Abstract
Heme oxygenase-1 (HO-1) confers cytoprotection against oxidative stress. A (GT)n dinucleotide repeat in the 5′-flanking region of human HO-1 gene shows length polymorphism, which was classified into S (< 27 GT), M (27-32 GT), and L alleles (≥ 33 GT). Polymorphism in the HO-1 gene promoter was shown to be associated with susceptibility to pulmonary emphysema and restenosis after angioplasty. However, the biologic mechanism underlying these associations is still unclear. To examine this issue, we established lymphoblastoid cell lines (LCLs) from subjects possessing S/S or L/L genotypes. HO-1 mRNA expressions and HO activities induced by oxidative stress were significantly higher in LCLs with S/S than those with L/L. Furthermore, LCLs with S/S were significantly more resistant to oxidant-induced apoptosis than those with L/L. These findings suggested that the polymorphism of the HO-1 gene is associated with the strength of antiapoptotic effects of HO-1, resulting in an association with susceptibility to oxidative stress–mediated diseases.
Introduction
Heme oxygenase (HO) catalyzes the rate-limiting step in the oxidative degradation of heme into carbon monoxide (CO), iron, and biliverdin,1 which is metabolized to bilirubin by biliverdin reductase. Whereas HO-2 is constitutively expressed, HO-1 is the only inducible isoform.1 HO-1 is highly induced under oxidative stress and confers protection against oxidant-induced injury in various tissues.2-6 There have been studies on the cytoprotective mechanisms of HO-1 against oxidative stress in recent years. Both biliverdin and bilirubin possess antioxidant properties.7 CO has been described as mediating the anti-inflammatory and antiapoptotic effects of HO-1 in vitro8 and in vivo.9
The human HO-1 gene has a polymorphic (GT)n repeat in the 5′-flanking region. The distribution of the number of (GT)n repeats was trimodal, with one peak located at 23 GT repeats and the other 2 peaks located close together at 30 and 33 GT repeats. Therefore, the allele type is grouped into 3 classes according to the (GT)n repeat size, as follows: short alleles (S: < 27 GT), middle alleles (M: 27-32 GT), and long alleles (L: ≥ 33 GT).10 We recently reported that the population of subjects with L allele was significantly higher in smokers with chronic pulmonary emphysema (CPE) than in smokers without CPE.10 Furthermore, it was published that patients with short (< 25) GT repeats showed a significantly reduced risk for restenosis after percutaneous transluminal angioplasty.11 These studies suggested that the 5′-flanking polymorphism in the HO-1 gene is associated with the development of oxidative stress–mediated diseases. However, the biologic mechanism underlying the association between the length polymorphism and susceptibility to disease is still unclear. In this study, we established lymphoblastoid cell lines (LCLs) from human subjects possessing S/S or L/L genotype in the HO-1 gene and examined the HO-1 mRNA expressions and HO-1 activities by hydrogen peroxide (H2O2) stimulation. Furthermore, we examined the oxidative stress–mediated apoptosis in the LCLs to assess the biologic significance of HO-1 activities.
Study design
Subjects and cell preparation
In our database of 1262 people, 206 had S/S alleles and 29 had L/L alleles. We recruited 12 healthy subjects (6 people with S/S and 6 people with L/L) who signed informed consent for the present study (Table 1). This study was approved by the Tohoku University Ethics Committee. LCLs were prepared by transforming peripheral lymphocytes from each subject using supernatant from B95-8 as described elsewhere.12 LCLs were further cloned by a limiting dilution method. Five different clones derived from each subject were used for analyses.
HO-1 mRNA expressions and HO activities induced by H2O2 in LCLs
. | . | . | . | . | HO-1 mRNA expression* . | . | HO-1 activity, nmol/mg/h* . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject . | Genotype . | Age, y . | Sex . | (GT)n repeats . | Baseline . | After H2O2 treatment . | Baseline . | After H2O2 treatment . | ||
| SS-1 | S/S | 64 | Male | 22/23 | 0.89 ± 0.02 | 1.61 ± 0.09† | 0.48 ± 0.18 | 1.12 ± 0.09† | ||
| SS-2 | S/S | 66 | Male | 16/23 | 0.74 ± 0.03 | 1.90 ± 0.09† | 0.69 ± 0.14 | 2.04 ± 0.58† | ||
| SS-3 | S/S | 77 | Male | 22/23 | 0.63 ± 0.07 | 0.94 ± 0.08† | 1.52 ± 0.82 | 2.28 ± 1.45† | ||
| SS-4 | S/S | 24 | Female | 24/25 | 0.59 ± 0.01 | 1.33 ± 0.05† | 1.50 ± 0.30 | 3.22 ± 0.46† | ||
| SS-5 | S/S | 38 | Male | 24/26 | 0.94 ± 0.20 | 2.63 ± 0.13† | 1.32 ± 0.31 | 3.06 ± 0.51† | ||
| SS-6 | S/S | 43 | Male | 23/24 | 0.72 ± 0.04 | 1.39 ± 0.07† | 1.29 ± 0.44 | 4.05 ± 1.40† | ||
| LL-1 | L/L | 62 | Male | 33/34 | 0.78 ± 0.08 | 0.80 ± 0.04 | 1.20 ± 0.64 | 1.02 ± 0.37 | ||
| LL-2 | L/L | 58 | Male | 33/38 | 0.73 ± 0.29 | 0.84 ± 0.36 | 0.71 ± 0.38 | 0.57 ± 0.19 | ||
| LL-3 | L/L | 68 | Male | 33/38 | 0.85 ± 0.09 | 0.79 ± 0.03 | 1.30 ± 1.00 | 1.41 ± 0.66 | ||
| LL-4 | L/L | 24 | Male | 34/37 | 0.90 ± 0.01 | 0.88 ± 0.04 | 1.64 ± 0.33 | 1.96 ± 0.83 | ||
| LL-5 | L/L | 70 | Male | 34/36 | 0.77 ± 0.09 | 0.89 ± 0.11 | 1.46 ± 0.39 | 1.50 ± 0.47 | ||
| LL-6 | L/L | 31 | Male | 34/40 | 0.80 ± 0.34 | 0.89 ± 0.06 | 1.31 ± 0.04 | 1.61 ± 0.30 | ||
. | . | . | . | . | HO-1 mRNA expression* . | . | HO-1 activity, nmol/mg/h* . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject . | Genotype . | Age, y . | Sex . | (GT)n repeats . | Baseline . | After H2O2 treatment . | Baseline . | After H2O2 treatment . | ||
| SS-1 | S/S | 64 | Male | 22/23 | 0.89 ± 0.02 | 1.61 ± 0.09† | 0.48 ± 0.18 | 1.12 ± 0.09† | ||
| SS-2 | S/S | 66 | Male | 16/23 | 0.74 ± 0.03 | 1.90 ± 0.09† | 0.69 ± 0.14 | 2.04 ± 0.58† | ||
| SS-3 | S/S | 77 | Male | 22/23 | 0.63 ± 0.07 | 0.94 ± 0.08† | 1.52 ± 0.82 | 2.28 ± 1.45† | ||
| SS-4 | S/S | 24 | Female | 24/25 | 0.59 ± 0.01 | 1.33 ± 0.05† | 1.50 ± 0.30 | 3.22 ± 0.46† | ||
| SS-5 | S/S | 38 | Male | 24/26 | 0.94 ± 0.20 | 2.63 ± 0.13† | 1.32 ± 0.31 | 3.06 ± 0.51† | ||
| SS-6 | S/S | 43 | Male | 23/24 | 0.72 ± 0.04 | 1.39 ± 0.07† | 1.29 ± 0.44 | 4.05 ± 1.40† | ||
| LL-1 | L/L | 62 | Male | 33/34 | 0.78 ± 0.08 | 0.80 ± 0.04 | 1.20 ± 0.64 | 1.02 ± 0.37 | ||
| LL-2 | L/L | 58 | Male | 33/38 | 0.73 ± 0.29 | 0.84 ± 0.36 | 0.71 ± 0.38 | 0.57 ± 0.19 | ||
| LL-3 | L/L | 68 | Male | 33/38 | 0.85 ± 0.09 | 0.79 ± 0.03 | 1.30 ± 1.00 | 1.41 ± 0.66 | ||
| LL-4 | L/L | 24 | Male | 34/37 | 0.90 ± 0.01 | 0.88 ± 0.04 | 1.64 ± 0.33 | 1.96 ± 0.83 | ||
| LL-5 | L/L | 70 | Male | 34/36 | 0.77 ± 0.09 | 0.89 ± 0.11 | 1.46 ± 0.39 | 1.50 ± 0.47 | ||
| LL-6 | L/L | 31 | Male | 34/40 | 0.80 ± 0.34 | 0.89 ± 0.06 | 1.31 ± 0.04 | 1.61 ± 0.30 | ||
Results are reported as means ± SDs among 5 lymphoblastoid cell clones from each subject.
Significantly greater than baseline value, P < .05.
Real-time quantitative PCRs
To quantify the expression of HO-1 mRNA, we applied the technique of real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) by using the ABI PRISM 7700 apparatus (Applied Biosystems, Foster City, CA). After exposing LCLs to 900 μM H2O2 for 10 hours, total RNA was isolated from LCLs using RNA-Bee (Tel-Test, Friendswood, TX). A one-step RT-PCR was performed with HO-1 forward primer (5′-AGGCCAAGACTGCGTTCCT-3′), reverse primer (5′-GGTGTCATGGGTCAGCAGC-3′), and TaqMan probe (5′-FAM TCAACATCCAGCTCTTTGAGGAGTTGCAG-3′-TAMURA) according to the manufacturer's protocol. At the same time, 18S rRNA as an internal control was amplified using a commercially available kit (rRNA primers and VIC-labeled probe, Applied Biosystems). The amount of mRNA in each sample was calculated as the ratio between the HO-1 and the endogenous control, 18S rRNA.
HO activity
HO activity was measured as described previously.13 After exposing human LCLs to H2O2 at 900 μM for 18 hours, cells were collected, and microsome fractions were prepared.14 Microsomes (100 μg) were incubated for 20 minutes at 37°C in 200 μL potassium phosphate buffer13 containing 15 μM hemin, and 100 μg/mL of a partially purified biliverdin reductase.14 The amount of bilirubin formed was measured with a double-beam spectrophotometer (U-2000, Hitachi, Tokyo, Japan) with an optical density at 464 to 530 nm (excitation coefficient, 43.5/mM/cm for bilirubin). HO activity was expressed as nanomoles of bilirubin formed per milligram of protein.
Apoptosis assay
Oxidant-induced apoptosis in LCLs was measured by TUNEL assay.15 Viability of each LCL was more than 90% before exposure to H2O2. Each LCL was cultured with H2O2 at 300, 600, or 900 μM for 24 hours. To detect DNA fragmentation in apoptosis, we used FACScaliber (Becton Dickinson, Franklin Lakes, NJ) and the APO-BRDU kit (Molecular Probes, Eugene, OR) according to the supplied protocol.16 To determine whether HO-1 was involved in oxidant-induced apoptosis, LCLs were preincubated with 10 μM zinc protoporphyrin IX (ZnPP-9; OxisResearch, Portland, OR), competitive inhibitors of HO-1, then H2O2 stimulation and apoptosis detection were performed as described.
Results and discussion
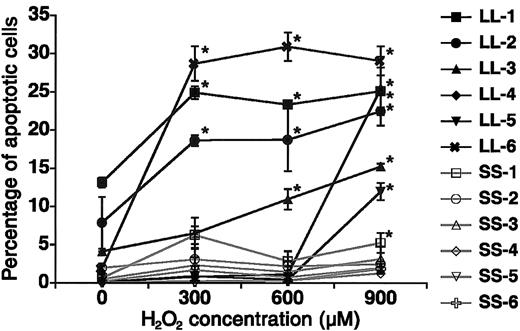
In this study, we established LCLs from each subject with S/S or L/L genotypes and examined the HO-1 mRNA expression, HO enzyme activity, and antiapoptotic effect against oxidative injury. Because LCLs have normal diploids in karyotypes and no tumorgenicity,17 studies on LCLs are expected to show endogenous promoter activities of the human genome. As shown in Table 1, there was no significant difference in HO-1 mRNA expression between S/S and L/L at baseline (0.75 ± 0.14 versus 0.81 ± 0.06, P > .50). After H2O2 stimulation, however, the S/S group showed a 2.10 ± 0.49-fold increase in HO-1 mRNA expression, whereas the L/L group showed only a 1.03 ± 0.17-fold increase (P < .05). Compatible with the results of HO-1 mRNA expression, HO activity was not significantly different between S/S and L/L at the baseline (1.27 ± 0.32 versus 1.13 ± 0.44 nmol bilirubin/mg protein/h; P > .50). After H2O2 stimulation, however, the S/S group showed a 2.4 ± 0.6-fold increase in HO activity, whereas the L/L group showed only a 1.0 ± 0.2-fold increase (P < .05). Treatment with H2O2 at different concentrations hardly induced any apoptosis in the S/S group even after 24 hours of culture (Figure 1). Conversely, marked cell death was observed in the L/L group. There were significant differences in apoptotic cell death between the S/S group and the L/L group at 900 μM H2O2 (4.33% ± 1.61% versus 21.56% ± 2.68%, P < .05). Adding ZnPP-9 increased apoptotic cell death in the S/S group (data not shown). These results demonstrated for the first time that the length polymorphism in the HO-1 gene promoter has a regulatory effect on the inductivity of HO-1 mRNA and HO activity and on the strength of the antiapoptotic effects of HO-1.
Apoptosis assay. LCLs from subjects with L/L (filled symbols) and with S/S (open symbols) were cultured for 24 hours with different concentrations of H2O2. Apoptotic cells were detected by the TUNEL method using a flow cytometer. Results are reported as means ± SDs among 5 lymphoblastoid cell clones from each subject. *Significantly greater than baseline (0 μM H2O2), P < .05.
Apoptosis assay. LCLs from subjects with L/L (filled symbols) and with S/S (open symbols) were cultured for 24 hours with different concentrations of H2O2. Apoptotic cells were detected by the TUNEL method using a flow cytometer. Results are reported as means ± SDs among 5 lymphoblastoid cell clones from each subject. *Significantly greater than baseline (0 μM H2O2), P < .05.
Recently, the first human case of HO-1 deficiency reported was a 6-year-old boy with growth retardation, anemia, iron deposition, and vulnerability to oxidative stress.18 The LCLs from the patient showed no HO-1 production and severe vulnerability to apoptosis induced by oxidative stress. Conversely, the subjects with the L/L genotype in our study had no anemia or abnormality of iron metabolism. Cultured LCLs with an L/L genotype showed a background expression of the HO-1 gene and marginal induction of it after H2O2 stimulation. Taken together, subjects with L/L have enough HO-1 expression to prevent iron metabolism abnormality and multiorgan failure in childhood. However, through years of life with oxidative circumstances or heavy smoking, subjects with L/L could be prone to accumulating tissue injury due to oxidative stress, resulting in higher susceptibility to pulmonary emphysema or cardiovascular disease. Analysis of the polymorphism of the HO-1 gene promoter as well as conventional risk factors could provide useful information for strategies in education and treatment for individuals with susceptibility to oxidative stress–mediated diseases.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2002-12-3733.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal