Abstract
We studied the feasibility and activity of adding sirolimus to tacrolimus and low-dose methotrexate as graft-versus-host disease (GVHD) prophylaxis in recipients of alternative donor transplants. Forty-one patients with hematologic malignancies were conditioned with cyclophosphamide and total body irradiation. Marrow stem cells were from an HLA-A, -B, and -DR compatible, unrelated donor (n = 26, 68%), froma5of6 antigen-matched unrelated donor (n = 8, 20%), or from a 5 of 6 antigen-matched family member (n = 5, 12%). Therapeutic serum levels of sirolimus were attained in most patients. All evaluable patients engrafted. An absolute neutrophil count of 500/μL was achieved on day +18 (range, 11-32 days). Sustained platelet counts of more than 20 000/ μL were attained on day +29 (range, 14-98 days). Grades 0-I acute GVHD occurred in 75% of patients. Grades II, III, and IV acute GVHD occurred in 13%, 8%, and 5%, respectively (total grades II-IV GVHD, 26%). Median survival is 366 days (95% CI 185, not estimable) and actuarial survival at 1 year is 52%. Oral sirolimus is tolerable, adequate blood levels are achievable, and there is a low rate of acute GVHD compared with historical data in this high-risk population. This novel agent is worthy of further study in allogeneic transplantation.
Introduction
Sirolimus is a macrocyclic lactone immunosuppressant derived from Streptomyces hygroscopicus that is similar in structure to tacrolimus. Sirolimus has excellent antirejection activity in organ transplantation,1 and combination therapy with cyclosporine or tacrolimus appears to be extremely effective in human organ allografting2-4 and mouse models of hematopoietic stem cell transplantation (HSCT).5,6 There is also a study demonstrating the activity of sirolimus in steroid-resistant graft-versus-host disease (GVHD).7
While both tacrolimus and sirolimus bind to FKBP12, there appear to be adequate binding sites for both molecules. Therefore, contrary to expectations, the drugs are not competitive, and, in fact, they appear to be synergistic.8-11 Sirolimus also is free of nephrotoxicity and neurotoxicity, making combination therapy appealing. Sirolimus affects lymphocyte activation at a later stage than either cyclosporine or tacrolimus, and activation stimuli that resist inhibition to the latter agents have been shown to be sensitive to sirolimus. Despite the activity observed in organ transplantation, its application to HSCT was delayed by concerns about bioavailability of an oral agent, as well as neutropenia and thrombocytopenia, which may complicate its use.
Transplantation from donors other than HLA-matched siblings is limited by a substantial risk of severe GVHD using conventional cyclosporine or tacrolimus-based prophylaxis.12-15 Combination therapy with sirolimus and tacrolimus may offer an advantage in GVHD prophylaxis. This combination would prevent T-cell activation and proliferation via separate but related mechanisms. Because they appear to be synergistic, lower doses of each drug may be used without enhancing toxicity. Because low-dose methotrexate has a known track record in GVHD prophylaxis,12-15 we explored the feasibility of adding sirolimus to tacrolimus plus low-dose methotrexate. We tested this hypothesis in a sequential phase 1/2 study to be sure adequate blood levels of oral sirolimus could be obtained.
Patients, materials, and methods
Study design
The trial was an open-label phase 1/2 trial of sirolimus, tacrolimus, and low-dose methotrexate in patients with hematologic disorders. The initial end point was to determine the feasibility of using oral sirolimus and to determine the risk of grades II-IV acute GVHD. We also were concerned that sirolimus might delay engraftment or have unanticipated toxicity in HSCT, so an early stopping rule was incorporated into the study based on the development of severe adverse events within 35 days of stem cell infusion. Once we were satisfied that oral administration was feasible, the trial was extended to collect toxicity and phase 2 outcome data. The Dana Farber Cancer Institute Office for the Protection of Research Subjects approved the study, and all patients provided informed consent.
Treatment protocol
All patients received cyclophosphamide 1800 mg/m2 on 2 consecutive days followed by total body irradiation, 14.0 Gy in 7 fractions. Tacrolimus was administered beginning on day –3 at 0.02 mg/kg intravenously by continuous infusion every 24 hours, with a target serum level of 5 to 10 ng/mL. Tacrolimus dosing was converted to oral capsules prior to discharge. Sirolimus was administered as a 12-mg oral loading dose beginning on day –3, followed by 4 mg per day orally in a single morning dose with a target trough level of 3-12 ng/mL by high-performance liquid chromatography (performed at The Children's Hospital, Boston, MA). Trough levels were measured 2-3 times per week. When the trial began, sirolimus was available only as a disagreeable-tasting liquid. It was administered either mixed in water or juice, or in some cases was reformulated by the hospital pharmacy into gelatin capsules. When tablets became available, the liquid was used only rarely. Bone marrow stem cells were infused on day 0. Methotrexate was given at a dose of 5 mg/m2 on days +1, +3, +6, and +11. Sirolimus and tacrolimus were projected to be tapered by 1/3 on weeks 9 and 17 and eliminated on week 26 if clinically feasible.All patients were treated in reverse isolation using fungal prophylaxis and oral nonabsorbable antibiotics for gastrointestinal bacterial decontamination. All patients received prophylactic acyclovir and Pneumocystis carinii prophylaxis consisting of atovaquone 750 mg twice a day or oral trimethoprim-sulfamethoxazole. Blood was obtained weekly for cytomegalovirus testing via hybrid capture, and patients were treated preemptively with ganciclovir or valgancyclovir if clinically indicated. Acute GVHD was graded according to the consensus grading scale.16
Patients
A total of 41 patients were enrolled on this study from the allogeneic transplantation program of Dana Farber Cancer Institute/Brigham & Women's Hospital between September 2000 and June 2002. Most patients (n = 36) had unrelated donors, and the rest had partially matched family-member donors (n = 5). Seven of the unrelated donors were mismatched at major histocompatibility complex (MHC) class I. Unrelated donors were obtained from the National Marrow Donor Program or international registries. Histocompatibility was determined by serology or intermediate resolution molecular typing for MHC class I and by high resolution molecular techniques for MHC class II (Table 1).
Patient characteristics
N | 41 |
| Median age, y (range) | 42 (19-62) |
| Sex, n, M/F | 26/15 |
| Stem cell source, n (%) | |
| Bone marrow | 41 (100) |
| Donor,* n (%) | |
| Histocompatible unrelated donor | 26 (68) |
| 5 of 6 unrelated donor | 8 (20) |
| 5 of 6 matched family member | 5 (12) |
| Diagnosis | |
| CML, n (%) | 12 (29) |
| Stable phase | 8 |
| Accelerated phase or second stable phase | 2 |
| Blast crisis | 2 |
| ALL, n (%) | 9 (22) |
| First remission | 4† |
| Second remission | 4 |
| Resistant relapse | 1 |
| AML, n (%) | 10 (24) |
| Second remission | 1 |
| Resistant relapse | 8 |
| Untreated relapse | 1 |
| Myelodysplasia, n (%) | 7 (17) |
| Non-Hodgkin lymphoma, n (%) | 2 (5) |
N | 41 |
| Median age, y (range) | 42 (19-62) |
| Sex, n, M/F | 26/15 |
| Stem cell source, n (%) | |
| Bone marrow | 41 (100) |
| Donor,* n (%) | |
| Histocompatible unrelated donor | 26 (68) |
| 5 of 6 unrelated donor | 8 (20) |
| 5 of 6 matched family member | 5 (12) |
| Diagnosis | |
| CML, n (%) | 12 (29) |
| Stable phase | 8 |
| Accelerated phase or second stable phase | 2 |
| Blast crisis | 2 |
| ALL, n (%) | 9 (22) |
| First remission | 4† |
| Second remission | 4 |
| Resistant relapse | 1 |
| AML, n (%) | 10 (24) |
| Second remission | 1 |
| Resistant relapse | 8 |
| Untreated relapse | 1 |
| Myelodysplasia, n (%) | 7 (17) |
| Non-Hodgkin lymphoma, n (%) | 2 (5) |
CML indicates chronic myeloid leukemia; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia.
Mismatch locus: Unrelated donor disparity: HLA-A (n = 4), HLA-B (n = 3), HLA-DR (n = 1). Related donor disparity: HLA-A (n = 4), HLA-DR (n = 1). Donor-recipient disparity at HLA-C (n = 8), HLA-DRB3 (n = 1), and HLA-DQ (n = 3) were not considered to be mismatched. Two unrelated donors were mismatched at both HLA-B and HLA-C, and one was mismatched at HLA-A and HLA-C. These were counted as single antigen mismatches.
All 4 patients had high-risk cytogenetics or clinical features.
Statistical analysis
Descriptive statistical analysis was performed to assess patient baseline characteristics, engraftment, GVHD, and relapse rate. Overall survival and event-free survival were calculated using the Kaplan-Meier method. Cumulative incidence rate of grades II-IV GVHD was estimated using a competing risk of 100-day mortality.17 Event-free survival was defined as the time between allogeneic transplant to relapse or death from any cause. Overall survival was defined as the time between allogeneic transplantation to death from any cause. If a patient was alive without relapse, then the duration of event-free survival was censored at the date of last contact. Smoothing spline curve estimation techniques were used to explore the patterns of longitudinal data on sirolimus and tacrolimus blood levels.
Results
Sirolimus and tacrolimus blood levels
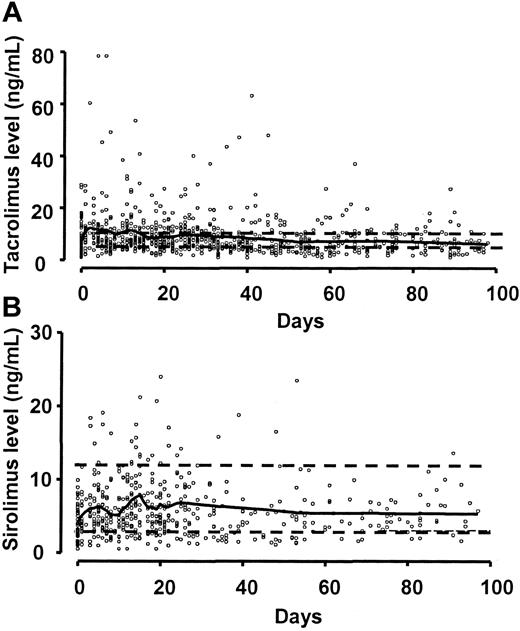
Sirolimus blood levels of 3-12 ng/mL and tacrolimus levels of 5-10 ng/mL were maintained in most patients (Figure 1). There were occasional patients who were unable to take liquid sirolimus because the disagreeable taste stimulated immediate vomiting. However, occasional missed doses had little effect on the levels because of the long half-life of the drug. Once the tablets became available, compliance became a much less significant concern. In 33 (80%) of 41 patients, one or more sirolimus measurements were below the therapeutic level. However, most patients promptly returned to the therapeutic level and only 2 (6%) of 33 patients were unable to sustain therapeutic level of sirolimus for most of the first month of treatment. Similarly, 32 (78%) of 41 of patients had one or more measurements of tacrolimus level that were below the therapeutic level, but all of them were able to sustain therapeutic levels of tacrolimus for most of the first month of treatment.
Sirolimus and tacrolimus blood levels. The solid line is a spline smoothing curve. (A) Tacrolimus target range was 5-10 ng/mL (dashed lines). (B) Sirolimus target range was 3-12 ng/mL (dashed lines). Compliance was substantially better when the tablet formulation became available.
Sirolimus and tacrolimus blood levels. The solid line is a spline smoothing curve. (A) Tacrolimus target range was 5-10 ng/mL (dashed lines). (B) Sirolimus target range was 3-12 ng/mL (dashed lines). Compliance was substantially better when the tablet formulation became available.
Engraftment
Two patients died of transplant-related toxicity prior to day 28 without attaining an absolute neutrophil count of at least 500 neutrophils/μL. Time to neutrophil and platelet recovery is shown in Table 2. Thirteen patients never reached a platelet count of 100 000 cells/μL. There were no graft failures.
Time to neutrophil and platelet recovery
Time . | n . | Median d (range) . |
|---|---|---|
| At least 500 neutrophils/μL | 39 | 18 (11-32) |
| At least 20 000 platelets/μL | 33 | 29 (14-98) |
| At least 100 000 platelets/μL | 26 | 52 (22-482) |
Time . | n . | Median d (range) . |
|---|---|---|
| At least 500 neutrophils/μL | 39 | 18 (11-32) |
| At least 20 000 platelets/μL | 33 | 29 (14-98) |
| At least 100 000 platelets/μL | 26 | 52 (22-482) |
Graft-versus-host disease
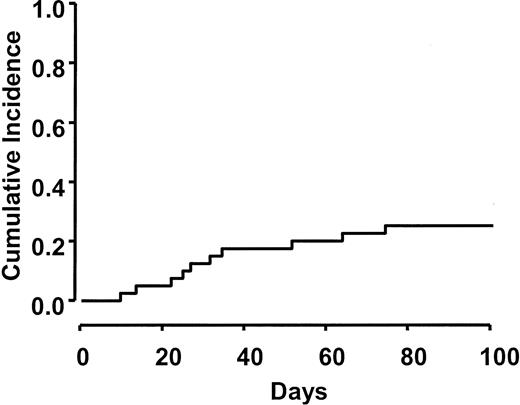
Table 3 shows the distribution of acute GVHD grades observed in this study. Moderate to severe GVHD (grades II-IV) developed in 10 (26%) of 39 evaluable patients. Severe GVHD (grades III/IV) was observed in 5 (13%) of 39. The cumulative incidence rate of grade II-IV GVHD (26%) using a competing risk of 100-day mortality is presented in Figure 2.
Graft-versus-host disease
. | n (%) . |
|---|---|
| Acute GVHD grade | |
| 0 | 21 (54) |
| I | 8 (21) |
| II | 5 (13) |
| III | 3 (8) |
| IV | 2 (5) |
| Grades II-IV GVHD | |
| Matched unrelated donor | 7 of 27 (26) |
| Mismatched unrelated donor | 2 of 7 (29) |
| Mismatched family member | 1 of 5 (20) |
. | n (%) . |
|---|---|
| Acute GVHD grade | |
| 0 | 21 (54) |
| I | 8 (21) |
| II | 5 (13) |
| III | 3 (8) |
| IV | 2 (5) |
| Grades II-IV GVHD | |
| Matched unrelated donor | 7 of 27 (26) |
| Mismatched unrelated donor | 2 of 7 (29) |
| Mismatched family member | 1 of 5 (20) |
Cumulative incidence of grades II-IV acute GVHD. Acute GVHD occurred in 26% of patients by day 100. Three cases of GVHD occurred after day 40 during the taper of immunosuppression.
Cumulative incidence of grades II-IV acute GVHD. Acute GVHD occurred in 26% of patients by day 100. Three cases of GVHD occurred after day 40 during the taper of immunosuppression.
A flare of acute GVHD occurred in 3 patients after day 40 during the taper of immunosuppressants. In 2 of these patients GVHD came under control promptly with corticosteroids. Acute GVHD was fatal in 2 patients, one occurred after day 40 during the tapering phase. There was no association between stem cell source and the risk of acute GVHD (Table 3). Chronic GVHD has been observed in 14 (3 limited, 11 extensive) (42%) of 33 evaluable patients.
Sirolimus-related toxicity
Hyperlipidemia is one of the principal toxicities of sirolimus. Cholesterol and triglyceride levels were elevated in 2 patients. One patient had National Cancer Institute Common Toxicity Criteria (NCI CTC) grade 2 elevation of cholesterol levels, and the other patient had grade 3 hypertriglyceridemia. Typically, the blood levels either required no specific therapy or they came under satisfactory control with an 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor. One patient developed hemolytic uremic syndrome (HUS). The HUS resolved with reduction in tacrolimus and sirolimus levels.
Relapse and survival
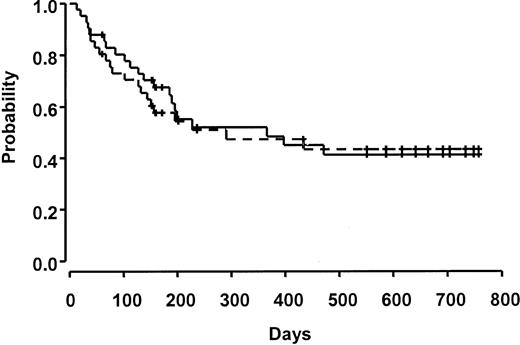
Ten patients relapsed over the course of the study. Survival at 100 days was 80%. Fifteen patients were considered to be in a good-risk group, and 26 patients were in a poor-risk group. There was no significant difference in death prior to day 100 in this small cohort based on risk group (3 [20%] of 15 good-risk patients versus 5 [16%] of 26 poor-risk patients). Median survival was 366 days (95% CI 185, not estimable), and overall and event-free survival were 52% and 45%, respectively, at 1 year (Figure 3). Other causes of death included hepatic veno-occlusive disease (n = 3, 7%), diffuse alveolar hemorrhage or interstitial pneumonia (n = 2, 5%), multiorgan failure (n = 1, 2%), infection (n = 3, 7%), and hemorrhage (n = 1, 2%).
Overall and event-free survival. All patients who relapsed have died. The solid line indicates overall survival, and the dashed line indicates event-free survival. Ticks represent censored observations.
Overall and event-free survival. All patients who relapsed have died. The solid line indicates overall survival, and the dashed line indicates event-free survival. Ticks represent censored observations.
Discussion
While hematopoietic stem cell transplantation (HSCT) may cure some patients with blood diseases, its use is associated with substantial risks. One of the most serious of these complications is acute GVHD. Strategies to reduce GVHD have been the subject of numerous trials over the past 25 years, but efforts have been incompletely effective and novel approaches are needed. Methotrexate has been a 2-edged sword in GVHD control. Its antiproliferative effect is an important adjunct to calcineurin inhibition, but its intrinsic toxicity prolongs time to count recovery and increases transplant-related toxicity. The objective of this trial was to pilot a novel approach to GVHD control, taking advantage of the synergistic interactions of sirolimus and tacrolimus. We chose to combine sirolimus with tacrolimus because the 2 drugs can be administered simultaneously, in contrast to cyclosporine, which is given 4 hours before sirolimus because of drug interactions. Moreover, phase 3 studies comparing cyclosporine to tacrolimus prophylaxis showed a better control of acute GVHD with tacrolimus.14,18 Since sirolimus is synergistic with tacrolimus, we believed it would be possible to control GVHD while minimizing regimen-related toxicity. We ultimately hoped to develop a prophylaxis regimen in which methotrexate administration could be minimized or perhaps eliminated altogether. We did not believe we could eliminate methotrexate at the outset in this high-risk group of patients, so we added sirolimus to a regimen of “minidose” methotrexate in which GVHD control appeared to be similar to the efficacy observed with conventional dose methotrexate prophylaxis.12,13
Our first goal was to determine whether sirolimus levels could be maintained, since the drug is available only in an oral formulation. At the beginning of the study, sirolimus was only available in liquid form, and it was quite distasteful. In patients with severe vomiting from conditioning regimen–related toxicity, maintaining blood levels required close nursing attention. Fortunately, sirolimus has a long half-life (t1/2 = 60 hours), and this resulted in modest declines in serum levels if a dose was missed. Once tablets became available, compliance was close to 100%. We chose serum levels of 3-12 ng/mL since levels above 15 ng/mL have been associated with higher rates of toxicity.
Prevention of acute GVHD remains a critical problem, particularly in the use of alternative donors. Sirolimus has been added to methylprednisolone as second-line therapy in steroid-resistant GVHD.7 Prior studies of cyclosporine and methotrexate in high-risk populations typically show rates of grades II-IV acute GVHD of 50% to 75%, and the risk of grades III-IV acute GVHD is 20%-50%.12-14,19-24 In contrast, the addition of sirolimus resulted in a 26% rate of grades II-IV GVHD and a 13% rate of grades III/IV GVHD. Moreover, there did not appear to be an increased risk of acute GVHD in patients with HLA mismatches, although there were insufficient patients to be confident of this observation. The rate of chronic GVHD was 42% compared with rates of 55% and 86% reported previously with tacrolimus and low-dose methotrexate.12,13
Excellent control of GVHD was observed despite using a dose of methotrexate that is approximately 50% of the dose used in most studies. The study was designed to assess feasibility rather than demonstrate synergy, but it appeared that the combination resulted in improved GVHD control compared with historical experience. The mechanism of improved GVHD control with sirolimus is suggested by the mechanism of action. Both sirolimus and tacrolimus bind to FKBP12. The tacrolimus:FKBP12 complex inhibits calcineurin phosphatase and prevents dephosphorylation of nuclear factor of activated T cells (NF-AT). This results in prevention of T-cell activation, failure to produce interleukin-2 (IL-2) and IL-2 receptor, and prevention of cell cycle progression at the G0 → G1 interface. In contrast, sirolimus complexed with FKBP12 binds to mammalian target of rapamycin (mTOR), which blocks cytokine-mediated signal transduction pathways that prevent G1 → S phase transition. This effect is mediated through a complex pathway involving inhibition of ribosomal protein synthesis at several levels, as well as effects on transcription and translation (reviewed by Sehgal25 ). It also may interfere with costimulation by inhibiting CD28-mediated blocking of IκBα and translocation of c-Rel to the nucleus.26,27 Sirolimus contributes to massive T-cell apoptosis if signals 1 and 2 of T-cell activation are blocked—an effect that is not seen with cyclosporine or tacrolimus.28 This effect may be related to its ability to inhibit the expression of bcl-2 and BAG-1.29,30 Finally, dendritic cells play an important role in the initiation of GVHD.31,32 Sirolimus has profound effects on dendritic cell function and survival that are distinct from the effects of corticosteroids and calcineurin inhibitors and that may contribute to prevention of GVHD.33-36
Control of GVHD is sometimes associated with an increased relapse rate due to loss of the graft-versus-leukemia effect. Despite the high-risk cohort studied here, the relapse rate was only 24%. The cohort is too small and heterogeneous to make a meaningful assessment of any effect of the addition of sirolimus on relapse rate.
There was also concern about the toxicity of sirolimus in stem cell transplantation. It has been noted to cause reversible neutropenia and thrombocytopenia in organ transplant recipients37,38 as well as in patients with psoriasis.39 However, we observed no delay in blood count recovery, in fact, the rate of neutrophil recovery was faster than our historical experience,40 most likely related to the lower methotrexate dose. Hemolytic uremic syndrome is a well-established complication of stem cell transplantation, and the use of high doses of sirolimus appeared to have increased the risk of HUS in patients with established GVHD.7 In kidney transplantation the combination of sirolimus with a calcineurin inhibitor increases the risk of HUS when the drug levels are high, but sirolimus alone seems to be less associated with HUS.41 We observed one case of HUS, and it was not clear whether HUS was related to the use of sirolimus per se since tacrolimus can also cause HUS; however careful monitoring of drug levels is likely to be critical in the safe use of this combination. Blood levels are particularly sensitive to the addition or discontinuation of drugs with effects on cytochrome P450 IIIA4 (CYP3A4) and P-glycoprotein. Hyperlipidemia was observed, although it was not a significant management problem. Sirolimus, tacrolimus, and prednisone all may be associated with hyperlipidemia, and clearly triglyceride and cholesterol levels need to be monitored, however, in no case did sirolimus need to be stopped. The hyperlipidemia responded to tapering of all 3 immunosuppressants and the institution of HMG CoA inhibitors, such as atorvastatin. Since hyperlipidemia due to sirolimus increases with duration of therapy, we speculate that the tapering schedule employed in this study prevented some of the expected hyperlipidemia. In patients with active GVHD in whom sirolimus tapering is much slower, hyperlipidemia may be a more serious problem.
In summary, sirolimus can be added safely to tacrolimus and methotrexate for GVHD prophylaxis. Despite its poor bioavailability and the lack of an intravenous formulation, adequate blood levels can be obtained in most patients. There is no apparent deleterious effect on neutrophil or platelet recovery, and toxicity attributable to sirolimus is manageable. Most important, GVHD prevention is excellent in a high-risk cohort of patients. This promising agent may have synergistic effects with tacrolimus that will allow a further reduction in both agents and perhaps the elimination of methotrexate.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2003-02-0489.
Supported in part by P01 HL070149 from the National Heart, Lung, and Blood Institute and unrestricted educational grants from Fujisawa Healthcare and Wyeth; and by unrestricted educational grants from Wyeth and Fujisawa Healthcare.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal