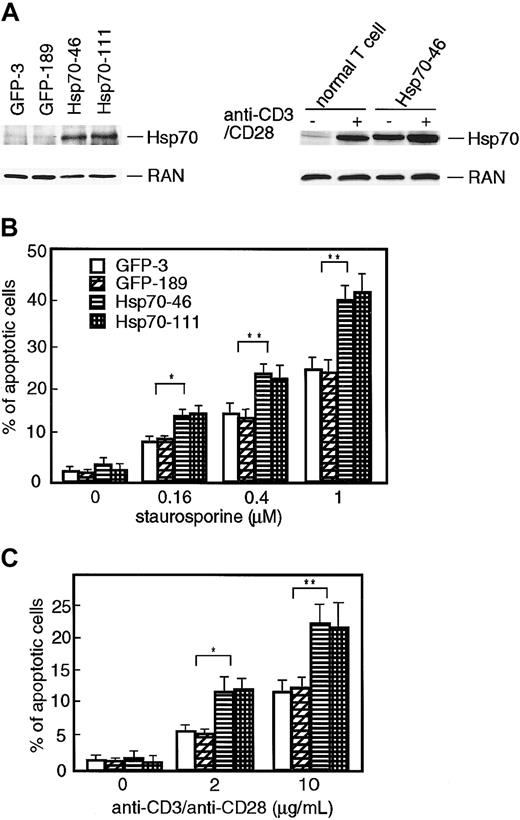
Liossis et al demonstrated that Hsp70 enhanced CD3- and Fasmediated apoptotic cell death in the Jurkat T-cell line, but protected the same cells from heat-induced cell death.27 In order to investigate the mechanism of the augmentation of apoptosis by Hsp70 in Jurkat T cells, we examined the effect of ectopic expression of Hsp70 on staurosporine- or CD3/CD28-induced apoptosis using TAg-Jurkat cells that stably express SV40 large T-antigen. We used TAg-Jurkat cells because the expression of SV40 large T-antigen augmented the expression of the products from the expression vector containing the DNA replication origin of SV40 DNA. To obtain the stable Hsp70 transfectants, Hsp70 cDNA in a retrovirus vector containing GFP cDNA was introduced into TAg-Jurkat cells by infection of the recombinant viruses. As a control, a retrovirus vector containing only GFP cDNA was introduced to the cells. The GFP-expressing cells were selected by cell sorting and cloned by limiting dilution. As shown in Figure 1A, Hsp70 transfectants (Hsp70-46 and -111) expressed a larger amount of Hsp70 compared with the control transfectants (GFP-3 and -189). When compared with normal T cells, the expression level of Hsp70 in Hsp70 transfectants was comparable with that of normal T cells stimulated with anti-CD3/CD28 Abs (Figure 1A). These transfectants were incubated with various doses of staurosporine or anti-CD3/CD28 Abs, and the percentages of apoptotic cells were determined by flow cytometry (Figure 1B-C). When the control transfectants were incubated with staurosporine or anti-CD3/CD28 Abs, the percentages of apoptotic cells were dose-dependently increased. When Hsp70 transfectants were stimulated with staurosporine or anti-CD3/CD28 Abs, the percentages of apoptotic cells were significantly increased compared with the control transfectants.
Ectopic expression of Hsp70 enhances staurosporine- and TCR-induced apoptotic cell death in TAg-Jurkat cells. (A) Left panel: expression of Hsp70 protein in GFP transfectants (GFP-3 and -189) and GFP-Hsp70 transfectants (Hsp70-46 and -111) was analyzed by immunobotting using anti-Hsp70 Ab. Expression of RAN (ubiquitously and abundantly expressed Ras-related nuclear protein) was assessed for a loading control. Right panel: normal T cells or one of the Hsp70 transfectants (Hsp70-46) was stimulated with or without anti-CD3/CD28 Abs. Expression of Hsp70 or RAN was analyzed as above. (B-C) Transfectants (5 × 105 cells) were cultured in the absence or presence of various amounts of staurosporine (B) or stimulated with various doses of anti-CD3/CD28 Abs (C), and percentages of apoptotic cell deaths were determined by flow cytometry after propidium iodide staining. Each value represents the mean ± SD of 3 independent experiments. *P < .05; **P < .01.
Ectopic expression of Hsp70 enhances staurosporine- and TCR-induced apoptotic cell death in TAg-Jurkat cells. (A) Left panel: expression of Hsp70 protein in GFP transfectants (GFP-3 and -189) and GFP-Hsp70 transfectants (Hsp70-46 and -111) was analyzed by immunobotting using anti-Hsp70 Ab. Expression of RAN (ubiquitously and abundantly expressed Ras-related nuclear protein) was assessed for a loading control. Right panel: normal T cells or one of the Hsp70 transfectants (Hsp70-46) was stimulated with or without anti-CD3/CD28 Abs. Expression of Hsp70 or RAN was analyzed as above. (B-C) Transfectants (5 × 105 cells) were cultured in the absence or presence of various amounts of staurosporine (B) or stimulated with various doses of anti-CD3/CD28 Abs (C), and percentages of apoptotic cell deaths were determined by flow cytometry after propidium iodide staining. Each value represents the mean ± SD of 3 independent experiments. *P < .05; **P < .01.
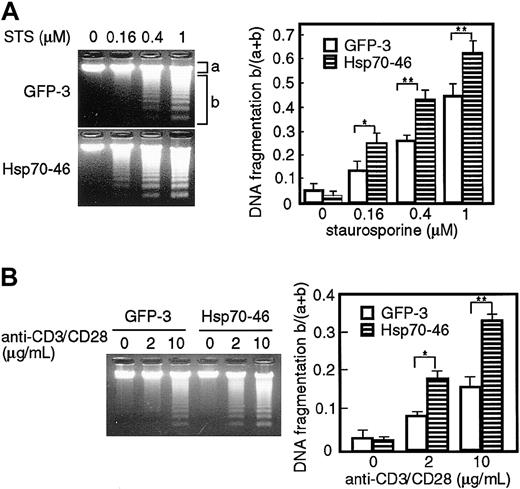
We then examined whether the ectopic expression of Hsp70 enhances DNA fragmentation in apoptotic cells. GFP transfectants or Hsp70 transfectants of TAg-Jurkat cells were stimulated with various doses of staurosporine or anti-CD3/CD28 Abs, and fragmentation of chromosomal DNA was analyzed with agarose gel electrophoresis. As shown in Figure 2, DNA fragmentation was induced dose-dependently by staurosporine or anti-CD3/CD28 Abs in the GFP tranfectants. The staurosporine- or CD3/CD28-induced fragmentation was significantly augmented in the Hsp70 transfectants, when compared with the GFP transfectants. These results show that ectopic expression of Hsp70 enhances staurosporine- or TCR-induced apoptosis in TAg-Jurkat cells.
Ectopic expression of Hsp70 augments DNA fragmentation induced by staurosporine and anti-CD3/CD28 Abs in TAg-Jurkat cells. Cells (5 × 105) of GFP transfectant (GFP-3) or Hsp70 transfectant (Hsp70-46) were treated with various concentrations of staurosporine (STS) (A) or anti-CD3/CD28 Abs (B) and DNA fragmentation was analyzed by electrophoresis on a 1.5% agarose gel. DNA was visualized with ethidium bromide (left panel). The intensity of the fluorescence of unfragmented DNA (a) and that of fragmented DNA (b) was measured by densitograph software (Atto, Tokyo, Japan), and the ratio of fragmented DNA and total DNA, b/(a+b), is shown on the right panel. Results are shown as mean ± SD of 3 independent experiments. *P < .05; **P < .01.
Ectopic expression of Hsp70 augments DNA fragmentation induced by staurosporine and anti-CD3/CD28 Abs in TAg-Jurkat cells. Cells (5 × 105) of GFP transfectant (GFP-3) or Hsp70 transfectant (Hsp70-46) were treated with various concentrations of staurosporine (STS) (A) or anti-CD3/CD28 Abs (B) and DNA fragmentation was analyzed by electrophoresis on a 1.5% agarose gel. DNA was visualized with ethidium bromide (left panel). The intensity of the fluorescence of unfragmented DNA (a) and that of fragmented DNA (b) was measured by densitograph software (Atto, Tokyo, Japan), and the ratio of fragmented DNA and total DNA, b/(a+b), is shown on the right panel. Results are shown as mean ± SD of 3 independent experiments. *P < .05; **P < .01.
Physical interaction of Hsp70 with CAD dissociated from ICAD upon apoptosis induction
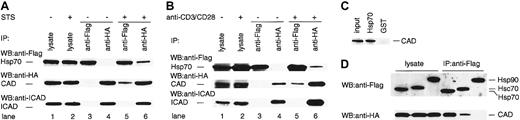
Hsp70 exerts its various effects by binding to its target molecules.33 In order to test the possibility that Hsp70 binds CAD in the apoptotic cells, we examined the physical interaction of CAD and Hsp70 using TAg-Jurkat cells. TAg-Jurkat cells were cotransfected with Flag-Hsp70, HA-CAD, and ICAD, and cultured with or without staurosporine or anti-CD3/CD28 Abs. Cell lysates were prepared, and Hsp70 and CAD were immunoprecipitated by either anti-Flag Ab or anti-HA Ab. Then, the precipitates were immunoblotted with anti-Flag Ab, anti-HA Ab, or anti-ICAD Ab. As shown in Figure 3A-B, anti-Flag Ab precipitated only Hsp70, and anti-HA Ab coprecipitated CAD and ICAD from the cell lysate of unstimulated TAg-Jurkat cells. From the cell lysate of both staurosporine- and anti-CD3/CD28 Ab–stimulated cells, anti-Flag Ab coprecipitated Hsp70 and CAD, but not ICAD, while anti-HA Ab coprecipitated CAD and Hsp70 as well as ICAD. These results suggest that stimulation of cells with staurosporine or TCR-signal induces dissociation of CAD from the CAD/ICAD complex, and Hsp70 binds the free CAD. The data also show that Hsp70 does not bind the CAD/ICAD complex. It is of note that only some ICAD was degraded and most ICAD still associated with CAD in staurosporine or anti-CD3/CD28 Ab–stimulated TAg-Jurkat cells (lane 6 vs lane 4, Figure 3A-B). Thus, it is conceivable that only a minor fraction of free CAD was associated with Hsp70 upon the stimuli.
Physical interaction of Hsp70 and CAD. TAg-Jurkat cells (5 × 107) that had been transfected with expression vectors for Flag-Hsp70, HA-CAD, and ICAD were stimulated without or with staurosporine (A) or anti-CD3/CD28 Abs (B), and Hsp70 or CAD was immunoprecipitated with anti-Flag Ab (lanes 3, 5) or anti-HA Ab (lanes 4, 6). Precipitates were immunoblotted with anti-Flag Ab or anti-HA Ab, or anti-ICAD Ab. As control for blotting, 5% of cell lysates were directly immunoblotted with Abs (lanes 1-2). (C) Pull-down analysis. Recombinant His-Hsp70 or GST was coupled to beads and incubated with CAD released from the purified CAD/ICAD complex. After washing, precipitates were analyzed by immunoblot with anti-CAD Ab. (D) TAg-Jurkat cells were transfected with either Flag-Hsp70, Flag-Hsc70, or Flag-Hsp90 expression vector, together with HA-CAD and ICAD expression vector, and then stimulated with anti-CD3/CD28 Abs. Hsp70, Hsc70, or Hsp90 was immunoprecipitated with anti-Flag Ab and the precipitates were immunoblotted with anti-Flag or anti-HA Ab.
Physical interaction of Hsp70 and CAD. TAg-Jurkat cells (5 × 107) that had been transfected with expression vectors for Flag-Hsp70, HA-CAD, and ICAD were stimulated without or with staurosporine (A) or anti-CD3/CD28 Abs (B), and Hsp70 or CAD was immunoprecipitated with anti-Flag Ab (lanes 3, 5) or anti-HA Ab (lanes 4, 6). Precipitates were immunoblotted with anti-Flag Ab or anti-HA Ab, or anti-ICAD Ab. As control for blotting, 5% of cell lysates were directly immunoblotted with Abs (lanes 1-2). (C) Pull-down analysis. Recombinant His-Hsp70 or GST was coupled to beads and incubated with CAD released from the purified CAD/ICAD complex. After washing, precipitates were analyzed by immunoblot with anti-CAD Ab. (D) TAg-Jurkat cells were transfected with either Flag-Hsp70, Flag-Hsc70, or Flag-Hsp90 expression vector, together with HA-CAD and ICAD expression vector, and then stimulated with anti-CD3/CD28 Abs. Hsp70, Hsc70, or Hsp90 was immunoprecipitated with anti-Flag Ab and the precipitates were immunoblotted with anti-Flag or anti-HA Ab.
To examine if Hsp70 directly binds CAD, active CAD preparation was incubated with either recombinant His-Hsp70 or glutathione-S–transferase–coupled beads. Then coprecipitation of CAD with each kind of bead was assessed by immunoblot. Figure 3C shows that CAD was coprecipitated specifically with Hsp70, demonstrating that Hsp70 directly binds free CAD.
Since various Hsps bind other proteins and function as chaperones,18-20 we further examined if other Hsps bind free CAD or not. To this end, TAg-Jurkat cells were transfected with Flag-Hsc70 or Flag-Hsp90 expression vector together with HA-CAD and ICAD vector, and stimulated with anti-CD3/CD28 antibodies. When Hsc70 or Hsp90 was immunoprecipitated from the cell lysate, CAD was coprecipitated with Hsc70 but not with Hsp90 (Figure 3D).
Effect of recombinant Hsp70 on CAD-induced DNA fragmentation in the cell-free system
Hsp interacts with various proteins in the cells and exerts its effect in various phases of apoptosis.20 To delineate the mechanism of Hsp70 on the enhancement of CAD-mediated DNA fragmentation at the molecular level, we examined the effect of Hsp70 on CAD activity in a cell-free system using recombinant molecules.17,34 For this purpose, the Flag-Hsp70 was purified from cell extracts of 293T cells that had been transiently transfected with Flag-Hsp70 vector using anti-Flag Ab-coated protein A beads. As shown in Figure 4A, Hsp70 was purified to homogeneity without obvious contaminants. Similarly, the HA-CAD/ICAD complex and GST-ICAD were purified using anti-HA Ab and glutathione-column, respectively, as described before.17 Active CAD was prepared by treating the CAD/ICAD complex with caspase-3. Chromatin isolated from mouse thymocytes was incubated with or without active CAD in the absence or presence of various doses of Hsp70, and DNA fragmentation was analyzed with agarose gel electrophoresis. As shown in Figure 4B, DNA fragmentation was induced in the presence of CAD and it was completely inhibited by ICAD. Purified Hsp70 markedly enhanced the CAD-induced DNA fragmentation activity in a dose-dependent manner, whereas it did not cause DNA fragmentation in the absence of CAD.
Effect of Hsp70 on CAD-induced chromatin DNA fragmentation in a cell-free system. (A) Homogeneity of Flag-Hsp70 protein purified from Flag-Hsp70–transfected 293T cells. Purified Hsp70 protein was electrophoresed in SDS-PAGE and stained with Coomassie brilliant blue. (B) Effect of purified Hsp70 on CAD-induced DNA fragmentation in a cell-free system. Caspase-3–activated CAD and various amounts (0-3 μL) of purified Hsp70 preparation were mixed. The volume of Hsp70 preparation was adjusted to become 3 μL by adding IgG immunoprecipitates (IgG ppt). The ability to induce chromatin DNA fragmentation was examined by electrophoresis in a 1.5% agarose gel (lanes 3-6). Chromatin DNA incubated without (lane 1) or with (lane 2) Hsp70 in the absence of CAD is shown. ICAD (0.3 μg) was incubated with CAD preparation to confirm that the DNA-fragmentation activity was dependent on CAD activity (lane 7). Percentages of DNA fragmentation calculated as in Figure 2 are shown at bottom.
Effect of Hsp70 on CAD-induced chromatin DNA fragmentation in a cell-free system. (A) Homogeneity of Flag-Hsp70 protein purified from Flag-Hsp70–transfected 293T cells. Purified Hsp70 protein was electrophoresed in SDS-PAGE and stained with Coomassie brilliant blue. (B) Effect of purified Hsp70 on CAD-induced DNA fragmentation in a cell-free system. Caspase-3–activated CAD and various amounts (0-3 μL) of purified Hsp70 preparation were mixed. The volume of Hsp70 preparation was adjusted to become 3 μL by adding IgG immunoprecipitates (IgG ppt). The ability to induce chromatin DNA fragmentation was examined by electrophoresis in a 1.5% agarose gel (lanes 3-6). Chromatin DNA incubated without (lane 1) or with (lane 2) Hsp70 in the absence of CAD is shown. ICAD (0.3 μg) was incubated with CAD preparation to confirm that the DNA-fragmentation activity was dependent on CAD activity (lane 7). Percentages of DNA fragmentation calculated as in Figure 2 are shown at bottom.
Functional domains of Hsp70 to associate with and activate CAD
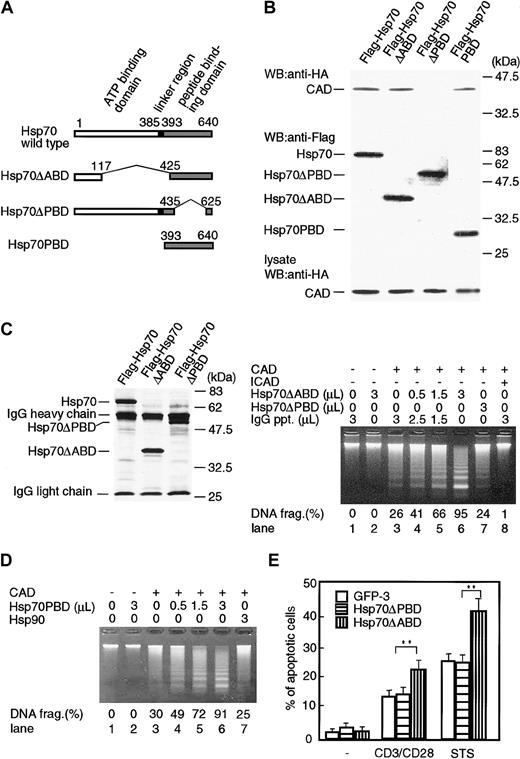
Hsp70 consists of mainly 2 functional domains: ATP-binding domain and peptide-binding domain (Figure 5A). In order to determine which domain is responsible for the binding of CAD as well as enhancement of its activity, expression vectors for ATP-binding domain–deleted mutant (Hsp70ΔABD) and peptide-binding domain–deleted mutant (Hsp70ΔPBD) of Hsp70 were prepared. Stimulated with staurosporine were the 293T cells that had been cotransfected with either Flag-Hsp70, Flag-Hsp70ΔABD, or Flag-Hsp70ΔPBD expression vector together with HA-CAD and ICAD expression vectors, and cell lysates were immunoprecipitated with anti-Flag Ab. Then the precipitates were immunoblotted with anti-HA or anti-Flag Ab. As shown in Figure 5B, CAD was coprecipitated with Hsp70 or Hsp70ΔABD, but not with Hsp70ΔPBD. The probing of the same membrane with anti-Flag Ab showed that a similar amount of Hsp70 or its mutants was expressed in the transfected cells. Furthermore, analysis of the cell lysates showed the existence of the similar amount of CAD. These results demonstrate that Hsp70 interacts with CAD via peptide-binding domain and that the ATP-binding domain is dispensable for Hsp 70/CAD association. In order to verify the above results, only the peptide-binding domain of Hsp70 (Hsp70PBD) was prepared (Figure 5A), and the coprecipitation with free CAD was examined. As shown in Figure 5B, CAD was coprecipitated with Hsp70PBD, confirming that the peptide-binding domain was enough for the association of Hsp70 with CAD.
Functional domains of Hsp70 to bind and activate CAD. (A) A scheme of wild-type Hsp70 and its deletion mutants. ATP-binding domain (1-385 amino acids), linker region (385-393 amino acids), and peptide-binding domain (393-640 amino acids) are indicated on the top. ATP-binding domain–deleted Hsp70 mutant (Hsp70ΔABD), peptide-binding domain–deleted Hsp70 mutant (Hsp70ΔPBD), and peptide-binding domain fragment (Hsp70PBD) are shown. (B) Association of CAD with Hsp70 and its mutants. Treated with 1 μM staurosporine were 293T cells that had been transfected with expression vectors for Flag-Hsp70 or its mutants (Hsp70ΔABD, Hsp70ΔPBD, or Hsp70PBD), together with expression vectors for HA-CAD and ICAD, and Hsp70 or its mutants were immunoprecipitated with anti-Flag Ab from cell lysates. Precipitates were immunoblotted with either anti-HA Ab or anti-Flag Ab. To estimate the expression of CAD protein in each cell lysate, an aliquot of each cell lysate was immunoblotted with anti-HA Ab. (C) Left panel: homogeneity of purified Hsp70 mutant proteins. Hsp70 or its mutant proteins (Hsp70ΔABD, Hsp70ΔPBD) were purified from 293T cell transfectants of their expression vectors with anti-Flag Ab-coated protein A beads. The purity of the Hsp70 and its mutant preparation was analyzed with SDS-PAGE, followed by silver staining. Right panel: effect of Hsp70 mutants (Hsp70ΔABD, Hsp70ΔPBD) on CAD-induced chromatin DNA fragmentation. Assay was performed as described in Figure 4. Percentages of DNA fragmentation calculated as in Figure 2 are shown at bottom. (D) Effect of peptide-binding domain fragment of Hsp70 (Hsp70PBD) on CAD-induced chromatin DNA fragmentation. Assay was performed as described above. Purified Hsp90 was used as a negative control. (E) Cells (5 × 105) of GFP transfectant (GFP-3), Hsp70ΔPBD transfectant, or Hsp70ΔABD transfectant were treated with 1 μM staurosporine (STS) or 10 μg/mL anti-CD3/CD28 Abs, and percentages of apoptotic cell deaths were determined by flow cytometry after propidium iodide staining. The data show the representative results using 2 clones of each transfectant. Each value represents the mean ± SD of 3 independent experiments. **P < .01.
Functional domains of Hsp70 to bind and activate CAD. (A) A scheme of wild-type Hsp70 and its deletion mutants. ATP-binding domain (1-385 amino acids), linker region (385-393 amino acids), and peptide-binding domain (393-640 amino acids) are indicated on the top. ATP-binding domain–deleted Hsp70 mutant (Hsp70ΔABD), peptide-binding domain–deleted Hsp70 mutant (Hsp70ΔPBD), and peptide-binding domain fragment (Hsp70PBD) are shown. (B) Association of CAD with Hsp70 and its mutants. Treated with 1 μM staurosporine were 293T cells that had been transfected with expression vectors for Flag-Hsp70 or its mutants (Hsp70ΔABD, Hsp70ΔPBD, or Hsp70PBD), together with expression vectors for HA-CAD and ICAD, and Hsp70 or its mutants were immunoprecipitated with anti-Flag Ab from cell lysates. Precipitates were immunoblotted with either anti-HA Ab or anti-Flag Ab. To estimate the expression of CAD protein in each cell lysate, an aliquot of each cell lysate was immunoblotted with anti-HA Ab. (C) Left panel: homogeneity of purified Hsp70 mutant proteins. Hsp70 or its mutant proteins (Hsp70ΔABD, Hsp70ΔPBD) were purified from 293T cell transfectants of their expression vectors with anti-Flag Ab-coated protein A beads. The purity of the Hsp70 and its mutant preparation was analyzed with SDS-PAGE, followed by silver staining. Right panel: effect of Hsp70 mutants (Hsp70ΔABD, Hsp70ΔPBD) on CAD-induced chromatin DNA fragmentation. Assay was performed as described in Figure 4. Percentages of DNA fragmentation calculated as in Figure 2 are shown at bottom. (D) Effect of peptide-binding domain fragment of Hsp70 (Hsp70PBD) on CAD-induced chromatin DNA fragmentation. Assay was performed as described above. Purified Hsp90 was used as a negative control. (E) Cells (5 × 105) of GFP transfectant (GFP-3), Hsp70ΔPBD transfectant, or Hsp70ΔABD transfectant were treated with 1 μM staurosporine (STS) or 10 μg/mL anti-CD3/CD28 Abs, and percentages of apoptotic cell deaths were determined by flow cytometry after propidium iodide staining. The data show the representative results using 2 clones of each transfectant. Each value represents the mean ± SD of 3 independent experiments. **P < .01.
We next examined if the ATP-binding domain is prerequisite for enhancing CAD activity. Hsp70ΔABD and Hsp70ΔPBD molecules were purified from the cell lysate of the 293T cell transfectants by immunoprecipitation with anti-Flag Ab and the homogeneity was assessed by SDS-PAGE, following silver staining (left panel, Figure 5C). We then examined the molecules' effects on CAD activity in the cell-free system as described in Figure 4. As shown in the right panel of Figure 5C, Hsp70ΔABD augmented the DNA-fragmentation activity of CAD in a dose-dependent manner, while Hsp70ΔPBD showed no effect. Furthermore, Hsp70PBD that had been purified from the cell lysate dose dependently augmented CAD activity, while Hsp90 purified from the cell lysate (used as a negative control) showed no enhancing effect (Figure 5D). These results suggest that the binding of Hsp70 to CAD via peptide-binding domain is required for the enhancement of CAD activity and that the ATP-binding domain of Hsp70 is dispensable for the augmentation of CAD activity. Silver staining profiles in Figure 5C showed no minor contaminants that exist in both Hsp70 and Hsp70ΔABD precipitates but not in Hsp70ΔPBD precipitates. The result suggests that other minor contaminants may not be involved in the reaction.
Then we analyzed the effect of Hsp70ΔPBD and Hsp70ΔABD on apoptosis-induction in vivo using stable TAg-Jurakat transfectants of Hsp70ΔPBD and Hsp70ΔABD. Both transfectants expressed high and comparable levels of GFP and Hsp70 mutant proteins (data not shown). They were stimulated with either anti-CD3/CD28 Abs or staurosporine, and apoptotic cells were analyzed by flow cytometer. As a negative control, a GFP transfectant was used. As shown in Figure 5E, the expression of Hsp70ΔABD significantly increased the percentage of apoptotic cells compared with the percentage in GFP transfectant, while Hsp70ΔPBD expression showed no apparent effect.
Effect of Hsp40 on the Hsp70/CAD complex formation and Hsp70-mediated enhancement of CAD activity
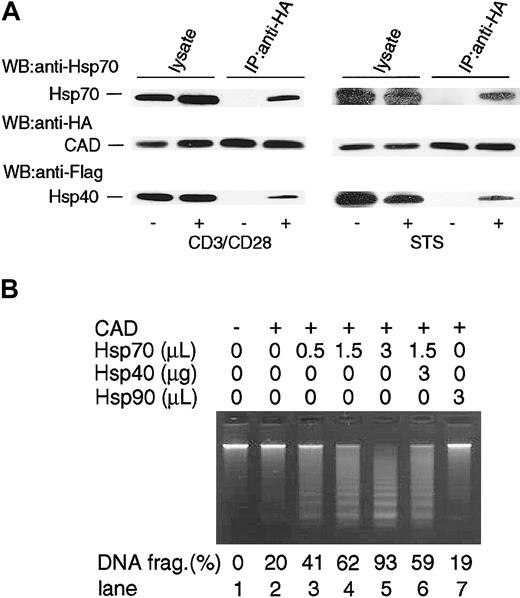
It has been shown that Hsp40 facilitates the chaperone activity of Hsp70 by augmenting theATPase activity of Hsp70,20 and Hsp40 is involved in the Hsc70 and CAD complex.35 To examine if Hsp40 is involved in the CAD/Hsp70 complex in our system, TAg-Jurkat cells were transfected with Flag-Hsp40 expression vector together with Hsp70, HA-CAD, and ICAD expression vector. Then the cells were stimulated with either anti-CD3/CD28 antibodies or staurosporine, and CAD was immunoprecipitated from the cell lysates. Then coprecipitation of Hsp70 and Hsp40 was examined by immunoblot. As shown in Figure 6A, Hsp40 was coprecipitated with CAD as well as Hsp70. The result suggests that Hsp40 can be associated with the CAD and Hsp70 complex in apoptotic TAg-Jurkat cells.
Interaction of Hsp40 with the Hsp70/CAD complex. (A) TAg-Jurkat cells (5 × 107) that had been transfected with expression vectors for Flag-Hsp40, Hsp70, HA-CAD, and ICAD were unstimulated (–) or stimulated (+) with anti-CD3/CD28 Abs or staurosporine (STS), and CAD was immunoprecipitated with anti-HA Ab. Precipitates were immunoblotted with anti-Hsp70 Ab, anti-HA Ab, or anti-Flag Ab. As control for blotting, cell lysates were directly immunoblotted with Abs. (B) Effect of Hsp40 on Hsp70-augmented CAD activity in a cell-free system. Caspase-3–activated CAD and various amounts (0-3 μL) of purified Hsp70 or purified Hsp90 preparation were mixed. At lane 6, 3 μg GST-Hsp40 purified from bacteria was added to the reaction. Then chromatin DNA was added to the reaction and DNA fragmentation was examined as in Figure 4. Percentages of DNA fragmentation calculated as in Figure 2 are shown at the bottom.
Interaction of Hsp40 with the Hsp70/CAD complex. (A) TAg-Jurkat cells (5 × 107) that had been transfected with expression vectors for Flag-Hsp40, Hsp70, HA-CAD, and ICAD were unstimulated (–) or stimulated (+) with anti-CD3/CD28 Abs or staurosporine (STS), and CAD was immunoprecipitated with anti-HA Ab. Precipitates were immunoblotted with anti-Hsp70 Ab, anti-HA Ab, or anti-Flag Ab. As control for blotting, cell lysates were directly immunoblotted with Abs. (B) Effect of Hsp40 on Hsp70-augmented CAD activity in a cell-free system. Caspase-3–activated CAD and various amounts (0-3 μL) of purified Hsp70 or purified Hsp90 preparation were mixed. At lane 6, 3 μg GST-Hsp40 purified from bacteria was added to the reaction. Then chromatin DNA was added to the reaction and DNA fragmentation was examined as in Figure 4. Percentages of DNA fragmentation calculated as in Figure 2 are shown at the bottom.
Next, we examined if Hsp40 affected CAD activity with Hsp70. To this end, GST-Hsp40 was prepared and added together with Hsp70 to the in vitro analysis of CAD activity (Figure 6B). There was no obvious effect of Hsp40 on Hsp70-augmented CAD activity.
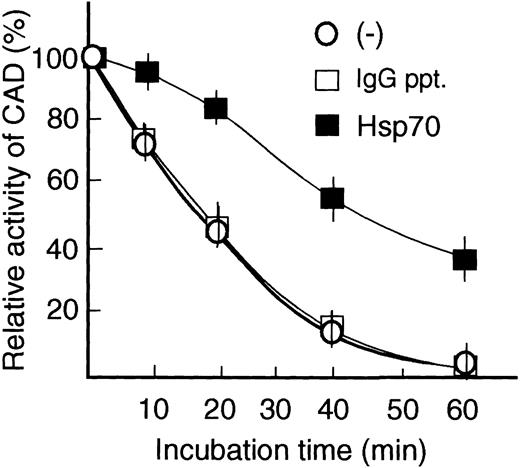
Stabilization of CAD activity in vitro by Hsp70
It has been shown that active CAD dissociated from ICAD easily loses its activity.36,37 To test the possibility that Hsp70 enhances CAD activity by stabilizing CAD, we prepared active CAD by treating the CAD/ICAD complex with caspase-3, and preincubated it with or without Hsp70 or control IgG immunoprecipitates in the reaction buffer at 4°C for 2 hours. After preincubation, the mixture was further kept at 37°C for various times. Then the ability of the mixture to induce chromatin DNA fragmentation was assessed by adding chromatin to the mixture. As shown in Figure 7, CAD activity started to decrease within 10 minutes, 50% of the activity was lost in 20 minutes, and most of the activity was lost in 60 minutes in the absence or presence of control IgG immunoprecipitates. In contrast, CAD activity decreased slowly in the presence of Hsp70, and 50% of its activity remained after 40 minutes of incubation. The result suggests that Hsp70 binds to CAD and stabilizes its activity in vitro.
Stabilization of CAD activity by Hsp70. Caspase-3–activated CAD was incubated without (–) or with either purified Hsp70 preparation (1 μL) or IgG immunoprecipitates (1 μL, control) in reaction buffer at 37°C for various times, and CAD activity was analyzed as in Figure 4. Initial CAD activity at 0 minute was indicated as 100%. Relative CAD activity is shown. Results are demonstrated as the mean ± SE of 2 independent experiments.
Stabilization of CAD activity by Hsp70. Caspase-3–activated CAD was incubated without (–) or with either purified Hsp70 preparation (1 μL) or IgG immunoprecipitates (1 μL, control) in reaction buffer at 37°C for various times, and CAD activity was analyzed as in Figure 4. Initial CAD activity at 0 minute was indicated as 100%. Relative CAD activity is shown. Results are demonstrated as the mean ± SE of 2 independent experiments.
DNA fragmentation–inducing activity of CAD associated with Hsp70 in vivo
We so far showed that Hsp70 binds and enhances CAD activity using the in vitro reconstitution system. In order to examine whether CAD associated with Hsp70 shows DNA-fragmentation activity in vivo, we purified Hsp70/CAD, Hsp70ΔABD/CAD, and Hsp70ΔPBD from 293T cells that had been transfected with either Flag-Hsp70, Hsp70ΔABD, or Hsp70ΔPBD together with HA-CAD and ICAD expression vectors and stimulated with staurosporine as described in Figure 5, and the DNA-fragmentation activity of these complexes was assessed. As shown in Figure 8, the purified Hsp70/CAD and Hsp70ΔABD/CAD but not Hsp70ΔPBD induced DNA fragmentation of the chromosomal DNA. Addition of ICAD to Hsp70/CAD and Hsp70ΔABD/CAD did not inhibit CAD activity, while it completely inhibited the activity of recombinant CAD. These data show that Hsp70- or Hsp70ΔABD-associated CAD in apoptotic cells has a DNA-fragmentation activity and that ICAD no longer interacts with and inhibits active CAD when the CAD forms a complex with Hsp70.
DNA-fragmentation activity of the Hsp70/CAD complex in the cells. Stimulated with staurosporine were the 293T cells that had been transfected with either Flag-Hsp70 or its mutant expression vectors (Hsp70ΔABD, Hsp70ΔPBD) together with HA-CAD and ICAD expression vectors. Hsp70 or its mutant proteins were immunoprecipitated as in Figure 5 B-C, and CAD activity was analyzed as in Figure 5D in the absence or presence of ICAD (lanes 1-6). To confirm the inhibitory activity of ICAD, caspase-3–activated CAD was incubated with or without ICAD and then DNA-fragmentation activity was analyzed (lanes 7-8).
DNA-fragmentation activity of the Hsp70/CAD complex in the cells. Stimulated with staurosporine were the 293T cells that had been transfected with either Flag-Hsp70 or its mutant expression vectors (Hsp70ΔABD, Hsp70ΔPBD) together with HA-CAD and ICAD expression vectors. Hsp70 or its mutant proteins were immunoprecipitated as in Figure 5 B-C, and CAD activity was analyzed as in Figure 5D in the absence or presence of ICAD (lanes 1-6). To confirm the inhibitory activity of ICAD, caspase-3–activated CAD was incubated with or without ICAD and then DNA-fragmentation activity was analyzed (lanes 7-8).
Discussion
In the immune system, apoptosis plays an important role regulating lymphocyte maturation and homeostasis,2 and it was demonstrated that Hsp70 enhanced TCR- and Fas-mediated apoptosis in Jurkat T cells.27 On the contrary, it was also reported that Hsp70 rather antagonized activation-induced cell death of Jurkat cells.37 In the present study, we intended to clarify the effect of Hsp70 on TCR-mediated apoptosis in Jurkat T cells using stable Hsp70-expressing Jurkat transfectants. Our results clearly demonstrated that ectopic expression of Hsp70 in Jurkat T cells augmented staurosporine- and CD3/CD28-induced cell death as well as DNA fragmentation (Figure 1, 2), supporting the result of Liossis et al27 and suggesting the possible important role of Hsp70 for DNA fragmentation in the TCR-mediated cell death of T cells.
It has been well characterized that CAD is responsible for DNA fragmentation in T cells upon stimulation with staurosporine or TCR engagement.10,14,15,17 We postulated and verified the possibility that Hsp70 binds CAD to augment its activity. Immunoprecipitation experiments showed that Hsp70 was coprecipitated with CAD, but not ICAD or the CAD/ICAD complex, after TCR stimulation of Jurkat T cells (Figure 3), indicating that Hsp70 physically binds free CAD that is dissociated from the CAD/ICAD complex after TCR engagement. This is the first demonstration that Hsp70 binds CAD and modulates its function. In this regard, Sakahira and Nagata showed that Hsc70, a constitutively expressed member of the Hsp70 family, was necessary for and facilitated functional CAD production in ribosomes.35 Our result in Figure 3D also showed that Hsc70 bound CAD in anti-CD3/CD28 Ab–stimulated TAg-Jurkat cells. Thus, the Hsp70 family may be involved in various steps of CAD function. Although we showed that Hsp90 did not interact with CAD after TCR stimulation, it is possible that other Hsps are involved in CAD function after TCR engagement. Regarding molecules other than the Hsp family, Liu et al showed that histone H1 augmented CAD activity by direct interaction with CAD.39 Toh et al also demonstrated that high-mobility group 1 (HMG-1), HMG-2, and DNA topoisomerase II functioned as an activator of CAD.40 We further verified the possibility that the enhancing effect of Hsp70 on CAD activity is due to the interaction of Hsp70 and CAD using a cell-free system (Figure 4). We have shown that purified Hsp70 significantly enhanced chromatin DNA fragmentation caused by activated recombinant CAD. This enhancement was no longer found by peptide-binding domain–deleted Hsp70 that could not bind CAD as shown in Figure 5, indicating that physical interaction of Hsp70 and activated CAD is prerequisite for augmentation of CAD-induced DNA fragmentation by Hsp70 in a cell-free system.
An ATP-binding domain and a peptide-binding domain, 2 major domains of Hsp70, functionally coupled each other in such a way that ATP-bound Hsp70 has a lower affinity for polypeptide substrate than the adenosine 5′-diphosphate (ADP)–bound protein,41,42 and, vice versa, the binding of polypeptide substrates to Hsp70 stimulates its ATPase activity,43 thus controlling the substrate-binding of Hsp70. Interestingly enough, it was shown that the ATP-binding domain of Hsp70 was not necessary for the binding of CAD and augmentation of its activity, whereas Hsp70 lacking the peptide-binding domain failed to bind and activate CAD (Figure 5). It was also demonstrated that CADs associated with ATP-binding domain–deleted Hsp70 in the cells were active (Figure 8). Our results indicate that the ATP-binding domain of Hsp70 seems not essential for augmenting CAD activity. In this context, it was demonstrated that Hsp70 bound to apoptosis-inducing factor or c-Jun N-terminal kinase and inhibited its function in the absence of ATP-binding domain.25,26 Thus, Hsp70 may be capable of regulating functions of other proteins by the alternative way independent on the ATP-binding domain.
Does Hsp70 directly interact with CAD or are other proteins involved in their interaction? In this regard, Sakahira and Nagata have indicated that Hsp40 is involved in the interaction of Hsc70 and CAD.35 We showed that Hsp40 was coprecipitated with CAD or the CAD/Hsp70 complex in our system (Figure 6A). However, in our in vitro CAD assay, Hsp40 did not obviously affect the Hsp70-augmented CAD activity (Figure 6B). Furthermore, as shown in Figure 5C-E, Hsp70 bound CAD and enhanced its activity in the absence of its ATP-binding domain that had been reported to play an important role for the functional interaction with Hsp40.20 These findings suggest that although Hsp40 binds CAD or even the CAD/Hsp70 complex in vivo, it may be dispensable for the augmentation of CAD activity by Hsp70. Further investigations are necessary to delineate the important role of Hsp40 in the context of Hsp70 and CAD during the process of TCR-induced apoptosis in T cells.
An interesting finding in the present study is that Hsp70 stabilized CAD activity in the cell-free system such that the half-life of CAD activity was prolonged to 2-fold in the presence of Hsp70 (Figure 7). The result indicates that augmentation of CAD activity by Hsp70 is due to the stabilization of its activity by Hsp70. In this regard, the Hsp70 family has been shown to play a pivotal role for protein stabilization. It was demonstrated that Hsc70 in the presence of U-box protein, carboxyl terminus of Hsp70-interacting protein (CHIP), promoted ubiquitination of cystic-fibrosis transmembrane-conductance regulator to facilitate its degradation in proteasome.44 Hsp70 was also shown to stabilize cystic-fibrosis transmembrane-conductance regulator in the presence of its cochaperone human DnaJ homologue (Hdj-1)/Hsp40.45
It is noteworthy that Hsp70- or Hsp70ΔABD-associated CAD has a DNA-fragmentation activity in vivo and that ICAD no longer interfered with CAD activity after forming the CAD/Hsp70 complex (Figure 8). The result poses 2 possibilities: (1) ICAD and Hsp70 may competitively bind the similar region in CAD and exert a similar function to stabilize CAD activity, or (2) the conformation of the Hsp70-associated CAD may be changed to be unable to interact with ICAD. In the latter case, free active CAD that is dissociated from ICAD may form a neutral conformation that binds to either ICAD or Hsp70 and is unstable. Regarding the interaction and inhibition of CAD with ICAD, Uegaki et al46 and Otomo et al47 have demonstrated that interaction of CAD and ICAD molecules by their N-terminal CAD domains are essential for correct folding of CAD in the complex and inhibition of CAD activity. They also showed that the charged amino acids in each CAD domain are prerequisite for their interaction. Further investigation of the Hsp70-binding domain of CAD may promote understanding of the mechanism of stabilization and activation of CAD by Hsp70.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2002-11-3499.
Supported by funding from Toyama Medical and Pharmaceutical University (H.K.); and the Japan Society for the Promotion of Science (H.K. and A.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr S. Nagata for mouse ICAD cDNA, and Dr G. Núñez for HA-CAD and Flag-ICAD expression vectors. We also appreciate the helpful assistance of Ms S. Hirota and Ms K. Hata. Q.-L.L. gratefully acknowledges Dr T. Nagata for his encouragement and support.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal