Abstract
The mechanism of action of farnesyltransferase inhibitors (FTIs) has not been fully clarified. We investigated the cytotoxic effects of various FTIs in chronic myeloid leukemia (CML), using LAMA cells and marrow cells from 40 CML patients in chronic phase. FTI-mediated cytotoxic effect was observed in LAMA cells and in 65% of primary CML cells, whereas marrow cells from controls were only weakly affected. Cytotoxic effects were partially related to enhanced apoptosis; however, Fas-receptor (FasR) and Fas-ligand (FasL) expression were not modified by FTIs. Susceptibility to FTI-mediated inhibition did not correlate with FasR/FasL expression in CD34+ CML cells. Moreover, intra-cellular activation of caspase-1 and -8 were not altered by FTIs, and their blockade did not reverse FTI toxicity. However, we observed FTI-induced activation of caspase-3, and its inhibition partially reverted FTI-induced apoptosis. FTIs did not modulate bcl2, bclxL, and bclxS expression, whereas they increased inducible nitric oxide (iNOS) mRNA and protein levels, resulting in higher NO production. Furthermore, C3 exoenzyme, a Rho inhibitor, significantly increased iNOS expression in CML cells, suggesting that FTIs may up-regulate NO formation at least partially through FTI-mediated inhibition of Rho. We conclude that FTIs induce selective apoptosis in CML cells via activation of iNOS and caspase-3.
Introduction
Mutations or overexpression of the ras proto-oncogene have been demonstrated in many solid tumors1-5 and in 20% to 30% of cases of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS).6-9 In malignant cells, constitutively expressed ras may lead to dysregulation of cell growth and apoptosis.10,11 Ras, a 21-kDa guanine nucleotide-binding protein, is farnesylated by the housekeeping enzyme farnesyltransferase (FTase). This reaction is the first obligatory step in the ras transduction pathway, enabling its link to the plasma membrane and its oncogenic activity.12,13 Consequently, FTase blockade was used as a biochemical target for growth suppression in tumors carrying ras mutations.1,2,7,14 Based on this concept, several potent and selective farnesyltransferase inhibitors (FTIs) have been developed and have been shown to block ras processing, signaling, and transformation. However, this effect is present not only in ras mutants15-21 but also in tumor cells containing the wild-type ras, suggesting that the farnesylation of other intracellular proteins may also be important for malignant proliferation.22,23 For example, farnesylation appears to be required for the activation of wild-type ras, Rho, and nuclear lamins. In several cell types, the antineoplastic action of FTIs has been proposed to be mediated by ras-independent effects.22-27 Moreover, FTIs have also been shown to modify the activity of other molecules that are not direct substrates of farnesylation, but depend on available pools of farnesol.28 In culture, FTIs have been shown to induce apoptosis in many malignant cell types, an effect that is considered to be ras-independent and associated with decreased expression of Fas.29-31
In addition to MDS and AML,6-9 ras appears to be involved in the leukemogenic transformation by the hybrid gene bcr/abl in chronic myelogenous leukemia (CML) by unclear mechanisms.32-34 ras overactivity in leukemias carrying bcr/abl may lead to abnormal transduction of proliferative signals.32-36 The bcr/abl oncogene activates ras and triggers the stress-activated protein kinase (SAPK, or Jun NH2-terminal kinase [JNK]) pathways; interruption of this pathway reduces bcr/abl-mediated transformation.37-39 In CML cells, ras exhibits redundant effects with several intracellular signaling proteins as STAT5, phosphatidylinositol 3–kinase, and Akt serine/threonine kinase.40,41 First-generation FTIs used in vitro on CML cells showed variable effects; more consistent inhibitory activity has been observed with some novel FTIs. As in other malignancies, it is possible that these effects are independent from the blockade of ras and involve other pathways, including Fas-receptor/Fas-ligand (FasR/FasL), bcl2, inducible nitric oxide synthase (iNOS), and Rho.
The biologic activity of FTIs in CML8,19,20 and their favorable toxicity profile suggest that FTase blockade may be used therapeutically in this disease. We hypothesized that FTIs' effects in CML involve reversal of the apoptotic pathway inhibition. Consequently, we studied the effects of FTIs on apoptosis and proliferation of CML cell lines and primary CML cells in vitro and investigated the mechanisms of their action by experimental blockade of various components of the apoptotic pathway.
Materials and methods
FTI compounds
The FTIs α-hydroxyfarnesylphosphonic acid (α-HFPA) and manumycin-A (Man-A) were purchased from Sigma (St Louis, MO). SCH66336 (SCH) was a gift from Schering-Plough Research Institute (Kenilworth, NJ). All these compounds were stored at –20°C as 10-mM stocks in dimethyl sulfoxide (DMSO; Sigma, St Louis, MO).
Cell lines and patient specimen collection
K562 and LAMA-84 bcr/abl+ cell-line cells were obtained from the American Type Culture Collection (Manassas, VA) and the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany), respectively. The cells were grown in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin, and 0.1 mg/mL streptomycin at 37°C in 5% CO2.
Heparinized bone marrow (BM) samples were obtained, after informed consent according to the procedures outlined by the ethical committee of our institution, from 10 healthy individuals at the time of allogeneic marrow donation and from 40 patients with CML in chronic phase at diagnosis during diagnostic procedures. CML diagnosis was documented by the cytogenetic finding of the Ph chromosome and the molecular finding of rearranged bcr/abl. Clinical characteristics of patients are summarized in Table 1. Bone marrow mononuclear cells (BMMNCs) were isolated by density gradient centrifugation using lymphocyte separation medium. After washing with phosphate-buffered saline (PBS), BMMNCs were resuspended in Iscove modified Dulbecco Medium (IMDM) supplemented with 10% heat-inactivated FCS. RPMI 1640, IMDM, PBS, FCS, l-glutamine, penicillin, streptomycin, and lymphocyte separation medium were purchased from Life Technologies (Gaithersburg, MD).
Main characteristics of CML patients
. | No., n = 40 . |
|---|---|
| Ph+ metaphases | |
| More than 95% | 37 |
| More than 80% | 3 |
| Sokal score (risk) | |
| Less than 0.8 (low) | 17 |
| 0.8-1.2 (intermediate) | 12 |
| Greater than 1.2 (high) | 11 |
| bcr/abl rearrangement | |
| b3/a2 | 33 |
| b2/a2 | 7 |
. | No., n = 40 . |
|---|---|
| Ph+ metaphases | |
| More than 95% | 37 |
| More than 80% | 3 |
| Sokal score (risk) | |
| Less than 0.8 (low) | 17 |
| 0.8-1.2 (intermediate) | 12 |
| Greater than 1.2 (high) | 11 |
| bcr/abl rearrangement | |
| b3/a2 | 33 |
| b2/a2 | 7 |
The mean age of the patients ± SD was 45.7 ± 15 years (range, 22-80 years); 18 were women and 22 were men.
Cultures
Cell lines and primary CML cells were washed 3 times with PBS and resuspended in RPMI containing 0.1% FCS 12 hours and 2 hours, respectively, before exposure to FTIs (or to solvent only, as control cultures). In some experiments, prior to exposure to FTIs, CML cells were preincubated for 2 hours with an esterified caspase inhibitor (caspase-1 inhibitor ZYVAD-(OMe)FMK, caspase-3 inhibitor Z-DEVD-(OMe)FMK, or caspase-8 inhibitor IETD-(OMe)FMK) or with the Fas-receptor–triggering inhibitor Fas:Fc (all purchased from Alexis, San Diego, CA, and used at 50 μM), or with the iNOS inhibitor NG-monomethyl-arginine (500 μM; γ-MM-arg; Calbiochem, San Diego, CA). In some experiments the effect on cell survival and apoptosis of the Rho inhibitor exoenzyme C3 transferase (Cytoskeleton, Denver, CO) at 50 μM for 2 hours before FTI addition was also assayed. All experiments were carried out in duplicate wells and repeated at least 3 times.
Viability assay
In vitro sensitivity of LAMA and primary BM CML cells to FTIs was determined by plating 5 × 105 cells in 500 μL medium containing several dilutions of FTI, in 24-well plates, without any specific growth factor. Controls were performed using identical concentrations of the solvent used for FTIs. After incubation for 2 days at 37°C with 5% CO2, cell viability was determined using the 3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT; Sigma) assay. Briefly, 20 μL MTT (5 mg/mL in PBS) was added to each well and, after shaking, incubated for 6 hours at 37°C. After centrifugation, the supernatant was removed and formazan crystals were dissolved in 200 μL DMSO. The optical density (OD) was measured at 562 nm using a microplate spectrophotometer (Titertek Multiscan, BioRad, Hercules, CA). FTI effect was measured as percentage of inhibition of cell viability by the following equation: 1 – [(OD treated well/mean OD control wells) × 100], after correction for the background OD of blank wells.
Hematopoietic colony assay
BMMNCs were plated in methylcellulose (Stem Cell Technologies, Vancouver, BC, Canada) at a concentration of 3 × 105 cells/mL medium (35 mm dishes; 1 mL medium per dish). In some experiments, purified CD34+ CML cells were obtained by using affinity columns (Cellpro, Bothel, WA), as previously described.42 The growth factor cocktail consisted of 10 ng/mL interleukin-3 (IL-3), 50 ng/mL granulocyte colony-stimulating factor (G-CSF), 50 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), 20 ng/mL stem cell factor (SCF), and 2 U/mL erythropoietin (EPO; Amgen, Thousand Oaks, CA). All cultures were performed in duplicate in endotoxin-free plasticware.
Apoptosis assay
DNA fragmentation was measured after low-molecular-weight DNA extraction from a constant number of cells. Briefly, 2 × 106 cells were resuspended in 900 μL 1 × Tris-EDTA (tris(hydroxymethyl)aminomethane–ethylenediaminetetraacetic acid) buffer and lysed with 25 μL 20% sodium dodecyl sulfate (SDS). The high-molecular-weight DNA fraction was precipitated for 6 hours with 5 M NaCl, pelleted by high-speed centrifugation; the fragmented DNA was extracted from the aqueous phase with phenol and chloroform and precipitated with ethanol. After resuspension in water, DNA was electrophoresed using 1.5% agarose gel and visualized by ethidium bromide staining.
Flow cytometry of cellular DNA was performed following propidium iodide (PI) staining. Briefly, washed cells were fixed in ice-cold 70% ethanol, rewashed, incubated in 1 mL PI staining solution (sample buffer with 50 μg/mL PI and 100 U/mL RNAse A [Boehringer Mannheim, Mannheim, Germany]) and stored in the dark at 4°C. A minimum of 60 000 events were counted per sample and the percentage of apoptotic cell nuclei containing hypodiploid DNA was evaluated.
Apoptotic cells were also measured by the terminal deoxynucleotidyl transferase (TdT) assay (Apotag; Oncor, Gaithersburg, MD), as previously described.42 Briefly, washed cells were fixed with 4% paraformaldehyde and spun onto siliconized slides. Apoptotic cells were identified according to the manufacturer's instructions.
Flow cytometry analysis
Phycoerythrin (PE)–conjugated monoclonal antibody (MoAb) to CD34 (clone HPCA-1; Becton Dickinson, Mountain View, CA) was used to identify CD34+ cells. A fluorescein isothiocyanate (FITC)–conjugated (Fab) fragment of a murine antihuman CD95 (clone UB2; Amac, West-brook, ME) or control mouse IgG1 FITC antibody (Becton Dickinson), combined with the PE-conjugated anti-CD34, was used to identify FasR on CD34+ cells. To detect membrane-bound FasL, CD34+ cells were incubated with PE-conjugated anti-CD34 for 30 minutes at 4°C and combined with a purified mouse antihuman FasL (IgG1, Nok1; Pharmingen, San Diego, CA) or control mouse IgG1 antibody (Becton Dickinson) for 1 hour at 4°C and then with the FITC-conjugated secondary antibody for 30 minutes at 4°C. Staining was performed in the presence of the metalloproteinase inhibitor KB8301 (10 μM; Pharmingen), which blocks FasL cleavage. For each flow cytometric acquisition (FACS, Becton Dickinson), a threshold was set to exclude cell debris from analysis.
Determination of caspase-1, -3, and -8
Activation of intracellular caspases was assayed by flow cytometry using fluorogenic caspase-specific substrates (WEHD-AMC for caspase-1, DEVDAMC for caspase-3, and IETD-AMC for caspase-8; Alexis), as previously described.43 Briefly, after treatment with FTI for 24 hours in the presence or absence of caspase-specific inhibitors, 1 × 106 cells were resuspended with 50 μL substrate buffer containing 10 mM dithiothreitol (DTT) and 10 μL fluorogenic caspase-specific substrate supplemented with 5 μL FCS. After centrifugation, cells were incubated at 37°C for 60 minutes. Then 500 μL ice-cold dilution buffer was added and the cells were subjected to flow cytometry, analyzing at least 10 000 events. Results were expressed as fold increase in fluorescence, relative to untreated control cells. Caspase-3 activity in CML cells was also detected by Western blotting (see “Immunoblotting of iNOS, bcl2, and bclxL/S proteins”) and by the CaspaTag Caspase activity assay (Intergen, Oxford, United Kingdom), which is based on the loss of outer membrane integrity following caspase activation. Briefly, 10 μL of cell-permeable FITC-labeled peptide FAM-VAD-FMK was added to 5 × 105/mL CML cells cultured for 24 hours with or without FTI. Cells were then incubated for 1 hour at 37°C under 5% CO2, protected from light. After 2 washings, followed by the addition of 2 μL PI to identify dead cells, at least 10 000 CML cells were analyzed by flow cytometry.
Reverse transcription–polymerase chain reaction (RT-PCR) for human iNOS RNA
Total RNA was extracted from a constant number of BMMNCs using TRIzol (Invitrogen, Carlsbad, CA). Contaminating DNA was digested using RNAse-free DNAse I (Promega, Madison, WI). RNA was reextracted with phenol/chloroform, precipitated with ethanol, and diluted in RNAse-free water. After reverse transcription using an oligo d(T)16 primer, human iNOS cDNA was amplified using the primer pairs 5′-CGG TGC TGT ATT TCC TTA CGA GGC GAA GAA GG-3′ and 5′-GGT GCT GCT TGT TAG GAG GTC AAG TAA AGG GC-3′, and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified using the primer pairs 5′-TTC ACC ACC ATG GAG AAG GCT-3′ and 5′-ACA GCC TTG GCA GCA CCA GT-3′. Reagents were supplied by Perkin Elmer (Foster City, CA). PCR products were electrophoresed in 1.2% agarose gel and bands visualized under ultraviolet (UV) light after staining with ethidium bromide.
Immunoblotting of iNOS, bcl2, and bclxL/S proteins
After the indicated treatment, washed CML cells were lysed for 10 minutes in ice in a buffer containing 0.15M NaCl, 1% NP40, 0.1% deoxycholic acid, 0.1% SDS, and 0.05 M Tris HCl (pH 8.00) supplemented with protease inhibitors (Sigma). Cell lysates were then diluted in Laemmli sample buffer (Novex, San Diego, CA) supplemented with 1% β-mercaptoethanol and boiled for 3 minutes at 90°C. Protein concentration of cell lysates was measured by a colorimetric method (BioRad, Richmond, CA). Cell lysate (100 μg), together with molecular-weight markers (Amersham, Little Chalfont, United Kingdom) and an iNOS-positive mouse macrophage lysate (Transduction Laboratories, Lexington, KY), was fractionated by 7.5% SDS–polyacrylamide gel electrophoresis (PAGE). Equal protein loading was assessed by Coomassie Blue staining of SDS-PAGE gels. Gels were equilibrated in transfer buffer (125 mM Tris-base, 960 mM glycine, 20% methanol) and electrically transferred to PVDF membrane filters (Millipore, Bedford, MA). Membranes were blocked in T-PBS (0.1% Tween 20 in PBS)–5% casein (Biorad) for 1 hour and incubated overnight at 4°C with 2 μg/mL mouse anti-iNOS, anti-bcl2, anti-bclxS/L (Transduction Laboratories), polyclonal rabbit anti–caspase-3 (Becton Dickinson), or antiactin MoAbs (Sigma), in T-PBS supplemented with 1% bovine serum albumin (BSA; Boehringer Mannheim). The blots were developed with horseradish peroxidase–conjugated goat antirabbit antibodies (BioRad, Richmond, CA) and developed by enhanced chemiluminescence (ECL; Amersham) according to the manufacturer's specifications.
Measurement of NO production in CML cells
After 3 washings with red phenol-free PBS supplemented with 15 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, and 0.1% BSA (Life Technologies), LAMA and primary CML cells were serum-starved for 24 hours and 2 hours, respectively. Serum-starved cells (5 × 105) were plated in the same medium in triplicate wells and loaded with the NO-reactive dye 4,5-diaminofluorescein diacetate (DAF-2 DA; Alexis; final concentration, 10 μM; 1 hour, 37°C). Then cells were exposed to FTI, kept in the dark, and maintained at 37°C for 1 hour. To inhibit NOS activity, wells were preincubated for 2 hours with γ-MM-arg (500 μM) prior to addition of DAF-2 DA and FTI. DAF-2 DA is a cell-permeable compound that is converted to DAF-2 by intracellular esterases. In the presence of NO, it forms a triazole derivative, which emits light at 515 nm upon excitation at 489 nm in proportion to the amount of NO present. Intracellular green fluorescence intensity of DAF-2 was quantified by flow cytometry and expressed as fold increase in mean fluorescence intensity, relative to untreated cells.
Statistical analysis
The 2-tailed Student t test was employed for flow cytometric analysis and tissue-culture experiments. A P value of .05 or less was considered statistically significant.
Results
Effects of FTIs on CML cells
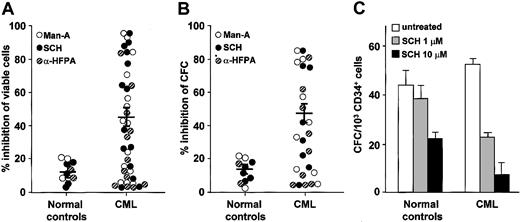
Initially, we investigated the effects of FTIs on the growth of the CML cell line LAMA. In MTT tests performed in the logarithmic growth phase, LAMA cell survival was inhibited by all FTIs in a dose-dependent manner. By linear regression analysis, we determined the 50% inhibitory concentration (IC50) after 48 hours' exposure to FTI. SCH66336 was more toxic on an equimolar basis (IC50: mean, 5 μM; range, 1-10 μM) than Man-A (IC50: mean, 50 μM; range, 25-75 μM) and α-HFPA (IC50: mean, 100 μM; range, 75-150 μM). In subsequent experiments with primary cells we used the mean IC50 of each FTI established in the LAMA cell line. FTI-induced inhibition of cell viability greater than 20% was present in 60% of CML samples, and the inhibitory effect was significantly stronger on CML marrow cells than on normal marrow cells (Figure 1A). In methylcellulose cultures, FTIs induced inhibition of colony formation greater than 20% in 65% of CML samples, with similar degrees of inhibition for both erythroid and myeloid progenitors, whereas healthy donor marrow cells were only weakly affected by FTI exposure (Figure 1B). Parallel results were obtained in colony cultures of highly purified marrow CD34+ CML cells after SCH exposure with an IC50 at 1 μM (mean inhibition of CFC ± SEM: 55.7% ± 2%). By contrast, marrow CD34+ cells from 3 healthy donors were less sensitive to colony inhibition by SCH, with an IC50 at 10 μM (mean inhibition ± SEM: 49% ± 1%; Figure 1C).
FTI-induced inhibition of cell viability and colony formation in normal and CML bone marrow cells measured by colorimetric MTT and methylcellulose colony-forming cell (CFC) assays. Each circle in panels A (colorimetric MTT assay) and B (methylcellulose CFC assay) represents a subject studied and indicates the percentage of inhibition of cell viability and colony formation by each FTI at the IC50 described in “Effects of FTIs on CML cells” (control cultures were considered 100%). Horizontal bars represent the mean; vertical bars, SEM. Cumulative mean inhibition of cell viability and of CFC, ± SEM, for any FTI used: 45% ± 5% and 47.7% ± 7% in CML cells versus 12.3% ± 2% and 15.5% ± 1% in normal cells; P = .03 and P < .001, respectively. (C) FTI effects on CFC from purified CD34+ cells of 3 CML patients and 3 healthy donors, measured by methylcellulose CFC assay. SCH at 1 μM (▦) was inoffensive on normal cells while causing more than 50% colony growth inhibition of CML cells; at 10 μM(▪) there was 50% colony growth inhibition of normal cells and 90% colony growth inhibition of CML cells. Error bars represent SEM. Statistical analysis (paired t test) showed P < .05 only in the CML group for no treatment (□) versus 1 μM and 10 μM SCH.
FTI-induced inhibition of cell viability and colony formation in normal and CML bone marrow cells measured by colorimetric MTT and methylcellulose colony-forming cell (CFC) assays. Each circle in panels A (colorimetric MTT assay) and B (methylcellulose CFC assay) represents a subject studied and indicates the percentage of inhibition of cell viability and colony formation by each FTI at the IC50 described in “Effects of FTIs on CML cells” (control cultures were considered 100%). Horizontal bars represent the mean; vertical bars, SEM. Cumulative mean inhibition of cell viability and of CFC, ± SEM, for any FTI used: 45% ± 5% and 47.7% ± 7% in CML cells versus 12.3% ± 2% and 15.5% ± 1% in normal cells; P = .03 and P < .001, respectively. (C) FTI effects on CFC from purified CD34+ cells of 3 CML patients and 3 healthy donors, measured by methylcellulose CFC assay. SCH at 1 μM (▦) was inoffensive on normal cells while causing more than 50% colony growth inhibition of CML cells; at 10 μM(▪) there was 50% colony growth inhibition of normal cells and 90% colony growth inhibition of CML cells. Error bars represent SEM. Statistical analysis (paired t test) showed P < .05 only in the CML group for no treatment (□) versus 1 μM and 10 μM SCH.
FTI-induced apoptosis of CML cells
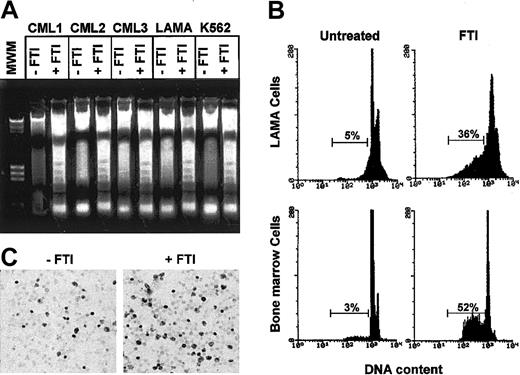
The inhibitory effect of FTIs could be due to inhibition of cell cycling or to induction of apoptosis.14,27 The analysis of low-molecular-weight DNA extracted from total BM CML cells cultured in the presence of any FTI showed a nucleosomal DNA degradation pattern on agarose gel electrophoresis characteristic of apoptosis (Figure 2A). By flow cytometry and TdT assay, FTIs strongly enhanced apoptosis of CML cells (n = 15; cumulative mean percentage of apoptotic cells ± SEM when any FTI was present, 31.8% ± 4%, vs 5.4% ± 1% in control cultures; P < .001; Figure 2B-C).
FTI-induced inhibition of cell viability is in part related to induction of apoptosis. (A) Agarose gel stained with ethidium bromide after electrophoresis of low-molecular-weight DNA extracted from a constant number of total marrow cells of 3 representative CML patients and from LAMA and K562 cells exposed for 48 hours to FTIs at the IC50 (CML1 to α-HFPA; CML2 to Man-A; CML3, LAMA, and K562 to SCH). (B) A representative flow cytometric detection of apoptic hypodiploid DNA peak-stained with propidium iodide derived from LAMA cells and total marrow cells of a CML patient exposed to SCH (1 μM). At least 6 × 105 events were counted per sample. Horizontal bars indicate hypodiploid DNA peak and its percentage. (C) In situ TdT assay to quantitate the number of apoptotic marrow cells from a CML patient after culture for 48 hours in absence and presence of SCH (1 μM). Darkly stained cells are apoptotic. Original magnification, × 40.
FTI-induced inhibition of cell viability is in part related to induction of apoptosis. (A) Agarose gel stained with ethidium bromide after electrophoresis of low-molecular-weight DNA extracted from a constant number of total marrow cells of 3 representative CML patients and from LAMA and K562 cells exposed for 48 hours to FTIs at the IC50 (CML1 to α-HFPA; CML2 to Man-A; CML3, LAMA, and K562 to SCH). (B) A representative flow cytometric detection of apoptic hypodiploid DNA peak-stained with propidium iodide derived from LAMA cells and total marrow cells of a CML patient exposed to SCH (1 μM). At least 6 × 105 events were counted per sample. Horizontal bars indicate hypodiploid DNA peak and its percentage. (C) In situ TdT assay to quantitate the number of apoptotic marrow cells from a CML patient after culture for 48 hours in absence and presence of SCH (1 μM). Darkly stained cells are apoptotic. Original magnification, × 40.
FTI-induced apoptosis of CML cells is independent from the Fas pathway
After FTI exposure, ras-transformed cells show FasR upregulation and enhanced Fas-induced apoptosis.29 To determine whether the Fas pathway is involved in FTI-induced apoptosis of CML cells, we first analyzed the modulation of FasR and FasL. Upon addition of any FTI, no increase in FasR and FasL was observed in LAMA cells (cumulative mean ± SEM of FasR and FasL expression induced by any FTI: 58% ± 1% and 45% ± 1% vs 59% ± 2% and 47% ± 2% in the absence and presence of FTIs, respectively; Figure 3A) and in CD34+ BM CML cells (25% ± 5% and 18% ± 7% vs 23% ± 2% and 17% ± 2% in the absence and presence of FTIs, respectively; data not shown). Theoretically, inability to detect FasR and FasL modulation on CML cells after in vitro FTI exposure could be due to changes in the composition of cell subsets in the culture. Therefore, we investigated whether the different susceptibility to FTI-mediated inhibition was dependent upon the expression of FasR and FasL in CD34+ CML cells. FTI-sensitive and FTI-resistant CML showed similar expression of FasR and FasL (Figure 3B). Using flow cytometry and intracellular caspase staining, we did not detect any modulation of caspase-8 and caspase-1 activity in CML cells after 48 hours' exposure to any FTI in vitro (Figure 3C). To further exclude the involvement of FasR/FasL in FTI-mediated apoptosis, prior to exposure to FTI, CML cells were preincubated with selective inhibitors of the FasR/FasL death pathway. Pretreatment of BM CML cells with a Fas-receptor antagonist, a caspase-8 inhibitor, and a caspase-1 inhibitor did not modify FTI-induced cytotoxicity (Figure 3D).
FTI-induced apoptosis in CML cells does not involve FasR/FasL signaling. (A) FTIs did not modify FasR and FasL expression in CML cells. A representative flow cytometric analysis of FasR and FasL expression, quantified as mean fluorescence intensity, in LAMA cells without and with in vitro exposure to SCH (1 μM) is shown. (B) FasR and FasL expression on CD34+ CML cells did not correlate with susceptibility to FTI-mediated inhibition of CML cell viability. Cumulative mean ± SEM CD34+ FasR+ and C34+ FasL+: 37% ± 1% and 29.9% ± 6% in FTI-sensitive CML versus 40% ± 3% and 36.6% ± 13% in FTI-resistant CML; P = .7 and P = .6, respectively. Horizontal bars represent mean values; vertical bars, SEM. (C) FTIs did not induce activation of caspase-1 and caspase-8. CML cells were cultured for 48 hours in medium alone containing the same concentration of DMSO used to dissolve SCH (untreated) or treated with a representative FTI (SCH [1 μM]). Caspase-1 and caspase-8 activities were measured by flow cytometry as described in “Materials and methods.” At least 1 × 104 cells were examined in each assay. (D) Inhibition of FasR triggering and caspase-1 and -8 did not revert FTI-mediated inhibition of CML cell growth. CML cells were grown for 48 hours with FTIs (IC50) and with FTIs + Fas-receptor–triggering inhibitor Fas:Fc, caspase-8 inhibitor IETD-FMK, or caspase-1 inhibitor ZYVAD-FMK (all used at 50 μM). CML cell viability was measured by the colorimetric MTT assay. Bars represent cumulative mean ± SEM of cell viability of 9 experiments performed with any FTI: 41.6% ± 3% versus 43.3% ± 3% without and with Fas:Fc, respectively (P = .6); 48.1% ± 1% versus 50.3% ± 3% without and with IETD-FMK, respectively (P = .3); 62.3% ± 3% versus 61.6% ± 3% without and with ZYVAD-FMK, respectively (P = .7).
FTI-induced apoptosis in CML cells does not involve FasR/FasL signaling. (A) FTIs did not modify FasR and FasL expression in CML cells. A representative flow cytometric analysis of FasR and FasL expression, quantified as mean fluorescence intensity, in LAMA cells without and with in vitro exposure to SCH (1 μM) is shown. (B) FasR and FasL expression on CD34+ CML cells did not correlate with susceptibility to FTI-mediated inhibition of CML cell viability. Cumulative mean ± SEM CD34+ FasR+ and C34+ FasL+: 37% ± 1% and 29.9% ± 6% in FTI-sensitive CML versus 40% ± 3% and 36.6% ± 13% in FTI-resistant CML; P = .7 and P = .6, respectively. Horizontal bars represent mean values; vertical bars, SEM. (C) FTIs did not induce activation of caspase-1 and caspase-8. CML cells were cultured for 48 hours in medium alone containing the same concentration of DMSO used to dissolve SCH (untreated) or treated with a representative FTI (SCH [1 μM]). Caspase-1 and caspase-8 activities were measured by flow cytometry as described in “Materials and methods.” At least 1 × 104 cells were examined in each assay. (D) Inhibition of FasR triggering and caspase-1 and -8 did not revert FTI-mediated inhibition of CML cell growth. CML cells were grown for 48 hours with FTIs (IC50) and with FTIs + Fas-receptor–triggering inhibitor Fas:Fc, caspase-8 inhibitor IETD-FMK, or caspase-1 inhibitor ZYVAD-FMK (all used at 50 μM). CML cell viability was measured by the colorimetric MTT assay. Bars represent cumulative mean ± SEM of cell viability of 9 experiments performed with any FTI: 41.6% ± 3% versus 43.3% ± 3% without and with Fas:Fc, respectively (P = .6); 48.1% ± 1% versus 50.3% ± 3% without and with IETD-FMK, respectively (P = .3); 62.3% ± 3% versus 61.6% ± 3% without and with ZYVAD-FMK, respectively (P = .7).
FTI-mediated apoptosis in CML cells involves activation of caspase-3
Since caspase-3 is the main “executioner caspase” required for apoptosis, we assessed the ability of FTIs to induce caspase-3 activation by flow cytometric measurement of the fluorescence generated after cleavage of the specific fluorogenic substrate DEVD-AMC. The FTIs clearly increased fluorescence intensity, indicating that caspase-3 was activated: mean percentage of fluorescent CML cells treated with any FTI was 35% ± 3% vs 5% ± 1% in control cultures (cumulative mean ± SEM of 5 experiments; Figure 4A). In addition, preincubation of CML cells with the caspase-3 inhibitor Z-DEVD-FMK partially abrogated FTI-mediated caspase activation (cumulative mean percentage ± SEM: 18% ± 5%; P = .03; Figure 4A). Caspase-3 triggering by FTIs in living CML cells was documented by flow cytometry–based analysis using the carboxyfluorescence probe FAM-VADFMK, which binds irreversibly to caspases in active configuration, in combination with propidium iodide, allowing us to distinguish dead cells from living cells (mean percentage of living CML cells showing caspase-3 activation treated with IC50 SCH was 52.5% ± 7%, vs 10.5% ± 2% in control cultures [mean of 3 experiments]; Figure 4B). In addition, quantification of Western blot films by densitometric scanning documented a 5-fold increase of active caspase-3 over background levels in CML cells after 6 hours and 24 hours of IC50 SCH treatment (Figure 4C). The involvement of caspase-3 in FTI-induced apoptosis was further confirmed by the ability of caspase-3 inhibitor to prevent FTI-induced cytotoxicity (Figure 4D) and apoptosis (cumulative mean percentage of apoptosis ± SEM after treatment with any FTI: 39% ± 6% vs 12.3% ± 2%, in absence and in presence of Z-DEVD-FMK, respectively; P = .003; Figure 4E).
FTI-induced inhibition of cell viability and apoptosis in CML cells is mediated by activation of caspase-3. (A) FTI-induced activation of caspase-3. Marrow cells from a patient with CML in chronic phase were cultured for 24 hours in medium alone (untreated) or treated with a representative FTI (1 μM SCH) or with SCH + caspase-3 inhibitor Z-DEVD-FMK (50 μM). Caspase-3 activity was measured by flow cytometry using the fluorogenic caspase-3–specific substrate DEVD-AMC as described in “Materials and methods.” At least 1 × 104 cells were examined in each assay. (B) Caspase-3 triggering by SCH in living CML cells. Marrow cells from a representative case of CML were cultured for 24 hours in control medium (– FTI) or treated with SCH (1 μM; + FTI). Caspase-3 was measured by flow cytometry using the cell-permeable FITC-labeled peptide FAM-VAD-FMK in combination with propidium iodide to distinguish dead cells from living cells: 65% of living CML cells (lower right quadrant) are stained with FAM-VAD-FMK. (C) Caspase-3 activation in CML cells is induced early after FTI exposure. Western blot analysis of active caspase-3 in CML cells treated for 6 and 24 hours with SCH (1 μM). (D) Inhibition of caspase-3 partially reverted FTI-induced inhibition of CML cell viability, as measured by the MTT assay. Bars represent the cumulative mean ± SEM after exposure to any FTI, expressed as percentage of control: 51.7% ± 6% versus 73.2% ± 2%, in absence and presence of Z-DEVD-FMK, respectively; P = .005. (E) Z-DEVD-FMK partially blocked FTI-induced apoptosis in marrow cells. Flow cytometric detection of apoptosis from a CML patient in chronic phase who showed in vitro susceptibility to a representative FTI (SCH [1 μM]). A minimum of 6 × 105 events were counted per sample. Horizontal bars indicate hypodiploid DNA peak and its percentage.
FTI-induced inhibition of cell viability and apoptosis in CML cells is mediated by activation of caspase-3. (A) FTI-induced activation of caspase-3. Marrow cells from a patient with CML in chronic phase were cultured for 24 hours in medium alone (untreated) or treated with a representative FTI (1 μM SCH) or with SCH + caspase-3 inhibitor Z-DEVD-FMK (50 μM). Caspase-3 activity was measured by flow cytometry using the fluorogenic caspase-3–specific substrate DEVD-AMC as described in “Materials and methods.” At least 1 × 104 cells were examined in each assay. (B) Caspase-3 triggering by SCH in living CML cells. Marrow cells from a representative case of CML were cultured for 24 hours in control medium (– FTI) or treated with SCH (1 μM; + FTI). Caspase-3 was measured by flow cytometry using the cell-permeable FITC-labeled peptide FAM-VAD-FMK in combination with propidium iodide to distinguish dead cells from living cells: 65% of living CML cells (lower right quadrant) are stained with FAM-VAD-FMK. (C) Caspase-3 activation in CML cells is induced early after FTI exposure. Western blot analysis of active caspase-3 in CML cells treated for 6 and 24 hours with SCH (1 μM). (D) Inhibition of caspase-3 partially reverted FTI-induced inhibition of CML cell viability, as measured by the MTT assay. Bars represent the cumulative mean ± SEM after exposure to any FTI, expressed as percentage of control: 51.7% ± 6% versus 73.2% ± 2%, in absence and presence of Z-DEVD-FMK, respectively; P = .005. (E) Z-DEVD-FMK partially blocked FTI-induced apoptosis in marrow cells. Flow cytometric detection of apoptosis from a CML patient in chronic phase who showed in vitro susceptibility to a representative FTI (SCH [1 μM]). A minimum of 6 × 105 events were counted per sample. Horizontal bars indicate hypodiploid DNA peak and its percentage.
Induction of iNOS expression and production of NO in CML cells by FTI
FTI-induced apoptosis is associated with cytochrome C release from the mitochondria.43 Because NO can change mitochondrial membrane potential, thus inducing cytochrome C release and caspase-3 activation, we tested whether FTIs may enhance iNOS expression in CML cells.44 Using PCR, we demonstrated the presence of iNOS mRNA in primary total CML BM cells. Expression of iNOS mRNA was also detectable in LAMA cells, suggesting that the iNOS signal in total BM CML cells was not due to the presence of accessory cells. A strong amplification of the iNOS signal was obtained after exposure of primary total BM CML and LAMA cells to any FTI (Figure 5A). Immunoblot of cell lysates further confirmed that CML cells express low levels of iNOS, and its expression is enhanced by any FTI in both primary total BM CML cells and in LAMA cells (Figure 5B). Quantification of protein bands by densitometry documented a 10-fold increase (range, 2- to 15-fold) of iNOS expression following FTI exposure in 5 CML patients. When intracellular NO production was measured directly, using the cell-permeable fluorescent indicator DAF-2 DA, any FTI increased NO levels by 50% as compared with the basal levels in LAMA cells and primary CML cells (P < .001). γ-MM-arg partially blocked FTI-mediated NO production in CML cells (Figure 5C). Since we previously reported that NO suppresses hematopoietic stem cell growth42 and FTIs increase iNOS expression and NO production in CML cells, we investigated whether the inhibitory effects of FTI were related to NO production. Inhibition of NO synthesis by pretreatment of CML cells with γ-MM-arg (500 μM), a competitive inhibitor of iNOS, only marginally reverted the inhibitory effect on cell viability (Figure 5D) and apoptosis of any FTI (cumulative mean ± SEM of apoptosis after FTI exposure at IC50: 31% ± 2% vs 39% ± 2% in absence and in presence of γ-MM-arg, respectively; P = .06; Figure 5E). However, when FTIs were used at IC10, γ-MM-arg partially prevented FTI-mediated inhibition of cell viability (Figure 5D) and apoptosis (cumulative mean ± SEM of apoptosis at IC10: 41.6% ± 2% vs 21% ± 2% in absence and in presence of γ-MM-arg, respectively; P = .03; Figure 5E). A similar effect was also obtained by using 500 μM of another iNOS inhibitor, N-nitro-l-arginine methyl ester (L-NAME; data not shown).
Involvement of NO in FTI-induced inhibition of CML cell viability. (A) iNOS mRNA signal was amplified in CML cells cultured for 48 hours in the presence of a representative FTI (SCH [1 μM]). Ethidium bromide staining of RT-PCR products performed with specific primers for iNOS and GAPDH. GAPDH documents equal RNA loading. The positive control was supplied by the manufacturer. (B) FTIs caused increased expression of iNOS protein in cells from 2 CML patients and in LAMA and K562 cells (CML1 and K562 treated with Man-A, 50 μM; CML2 treated with SCH [1 μM]). Actin is used as control for constant protein loading. (C) A representative FTI (SCH [1 μM]) caused intracellular NO production in LAMA cells. NO production was measured using the cell-permeable fluorescent indicator DAF-2 DA. γ-MM-arg, a competitive inhibitor of iNOS, partially abrogated FTI-mediated NO production. (D) Inhibition of iNOS partially blocks FTI-induced inhibition of CML cell viability. γ-MM-arg (500 μM), a competitive inhibitor of iNOS, induced a partial reversion of CML cell viability inhibition induced by all FTIs. Bars represent mean ± SEM of 3 experiments. Cumulative mean ± SEM of cell viability after FTI exposure at IC50 and IC10: 54.3% ± 3% and 66.3% ± 3% versus 62.6% ± 3% and 86.6% ± 3% in absence and in presence of γ-MM-arg; P = .056 and P = .01, respectively. (E) γ-MM-arg partially prevented FTI-mediated apoptosis. Flow cytometric detection of apoptotic hypodiploid DNA derived from a representative CML patient treated with SCH (1 μM). Horizontal bars indicate hypodiploid DNA peak and its percentage.
Involvement of NO in FTI-induced inhibition of CML cell viability. (A) iNOS mRNA signal was amplified in CML cells cultured for 48 hours in the presence of a representative FTI (SCH [1 μM]). Ethidium bromide staining of RT-PCR products performed with specific primers for iNOS and GAPDH. GAPDH documents equal RNA loading. The positive control was supplied by the manufacturer. (B) FTIs caused increased expression of iNOS protein in cells from 2 CML patients and in LAMA and K562 cells (CML1 and K562 treated with Man-A, 50 μM; CML2 treated with SCH [1 μM]). Actin is used as control for constant protein loading. (C) A representative FTI (SCH [1 μM]) caused intracellular NO production in LAMA cells. NO production was measured using the cell-permeable fluorescent indicator DAF-2 DA. γ-MM-arg, a competitive inhibitor of iNOS, partially abrogated FTI-mediated NO production. (D) Inhibition of iNOS partially blocks FTI-induced inhibition of CML cell viability. γ-MM-arg (500 μM), a competitive inhibitor of iNOS, induced a partial reversion of CML cell viability inhibition induced by all FTIs. Bars represent mean ± SEM of 3 experiments. Cumulative mean ± SEM of cell viability after FTI exposure at IC50 and IC10: 54.3% ± 3% and 66.3% ± 3% versus 62.6% ± 3% and 86.6% ± 3% in absence and in presence of γ-MM-arg; P = .056 and P = .01, respectively. (E) γ-MM-arg partially prevented FTI-mediated apoptosis. Flow cytometric detection of apoptotic hypodiploid DNA derived from a representative CML patient treated with SCH (1 μM). Horizontal bars indicate hypodiploid DNA peak and its percentage.
FTI cytotoxicity does not correlate with changes in bcl-2 and bclxL/S proteins
To determine whether FTIs induce apoptosis either through decreased expression of antiapoptotic bcl2 and bclxL or through increased proapoptotic bclxS proteins, we incubated CML cells with each FTI and assessed bcl2 and bclxL/S protein expression. Figure 6A depicts bcl2 expression in a sample from a CML patient and in LAMA and K562 cells and illustrates the negligible changes in bcl2 protein expression. Quantification of protein banding by densitometry in 3 CML patients demonstrated only marginal modification of expression of bcl2 following FTI exposure, while the concurrent apoptosis rate was high. The expression of bclxL and bclxS proteins was also unaffected by FTIs (Figure 6B).
Expression of bcl2 and its family members bclxL and bclxS is not modified by FTI exposure. Immunoblotting of bcl2, bclxL, and bclxS from total marrow cells of a CML patient and from LAMA and K562 cells cultured for 48 hours in absence or presence of a representative FTI (SCH [1μM]). (A) bcl2. (B) bclxL and bclxS.
Expression of bcl2 and its family members bclxL and bclxS is not modified by FTI exposure. Immunoblotting of bcl2, bclxL, and bclxS from total marrow cells of a CML patient and from LAMA and K562 cells cultured for 48 hours in absence or presence of a representative FTI (SCH [1μM]). (A) bcl2. (B) bclxL and bclxS.
Inhibition of Rho induces iNOS expression and apoptosis in CML cells
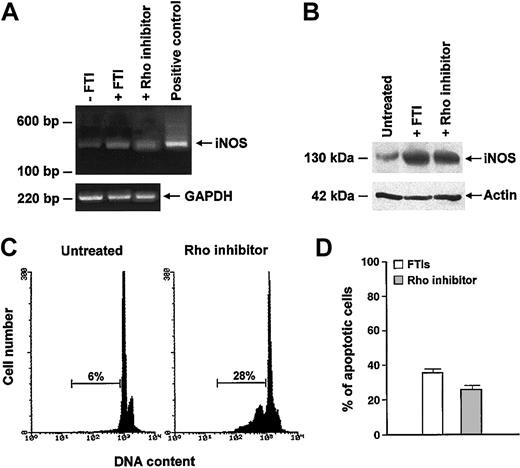
RhoB may be involved in the apoptosis mechanisms triggered by FTIs.22 To determine whether inhibition of Rho was also associated with up-regulation of iNOS, we incubated CML cells for 48 hours with C3 exoenzyme, which adenosine 5′-diphosphate (ADP) ribosylates asparagine 41 of RhoA and RhoB, rendering them biologically inactive. Compared with the results obtained after exposure to any FTI, a stronger amplification signal for iNOS mRNA was observed in CML cells pretreated with C3 exoenzyme, while only a weak signal was detected in control cells (Figure 7A). A Western blot confirmed the induction of iNOS in cultured CML cells after Rho inhibition (Figure 7B). In addition, we documented that C3 exoenzyme was also capable of triggering apoptosis in CML cells, suggesting that the effect of FTIs on iNOS expression might be related to inhibition of Rho (Figure 7C-D). Indeed, a combination of Rho inhibitor and FTIs did not increase the apoptotic rate of CML cells (cumulative mean ± SEM, 33.2% ± 5% vs 27.2% ± 10% with FTI and with FTI + Rho inhibitor, respectively; P = .6; data not shown).
Inhibition of Rho induces iNOS expression and apoptosis in CML cells. (A-B) Rho inhibitor C3 exoenzyme significantly increased iNOS mRNA (A) and protein (B) in total marrow cells from a CML patient. SCH (1 μM) was used to compare the iNOS-enhanced expression by Rho inhibitor and FTIs. (C-D) Inhibition of Rho-induced apoptosis of CML cells. (C) Flow cytometric detection of apoptotic hypodiploid DNA derived from a CML patient. Horizontal bars indicate hypodiploid DNA peak and its percentage. (D) Bars represent percentage of apoptotic cells, enumerated by the TdT assay, after exposure to FTIs (□) and to Rho inhibitor (▦) (cumulative mean percentage ± SEM: 36.2% ± 3% vs 27% ± 2%, respectively; P = .03).
Inhibition of Rho induces iNOS expression and apoptosis in CML cells. (A-B) Rho inhibitor C3 exoenzyme significantly increased iNOS mRNA (A) and protein (B) in total marrow cells from a CML patient. SCH (1 μM) was used to compare the iNOS-enhanced expression by Rho inhibitor and FTIs. (C-D) Inhibition of Rho-induced apoptosis of CML cells. (C) Flow cytometric detection of apoptotic hypodiploid DNA derived from a CML patient. Horizontal bars indicate hypodiploid DNA peak and its percentage. (D) Bars represent percentage of apoptotic cells, enumerated by the TdT assay, after exposure to FTIs (□) and to Rho inhibitor (▦) (cumulative mean percentage ± SEM: 36.2% ± 3% vs 27% ± 2%, respectively; P = .03).
Discussion
Phase 1 and some phase 2 clinical trials documented that FTIs may have beneficial effects in CML,45-47 AML,16-18 MDS,21 and juvenile myelomonocytic leukemia.48 Most significantly, antitumor activity of FTIs was not restricted to cells overexpressing ras or to those with activating ras mutation, providing a rationale for the application of FTIs in a wider spectrum of indications.
Here we have studied the mechanism of action of FTIs in CML cells and our results demonstrate their biologic activity in this disease. FTIs exhibited significant inhibitory activity in CML cell lines and in primary marrow progenitor cells from 60% to 65% of CML patients, while normal bone marrow progenitor cells appeared to be more resistant. These findings are in agreement with those reported by Peters et al19 and do not disagree with those reported by Morgan et al,20 who found loss of viability of normal CD34+ cells with a different FTI (FTI-277) at a concentration of 20 μM or higher.
Consistent with previous reports,19,43 we found that the FTI-induced CML cell toxicity was observed only under low serum conditions; this may be due to FTI binding to serum proteins, decreasing its bioavailability in culture media, or to requirement by the FTIs of an additional stress signal to induce inhibition of CML cell viability.
Bcr/abl expression in leukemic cells confers resistance to apoptosis caused by antileukemic drugs.49 Interferon-α (IFN-α) was shown to promote apoptosis of CML cells via overexpression of Fas constitutively expressed in bcr/abl cells.50-52 Recently, ras-transformed cells were shown to up-regulate FasR expression and to enhance Fas-induced apoptosis after FTI exposure.29 However, in CML cells we were unable to detect changes in FasR or FasL after exposure to FTIs. Moreover, no differential susceptibility to FTI-induced inhibition of CML viability was seen in correlation with FasR and FasL expression in CD34+ CML cells, suggesting that Fas-mediated effects are not involved in FTI-induced apoptosis of CML cells. In agreement with the lack of modulation of FasR/FasL, we found that FTI-mediated apoptosis in CML cells was independent of caspase-1 and -8 and that the inhibition of caspase-1 and -8 was not associated with rescue of FTI-treated CML cells. Consequently, we concluded that other cellular events induced by FTIs are triggers of apoptosis.
As the mechanisms of bcr/abl-mediated inhibition of apoptosis may involve blockade of the mitochondrial release of cytochrome C and subsequent activation of caspase-3,53-56 we investigated whether caspase-3 is activated during FTI-induced apoptosis. Our results demonstrated that FTIs did induce caspase-3 activation in CML cells. These findings were further confirmed by the ability of Z-DEVD-FMK, an agent interfering with caspase-3 activity, to partially inhibit FTI-induced apoptosis in CML. Consequently, we have studied the apoptotic transduction pathways, which may directly activate caspase-3. Bcl2 family members are known key mediators of common final pathways involved in the regulation of the mitochondrial outer membrane depolarization, cytochrome C release, and caspase-3 activation.57 Moreover, through modification of antiapoptotic members of the bcl2 family (eg, up-regulation of bclxL), bcr/abl protects mitochondria from proapoptotic signals (eg, those induced by DNA damage), thus preventing release of cytochrome C and activation of caspase-3.49,58 As neither down-regulation of antiapoptotic molecules such as bcl2 and bclxL nor up-regulation of the proapoptotic bclxS was detected after FTI treatment, we concluded that FTI-induced apoptosis in CML cells was not caused by modulation of the bcl2 family members. This finding is consistent with a recent report showing that bcl2 and its homolog bclxL were not degraded in FTI-treated myeloma cells.59
Apart from its many regulatory and effector functions, NO produced by activated macrophages or delivered by NO donors is one of the cytokine-regulated effector mechanisms responsible for apoptosis in normal and leukemic hemopoietic progenitor cells.44,60-62 Based on previous reports indicating that NO can change mitochondrial membrane potential and induce cytochrome C release and caspase activation, resulting in apoptosis,42,43 we studied the expression of iNOS and production of NO in CML cells exposed to FTIs. Our results demonstrate that CML cells expressed higher levels of iNOS upon FTI treatment. This effect was associated with a significant up-regulation of NO synthesis in these cells. Moreover, inhibition of NO synthesis partially abrogated the effects of FTIs on NO production and apoptosis, suggesting that iNOS induction and NO production may be involved in the mechanisms of action of FTIs. These results are in agreement with a recent report showing that cytochrome C release by NO is potentiated by FTIs and that FTIs in combination with NO are effective in inducing apoptosis of breast cancer cell lines.63
Although initially FTIs were developed to inhibit the oncogenic activity of ras, recent experimental evidence suggests that other farnesylated proteins may be responsible for their antitrasforming activity. Recently, a number of novel farnesylated proteins such as Rheb, CENP-E, and CENP-F have been documented to play a role in the cytotoxic effect of FTIs through their ability to induce cancer cell growth arrest at the G1/S and G2/M phases of the cell cycle.64 Among the farnesylated small guanosine 5′-triphosphate (GTP)–binding proteins, farnesylated RhoB (RhoB-F) has been proposed to be linked to the inhibition of oncogenic signaling, induction of apoptosis, and suppression of human tumor growth.25-31 However, recent work concerning induction of apoptosis by FTIs has established that overexpression of RhoB-F or geranylgeranylated RhoB (RhoB-GG) is a target for the FTI-induced apoptosis of tumor cells.14,27,65 To assess the contribution of Rho to FTI-mediated apoptosis in CML cells, we used the Clostridium botulinum C3 exoenzyme, which ADP-ribosylates Rho in its effector domain and keeps the G protein in an inactive conformation. Inhibition of Rho by C3 exotoxin resulted in induction of iNOS and apoptosis in CML cells. This effect appears to mirror the up-regulation of iNOS and apoptosis seen in CML cells treated with FTIs. Although there is still controversy about the role of Rho as a key target for FTIs,22,65 our findings suggest that FTIs can up-regulate iNOS expression in CML cells through inhibition of Rho; indeed, we observed no increase in apoptotic CML cells after simultaneous exposure to Rho inhibitor and FTIs.
In conclusion, our results demonstrate, as reported in other experimental systems,63 that FTI-induced apoptosis in CML is not accompanied by FasR/FasL or bcl2 modulation; rather, it involves induction of iNOS expression in the target cells. It is possible that several FTI mechanisms may have additive antineoplastic effects and can be further enhanced by other agents. For example, IFN-α has been shown to increase iNOS expression and may potentiate FTI effects.44,62 In addition, it has recently been hypothesised that FTI treatment may sensitize bcr/abl cells to imatinib mesylate–induced apoptosis and may allow imatinib resistance due to gene amplification of primary CML cells to be overcome.66 Our data support the ongoing clinical trials of FTIs as single agents or in combination with other molecules in CML patients.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2003-01-0178.
Supported in part by grants from the Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST), the Consiglio Nazionale delle Ricerche (CNR), and Associazione Italiana contro le Leucemie (AIL), Sezioni di Napoli e Salerno.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 3. FTI-induced apoptosis in CML cells does not involve FasR/FasL signaling. (A) FTIs did not modify FasR and FasL expression in CML cells. A representative flow cytometric analysis of FasR and FasL expression, quantified as mean fluorescence intensity, in LAMA cells without and with in vitro exposure to SCH (1 μM) is shown. (B) FasR and FasL expression on CD34+ CML cells did not correlate with susceptibility to FTI-mediated inhibition of CML cell viability. Cumulative mean ± SEM CD34+ FasR+ and C34+ FasL+: 37% ± 1% and 29.9% ± 6% in FTI-sensitive CML versus 40% ± 3% and 36.6% ± 13% in FTI-resistant CML; P = .7 and P = .6, respectively. Horizontal bars represent mean values; vertical bars, SEM. (C) FTIs did not induce activation of caspase-1 and caspase-8. CML cells were cultured for 48 hours in medium alone containing the same concentration of DMSO used to dissolve SCH (untreated) or treated with a representative FTI (SCH [1 μM]). Caspase-1 and caspase-8 activities were measured by flow cytometry as described in “Materials and methods.” At least 1 × 104 cells were examined in each assay. (D) Inhibition of FasR triggering and caspase-1 and -8 did not revert FTI-mediated inhibition of CML cell growth. CML cells were grown for 48 hours with FTIs (IC50) and with FTIs + Fas-receptor–triggering inhibitor Fas:Fc, caspase-8 inhibitor IETD-FMK, or caspase-1 inhibitor ZYVAD-FMK (all used at 50 μM). CML cell viability was measured by the colorimetric MTT assay. Bars represent cumulative mean ± SEM of cell viability of 9 experiments performed with any FTI: 41.6% ± 3% versus 43.3% ± 3% without and with Fas:Fc, respectively (P = .6); 48.1% ± 1% versus 50.3% ± 3% without and with IETD-FMK, respectively (P = .3); 62.3% ± 3% versus 61.6% ± 3% without and with ZYVAD-FMK, respectively (P = .7).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/4/10.1182_blood-2003-01-0178/6/m_h81634761003.jpeg?Expires=1769158575&Signature=oleK7qw9lUOmfeG~RQvuDg2E2DC-YDqWF9zoEwW1wJb0WyJf~cVCu2ZJ3HVmcBg1kkQuvHfyMCjGGy1n-Og1HJeLOcLKl84OnHBMbb8kBbTGytUZO5LjGePjzCvKbgaTyNSljAkKwysbbvq98eI2E1K125iVZGIc4914WhtLS4HPSj9RPlFPm3RIjDbpH-CFdzcUO~XeUkZaJnbW0Xhs0yEv~PRUqH9V-znhmIiV5risIUKKMr-ObkEr3v9ehxrX12SHNVKkgNBZuWkVJefjG7D1reD1xH9fqL1coTRs7xppPsslF16nNqlV2JWd3mYFQm2cu4wAM37t0yEeSpdfPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. FTI-induced inhibition of cell viability and apoptosis in CML cells is mediated by activation of caspase-3. (A) FTI-induced activation of caspase-3. Marrow cells from a patient with CML in chronic phase were cultured for 24 hours in medium alone (untreated) or treated with a representative FTI (1 μM SCH) or with SCH + caspase-3 inhibitor Z-DEVD-FMK (50 μM). Caspase-3 activity was measured by flow cytometry using the fluorogenic caspase-3–specific substrate DEVD-AMC as described in “Materials and methods.” At least 1 × 104 cells were examined in each assay. (B) Caspase-3 triggering by SCH in living CML cells. Marrow cells from a representative case of CML were cultured for 24 hours in control medium (– FTI) or treated with SCH (1 μM; + FTI). Caspase-3 was measured by flow cytometry using the cell-permeable FITC-labeled peptide FAM-VAD-FMK in combination with propidium iodide to distinguish dead cells from living cells: 65% of living CML cells (lower right quadrant) are stained with FAM-VAD-FMK. (C) Caspase-3 activation in CML cells is induced early after FTI exposure. Western blot analysis of active caspase-3 in CML cells treated for 6 and 24 hours with SCH (1 μM). (D) Inhibition of caspase-3 partially reverted FTI-induced inhibition of CML cell viability, as measured by the MTT assay. Bars represent the cumulative mean ± SEM after exposure to any FTI, expressed as percentage of control: 51.7% ± 6% versus 73.2% ± 2%, in absence and presence of Z-DEVD-FMK, respectively; P = .005. (E) Z-DEVD-FMK partially blocked FTI-induced apoptosis in marrow cells. Flow cytometric detection of apoptosis from a CML patient in chronic phase who showed in vitro susceptibility to a representative FTI (SCH [1 μM]). A minimum of 6 × 105 events were counted per sample. Horizontal bars indicate hypodiploid DNA peak and its percentage.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/4/10.1182_blood-2003-01-0178/6/m_h81634761004.jpeg?Expires=1769158575&Signature=XNB8IvvNlimxtlQvf75~g7Sk7Vds0UH-X7M-SWSwQyDzgaRcDerdbBAlYAqCDoxQy04AaBupyuvuOm0g4KIrfJqNJYi6e-aolOrAOS-eAPDg~wzYxZWrIQzL~BYYVj7gC7Y-8jdp9tfATroEtOE5594rvkxYZfaHycZicLvgaZQVUJsVeEDzGqHgfr1e0HYO40RvjakUvTW9VtAQM6DEoLZ4SqIzKn0iPyY4RM-5q1WVBxqhksmd8SEXLBRcEsQCw1OQf8nO6DlbCIlftU578FovSz8muW-1ZY1DQ-XIuL7TvLM1Q74lC94mMYmKN3TjK~COPL42dpDFAlNgexvqFw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Involvement of NO in FTI-induced inhibition of CML cell viability. (A) iNOS mRNA signal was amplified in CML cells cultured for 48 hours in the presence of a representative FTI (SCH [1 μM]). Ethidium bromide staining of RT-PCR products performed with specific primers for iNOS and GAPDH. GAPDH documents equal RNA loading. The positive control was supplied by the manufacturer. (B) FTIs caused increased expression of iNOS protein in cells from 2 CML patients and in LAMA and K562 cells (CML1 and K562 treated with Man-A, 50 μM; CML2 treated with SCH [1 μM]). Actin is used as control for constant protein loading. (C) A representative FTI (SCH [1 μM]) caused intracellular NO production in LAMA cells. NO production was measured using the cell-permeable fluorescent indicator DAF-2 DA. γ-MM-arg, a competitive inhibitor of iNOS, partially abrogated FTI-mediated NO production. (D) Inhibition of iNOS partially blocks FTI-induced inhibition of CML cell viability. γ-MM-arg (500 μM), a competitive inhibitor of iNOS, induced a partial reversion of CML cell viability inhibition induced by all FTIs. Bars represent mean ± SEM of 3 experiments. Cumulative mean ± SEM of cell viability after FTI exposure at IC50 and IC10: 54.3% ± 3% and 66.3% ± 3% versus 62.6% ± 3% and 86.6% ± 3% in absence and in presence of γ-MM-arg; P = .056 and P = .01, respectively. (E) γ-MM-arg partially prevented FTI-mediated apoptosis. Flow cytometric detection of apoptotic hypodiploid DNA derived from a representative CML patient treated with SCH (1 μM). Horizontal bars indicate hypodiploid DNA peak and its percentage.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/4/10.1182_blood-2003-01-0178/6/m_h81634761005.jpeg?Expires=1769158575&Signature=Qi3Y-ncvISGJY85O~JkRwOxk2lFKDKvBRxBPX7g9esAKrCrx9MCN7mQVYm45Fq4qKSLgp1209~ecSNo7~o6LX0uui5bh9nqx6BiWk5OZlnr7DXQgSGtanMof~2Rw0FDGXqfLm~8iKnOY6YLpx0XeaQ300c5pWjTvoSJrxuTHJxeFX77iFQK9Rg-1RriwPxbYmB0yqjZG2raNf7NaQQ-GROj7X2hNGkOcNmBQHqqRQK1RLFRENH2VeR7EGvN-dFVfJe-BR6pvq8dw8W2eFA2sV1RMHVWJvHfvsmcWuLmoi44LRLWtSLCb9WtVWWwAD6QJ~X6KOawMx7as6pqKTezjCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Expression of bcl2 and its family members bclxL and bclxS is not modified by FTI exposure. Immunoblotting of bcl2, bclxL, and bclxS from total marrow cells of a CML patient and from LAMA and K562 cells cultured for 48 hours in absence or presence of a representative FTI (SCH [1μM]). (A) bcl2. (B) bclxL and bclxS.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/4/10.1182_blood-2003-01-0178/6/m_h81634761006.jpeg?Expires=1769158575&Signature=CXkY9wRiMi7cLY2SGxGASTIvy7Y-lLdJpwu96EaBs7rzyli-iW38zmWaus1RgQnAnJPhy~RaQgWS~88YlsqfT-KF9CfqQro8voeJUAH9DXElWRnnNWHvJE22ZdxM44dh698gk94v80MqGw~CaqxtK19wMjAYBXxOA6F3ow9OMSe60nZN7J70BXRdNbvPEbxn7MJqFP-KvD5FHi9WCrnFt0sJrlDiPwzi1Et~CZUul1pBB5Suqy4fDRUvvV60TpDOlpUgs3tV0a~5OJmpE2P0t6-dNHVjGR-b1T3v29LyPXqdCg0~Ly~DBpMvAfvt-IGsAO2I9BufJq-KAabEwqGr0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal