Abstract
The mechanisms that regulate the growth and survival of acute myeloid leukemia (AML) cells are largely unknown. We hypothesized that constitutive activation of phosphatidyl-inositide 3 kinase (PI3 kinase) could regulate survival in primary cells from patients with AML. Here we demonstrate that Akt, a critical substrate of PI3 kinase, is activated in AML blasts. In a short-term culture system, most AML patient samples showed a dose-dependent decrease in survival after incubation with the PI3 kinase inhibitor LY294002. This decrease in survival was partially due to the induction of apoptosis. Furthermore, we have shown that p70 S6 kinase and 4EBP-1, downstream mediators of Akt signaling, also are phosphorylated in AML blasts. Phosphorylation of these proteins is inhibited by the mTOR inhibitor RAD001. Incubation of AML blasts with RAD001 induces only a small decrease in survival of the cells; however, when combined with Ara-C, RAD001 enhances the toxicity of Ara-C. These results demonstrate that constitutive activation of the PI3 kinase pathway is necessary for the survival of AML blasts and that targeting of this pathway with pharmacologic inhibitors may be of clinical benefit in treatment of AML.
Introduction
Acute myeloid leukemia (AML) is characterized by the accumulation of immature myeloid cells in the bone marrow and the suppression of normal hematopoiesis. AML results from genetic alterations that lead, in part, to the expression of transcription factor fusion proteins.1,2 Several of these proteins block myeloid differentiation3,4 ; however, data from animal models demonstrate that expression of transcription factor fusion proteins may not be sufficient to cause leukemogenesis.5-7 Furthermore, recent reports demonstrate that 25% to 30% of AML cases are associated with mutations in the Flt3 gene, leading to the expression of a constitutively activated Flt3 receptor,8-10 which may act as a growth stimulatory protein in AML.11 This and other data have suggested a model whereby transcription factor fusion proteins cooperate with altered growth or survival signaling to generate a fully transformed phenotype.12,13 However, the nature of the survival signals in most AML cases is not understood.
It has been hypothesized that AML cells may have constitutive activation of cytokine-dependent signal transduction pathways. Gouilleux-Gruart et al initially reported activation of signal transducers and activators of transcription 3 (STAT3) and STAT5 in blast cells of AML samples.14 Others have described activation of STAT1, STAT3, and STAT5.15 The percentage of samples showing activation of STAT proteins is variable, perhaps due to differences in preservation and preparation of the samples, and the functional significance of STAT activation in AML is unknown. In addition, 2 reports have described activation of mitogen-induced extracellular kinase (MEK) or mitogen-activated protein (MAP) kinase proteins in AML. Towatari et al reported phosphorylation of MAP kinase in 40% of AML samples.16 A second study also demonstrated kinase activity of MAP kinase and correlated the degree of activation with expression of the phosphatase, PAC1.17 Recent work suggests that most AML blasts may depend on MEK activation for survival as incubation of the cells with pharmacologic MEK inhibitors leads to an increase in spontaneous and chemotherapy-induced apoptosis.18 Finally, activation of nuclear factor κB (NFκB) and a requirement for NFκB in survival of leukemic cells have been described.19,20
In order to understand growth regulation in AML better, we have focused on the phosphatidyl-inositide 3 (PI3) kinase signaling pathway. PI3 kinase is a lipid kinase that is regulated in response to multiple hematopoietic cytokines and chemokines,21 including Flt ligand (FL), the physiologic ligand for the Flt3 receptor.22,23 Many of the downstream effects of PI3 kinase are mediated through the serine-threonine kinase protein kinase B (PKB)/Akt (hereafter Akt).24,25 Akt interacts with a myriad of downstream effectors, which, in various cell types, lead to regulation of cell proliferation, cell growth, and cell survival.26 One of these downstream targets is mTOR (mammalian target of rapamycin), which regulates both nutrient sensing and protein translation through phosphorylation of its substrates, p70 S6 kinase and 4EBP-1.27 Numerous studies have implicated activation of Akt in carcinogenesis,26 but whether the Akt signaling pathway is activated in primary cells from patients with AML has not been previously addressed.
Here we demonstrate that primary cells from patients with AML have constitutive activation of the PI3 kinase mediator Akt. Inhibition of PI3 kinase in a short-term culture system leads to decreased growth in most AML samples and is associated with the induction of apoptosis in these cells. This is in contrast to normal human CD34 cells, which show only low levels of Akt activation and are not induced to undergo apoptosis by incubation with the PI3 kinase inhibitor LY294002. In addition, both mTOR substrates, p70 S6 kinase and 4EBP-1, are constitutively phosphorylated in AML blasts, demonstrating that the signaling pathway is activated at multiple levels. Expression of the phosphatase Src homology 2 domain-containing inositol 5-phosphatase (SHIP) was seen in all samples, but expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) was absent or decreased, suggesting that PI3 kinase may be dysregulated by decreased expression of PTEN.28 These results demonstrate that constitutive activation of the PI3 kinase pathway is a critical event in the evolution of AML and suggest that targeting of this pathway may have an important therapeutic effect in this disease.
Materials and methods
Cells
Patient samples were obtained from patients presenting with acute leukemia at the hospital of the University of Pennsylvania after informed consent in accordance with institutional guidelines. Bone marrow or peripheral blood samples were collected as indicated. Samples were prepared by Ficoll gradient centrifugation. Mononuclear cells were frozen as viable cells in fetal calf serum and dimethyl sulfoxide (DMSO) and stored in liquid nitrogen. Percentage of blasts in the unmanipulated samples as determined morphologically and by flow cytometry was recorded. Samples were selected only if multiple samples were available for repeat analysis.
CD34 cells were purified from either adult bone marrow or human umbilical cord blood using immunomagnetic separation and a Miltenyi AutoMacs machine according to the manufacturer's recommendations (Miltenyi Biotec, Auburn, CA). CD34-depleted mononuclear cells were collected from the negative channel of the CD34 selection. Both leukemia samples and cord blood were obtained from the Stem Cell and Leukemia Core Facility at the University of Pennsylvania Cancer Center. All cells were frozen in 10% DMSO and stored in liquid nitrogen. MO7e cells were a kind gift of Dr Stephen Emerson (University of Pennsylvania) and were maintained in Dulbecco modified Eagle medium (DMEM) with 10% fetal calf serum (FCS) and granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems, Minneapolis, MN).
Tissue culture
Samples were thawed rapidly at 37°C and diluted with DMEM supplemented with bovine serum albumin (BSA), insulin, and transferrin (BIT 9500), serum substitute (Stem Cell Technologies, Vancouver, BC, Canada). Cells were then respun and plated in the same medium at 2 × 106 cells/mL. For short-term experiments (up to 6 hours), no exogenous cytokines were added. For longer experiments, cells were plated in serum-free medium with interleukin 3 (IL-3; 5 ng/mL), stem cell factor (SCF; 50 ng/mL), and IL-6 (10 ng/mL; R&D Systems). For Akt assays, cells were collected by centrifugation without further manipulation. For growth curves, relative cell survival was compared using a (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) (XTT) assay, a colorimetric assay for viable cell number, according to the manufacturer's recommendations (Molecular Probes, Eugene, OR). Results were quantitated in triplicate using absorption at 450 nm. LY294002 was purchased from Calbiochem (San Diego, CA), dissolved in DMSO, stored frozen, and used within 4 weeks. RAD001 was a kind gift of Heidi Lane (Novartis Pharmaceuticals, Basel, Switzerland).
Western blotting
Cells were pelleted by centrifugation, washed once in cold phosphate-buffered saline (PBS), and repelleted. Cells were lysed in lysis buffer containing 1% Triton-X 100, 150 mM NaCl, 50 mM Tris [tris(hydroxymethyl)aminomethane]-HCl with protease and phosphatase inhibitors (10 μg/mL aprotinin, 10 μg/mL leupeptin, 1 μg/mL pepstatin, 5 mM sodium orthovanadate, 50 mM NaF, 50 mM Na-pyrophosphate, 150 μM phenylmethylsulfonyl fluoride [PMSF]) for 5 minutes on ice. Per sample, 100 μg total cell lysate was loaded on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel and separated by electrophoresis. Proteins were blotted onto nitrocellulose using a semidry transfer apparatus (Bio-Rad, Hercules, CA). Blots were blocked in 5% dried milk solution diluted in TBST (10 mM Tris, pH 7.5, 100 mM NaCl, 0.1% Tween20), incubated with indicated antibody, and signal detected with a horseradish peroxidase–conjugated secondary antibody and enhanced chemiluminescence (ECL) detection. Blots were stripped with 2% SDS at 50°C for 30 minutes and reprobed for total Akt. All antibodies were from Cell Signaling (Beverly, MA) except SHIP antibody, which was from Santa Cruz Biotech (Santa Cruz, CA).
Akt kinase assay
Assay was done using the Akt kinase kit essentially according to manufacturer's directions (Cell Signaling). Briefly, cells were lysed in lysis buffer and protein concentration measured; then 400 μg total cell lysate was incubated with anti-Akt antibody and Akt immunoprecipitated using sepharose beads. Beads were resuspended in kinase buffer with a purified glycogen synthase kinase 3 (GSK3) substrate and adenosine triphosphate added. The reaction was stopped by boiling in loading buffer. Total kinase reaction was loaded onto SDS-PAGE and blotted onto nitrocellulose. Phospho-GSK3 was quantitated by Western blot using anti–phospho-GSK3 antibody.
Annexin V assay
Cells were incubated in DMSO alone or the PI3 kinase inhibitor LY294002 at indicated concentrations for 24 to 48 hours. Cells were pelleted by centrifugation and incubated with anti–annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (PI). Single-cell suspensions were analyzed by FACScan (Becton Dickinson, San Jose, CA). Apoptotic cells were scored as annexin V+, PI- to exclude necrotic cells.
NOD/SCID engraftment studies
Six- to eight-week-old animals were sublethally irradiated one day prior to injection. For each animal, 5 × 106 total mononuclear cells from leukemic samples was allowed to recover in medium for 2 hours and then either immediately injected or incubated overnight in appropriate conditions prior to injection. Animals were monitored for signs of illness and humanely killed 7 weeks after injection. Both femurs were flushed with normal saline to collect bone marrow cells. Bone marrow red blood cells were removed using hypotonic buffer and remaining cells stained with directly conjugated human antigen specific antibodies (CD45, CD33, CD19, all from Pharmingen, San Diego, CA). Cells were analyzed on a FACSCalibur using Cellquest software.
Mutational analysis
Cells from the leukemia tissue bank were thawed and genomic DNA prepared using the Puregene Gentra System (Gentra Systems, Minneapolis, MN) according to the manufacturer's directions. Analysis for both internal tandem duplication (ITD) length mutations and D835 point mutations was performed as described previously.29,30 Briefly, Flt3 exon 14-15 (formerly designated exons 11 and 12) ITD mutations were analyzed by polymerase chain reaction (PCR) using the primers 11F = 5′-GCA ATT TAG GTA TGA AAG CCA GC -3′ and 12R = 5′-CTT TCA GCA TTT TGA CGG CAA CC-3′. PCR products were separated on 2.5% agarose gel and stained with ethidium bromide. Wild-type Flt3 samples were identified by the presence of the expected ∼340–base pair (bp) band, and ITD samples were identified by the presence of a larger amplified DNA fragment. To detect point mutations at D835, we employed a restriction enzyme–based PCR assay. Exon 20 (previously designated exon 17) of the FLT3 gene was amplified by genomic PCR using the primers 17F, 5′-CCGCCAGGAACGT-GCTTG-3′, and 17R, 5′-GCAGCCTCACATTGCCCC-3′. Amplified products were digested with Eco RV and separated by electrophoresis on a low melting point agarose gel. The wild-type products were digested to 2 bands (68 bp and 46 bp) by EcoRV, while those containing the D835 mutation yielded an undigested band (114 bp). A known positive patient specimen was used as a positive control for this assay.
Results
Patient sample characteristics
Characteristics of patient samples are shown in Table 1. Samples were collected from all patients seen at the University of Pennsylvania Hospital for a diagnosis of acute leukemia who consented to have blood or bone marrow taken for research purposes. Most samples were obtained at the time of diagnosis and prior to initiation of therapy. Multiple forms of AML were examined, but M6 and M7 were not represented. The classification system for AML recently has been changed. Most samples were classified according to the French-American-British (FAB) system, but a few recent samples were classified using the revised World Health Organization (WHO) nomenclature. Samples were selected from the tissue bank if they contained an adequate number of cells to complete multiple studies. Two samples initially submitted with a diagnosis of AML (nos. 75 and 82) were subsequently recharacterized as acute lymphoblastic leukemia (ALL) or chronic myeloid leukemia (CML) in lymphoid blast crisis, based on immunophenotypic and molecular studies. Rather than editing blots, which are made with limited patient material, results for these samples have been shown in Figures 1 and 2, but these samples are not included in functional studies or data analysis.
Characteristics of patient samples
Collection ID . | Diagnosis . | Subtype . | Sample type . | Estimated blasts, %* . | Flt 3 ITD† . |
|---|---|---|---|---|---|
| 53 | AML | M2 | Pheresis | 98 | ND |
| 55 | AML | M2 | PB | 78 | ND |
| 61 | AML | M2 | PB | 17 | - |
| 72 | AML | With dysplasia | PB | 53 | - |
| 75 | ALL | Precursor-B cell | PB | 74 | ND |
| 78 | AML | M2 | Pheresis | 98 | ND |
| 79 | AML | M4 | Pheresis | 70 | ND |
| 80 | AML | M5 | PB | 53 | ND |
| 82 | CML | Lymphoid blast crisis | Pheresis | 89 | ND |
| 85 | AML | M2 | BM | 90 | - |
| 87‡ | AML | Biphenotypic | PB | 73 | + |
| 88‡ | AML | Biphenotypic | BM | 73 | + |
| 109 | AML | M1 | Pheresis | 89 | + |
| 125 | AML | M1 | PB | 80 | + |
| 128 | AML | M2 | Pheresis | 55 | - |
| 132 | AML | With dysplasia | PB | 76 | - |
| 134 | AML | § | Pheresis | 78 | + |
| 144 | AML | With dysplasia | Pheresis | 98 | - |
| 146 | AML | M2 | Pheresis | 97 | ND |
| 147 | AML | With dysplasia | PB | 58 | - |
| 149 | AML | M1 | BM | 91 | + |
| 150 | AML | M2 | PB | 88 | ND |
| 151 | AML | M4 | PB | 22 | - |
| 152 | AML | M3 | PB | 96 | + |
| 159 | AML | M2 | PB | 59 | - |
Collection ID . | Diagnosis . | Subtype . | Sample type . | Estimated blasts, %* . | Flt 3 ITD† . |
|---|---|---|---|---|---|
| 53 | AML | M2 | Pheresis | 98 | ND |
| 55 | AML | M2 | PB | 78 | ND |
| 61 | AML | M2 | PB | 17 | - |
| 72 | AML | With dysplasia | PB | 53 | - |
| 75 | ALL | Precursor-B cell | PB | 74 | ND |
| 78 | AML | M2 | Pheresis | 98 | ND |
| 79 | AML | M4 | Pheresis | 70 | ND |
| 80 | AML | M5 | PB | 53 | ND |
| 82 | CML | Lymphoid blast crisis | Pheresis | 89 | ND |
| 85 | AML | M2 | BM | 90 | - |
| 87‡ | AML | Biphenotypic | PB | 73 | + |
| 88‡ | AML | Biphenotypic | BM | 73 | + |
| 109 | AML | M1 | Pheresis | 89 | + |
| 125 | AML | M1 | PB | 80 | + |
| 128 | AML | M2 | Pheresis | 55 | - |
| 132 | AML | With dysplasia | PB | 76 | - |
| 134 | AML | § | Pheresis | 78 | + |
| 144 | AML | With dysplasia | Pheresis | 98 | - |
| 146 | AML | M2 | Pheresis | 97 | ND |
| 147 | AML | With dysplasia | PB | 58 | - |
| 149 | AML | M1 | BM | 91 | + |
| 150 | AML | M2 | PB | 88 | ND |
| 151 | AML | M4 | PB | 22 | - |
| 152 | AML | M3 | PB | 96 | + |
| 159 | AML | M2 | PB | 59 | - |
Blast percentages are determined in clinical laboratories based on differential of peripheral blood or bone marrow aspiration prior to Ficoll sedimentation. Numbers may underestimate actual percent blasts in mononuclear cell fraction used for experiments
PB indicates peripheral blood; BM, bone marrow; ND, not done; -, negative; and +, positive
Analysis of D835 mutation also was performed, but none of these samples was positive. Although 7 of 16 samples are positive for Flt3 ITDs in this group, this was not representative of the tissue bank in which 11 of 57 (18%) of samples are positive
Samples 87 and 88 are peripheral blood and bone marrow, respectively, from the same patient
Patient presented with an elevated white blood cell count and clinical evidence of leukostasis. Diagnosis of acute myeloid leukemia was made based on morphologic appearance of blast cells, but the patient died before these blasts could be immunophenotypically and cytochemically characterized, precluding a definitive pathologic subclassification
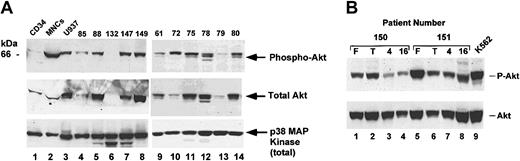
Akt phosphorylation in AML cells. (A) Patient samples were thawed, washed, harvested by centrifugation, and lysed in 1% Triton-X 100 lysis buffer; 100 μg cell lysates were loaded on SDS-PAGE gel and analyzed by Western blotting. Blots were probed with phospho-Akt antibody (top panel) and then stripped and reprobed with total Akt antibody (middle panel). As a loading control, lower portion of blot was separately probed with antibody against p38 MAP kinase (bottom panel). As a positive control for Akt phosphorylation, the human leukemia cell line U937 was analyzed. CD34+ cells and CD34-depleted MNCs were purified as described and lysed in parallel. (B) Fresh patient samples were purified by Ficoll gradient and MNCs obtained prior to freezing (lanes 1 and 5) or frozen and then thawed. Samples after thawing were collected immediately (lanes 2 and 6) or incubated in serum-free medium with no cytokines for the indicated times (lanes 3-4, 7-8). K562 cells were used as positive control (lane 9).
Akt phosphorylation in AML cells. (A) Patient samples were thawed, washed, harvested by centrifugation, and lysed in 1% Triton-X 100 lysis buffer; 100 μg cell lysates were loaded on SDS-PAGE gel and analyzed by Western blotting. Blots were probed with phospho-Akt antibody (top panel) and then stripped and reprobed with total Akt antibody (middle panel). As a loading control, lower portion of blot was separately probed with antibody against p38 MAP kinase (bottom panel). As a positive control for Akt phosphorylation, the human leukemia cell line U937 was analyzed. CD34+ cells and CD34-depleted MNCs were purified as described and lysed in parallel. (B) Fresh patient samples were purified by Ficoll gradient and MNCs obtained prior to freezing (lanes 1 and 5) or frozen and then thawed. Samples after thawing were collected immediately (lanes 2 and 6) or incubated in serum-free medium with no cytokines for the indicated times (lanes 3-4, 7-8). K562 cells were used as positive control (lane 9).
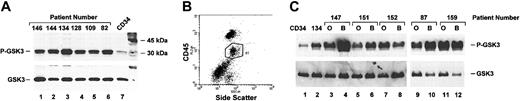
Activation of Akt kinase in AML cells. (A) Cells from pheresis pack were thawed, washed, and immediately lysed. Lysates were analyzed for activated Akt kinase using an in vitro kinase assay as described in “Materials and methods.” CD34+ cells were purified as described and used as a control. Presence of phosphorylated GSK3 in the kinase reaction was detected by Western blotting using an anti–phospho-GSK3 antibody. To ensure that substrate was not lost in handling, blot was stripped and reprobed using an antibody that recognized total GSK3 (lower panel). (B) Patient samples were thawed, washed, and suspended in FACS buffer. Cells were stained with anti-CD45 antibody and analyzed for the presence of CD45+ side scatter intermediate cells. A representative dot plot is shown. Blast cells are within the gated population indicated by the circle. (C) Blast (B) and other cells (O) were sorted into separate populations. Cells were lysed immediately after sorting and Akt kinase assay performed as above. Samples from patient no. 134, which contains 98% blasts, were sorted in parallel and used as a control (lane 2).
Activation of Akt kinase in AML cells. (A) Cells from pheresis pack were thawed, washed, and immediately lysed. Lysates were analyzed for activated Akt kinase using an in vitro kinase assay as described in “Materials and methods.” CD34+ cells were purified as described and used as a control. Presence of phosphorylated GSK3 in the kinase reaction was detected by Western blotting using an anti–phospho-GSK3 antibody. To ensure that substrate was not lost in handling, blot was stripped and reprobed using an antibody that recognized total GSK3 (lower panel). (B) Patient samples were thawed, washed, and suspended in FACS buffer. Cells were stained with anti-CD45 antibody and analyzed for the presence of CD45+ side scatter intermediate cells. A representative dot plot is shown. Blast cells are within the gated population indicated by the circle. (C) Blast (B) and other cells (O) were sorted into separate populations. Cells were lysed immediately after sorting and Akt kinase assay performed as above. Samples from patient no. 134, which contains 98% blasts, were sorted in parallel and used as a control (lane 2).
Akt is activated in mononuclear cells from patients with AML
To determine if Akt activation in leukemic blasts was detectable, we analyzed total mononuclear cell fractions of blood and bone marrow from patients with AML. For these experiments, cells were thawed and analyzed immediately upon thawing. Cells were compared to a cell line with constitutive activation of Akt (U937 cells, a human AML cell line; Figure 1A, lane 3) and to purified CD34+ cells and the residual mononuclear cell fraction after CD34 depletion from a healthy donor (Figure 1A, lanes 1-2). CD34+ cells represent a population of partially purified hematopoietic stem cells, and the residual mononuclear cells contain a mixed population of lymphocytes, monocytes, and a few progenitor cells. As shown in Figure 1A, CD34+ cells have a detectable but low level of Akt phosphorylation (lane 1), consistent with previous results,31 whereas the mononuclear cell fraction is consistently positive (lane 2). Analysis of leukemic samples demonstrated that most samples analyzed contained phosphorylated Akt (Figure 1A, lanes 4-14, and data not shown). Expression of total Akt in leukemic samples was variable as shown when the blot was stripped and reprobed with antibody for total Akt protein (Figure 1A, middle panel). However, the level of phosphorylation in general correlated with the level of expression, although one sample (sample no. 72, Figure 1, lane 1) showed strong phosphorylation despite a modest level of expression. Variation in Akt expression was not a technical artifact, as stripping and reprobing for p38 MAP kinase showed equal levels of loading in all lanes. This experiment demonstrates that Akt phosphorylation can be detected in primary patient cells using Western blotting but raised 2 questions. First, is there an effect of freeze/thawing or in vitro manipulation on Akt activation, and second, is Akt activated specifically in the blast population? Akt activation was detectable in one sample with a low blast count (sample no. 61, Figure 1A, lane 9), suggesting that the detection of activation did not depend on the presence of leukemic blasts in this assay. To address these issues, several experiments were performed.
In order to address these methodologic issues, several patient samples were examined in detail (Figure 1B). Fresh cells from patients with leukemia were acquired and mononuclear cells purified by Ficoll gradient. One aliquot of cells was removed and lysed prior to cell freezing. Lysate was frozen and stored for later analysis. Remaining cells were frozen at -140°C for at least one week. Cells were then thawed and washed. A second aliquot of cells was removed and immediately lysed, and other cells were incubated for 4-16 hours. Lysates were then compared for phosphorylation of Akt at different times of incubation (Figure 1B). In most samples, Akt phosphorylation was equivalent between lysates from fresh cells and those prepared immediately after thawing (Figure 1B, compare lanes 1 and 2). Some samples showed a stronger signal on lysates prepared from fresh cells (Figure 1B, lanes 5 and 6). Importantly, no samples showed either a complete gain or loss of phosphorylation of Akt between these conditions, although some quantitative differences could be detected. Incubation in serum-free medium with no cytokines leads to a decrease in Akt phosphorylation in about 50% of samples (Figure 1B, compare lane 2 to lanes 3 and 4). In other samples, there was no change in Akt phosphorylation during culture (Figure 1B, compare lane 6 to lanes 7 and 8). Given these findings, we performed remaining experiments as follows. To determine if a given sample contained activated Akt, cells were lysed immediately after thawing (this allows for collection and comparison of multiple samples). To determine the effects of signal transduction inhibitors, cells were analyzed both after thawing and after incubation in the absence of signal transduction inhibitors to control for the variability seen in some samples.
Akt is activated in purified blast populations
To determine if Akt is activated in purified populations of blast cells, we performed 2 experiments. Initially, we analyzed cells from pheresis packs. These cells are isolated using leukopheresis from patients with high white blood cell counts and represent highly purified populations of blasts. For these experiments, we used an Akt kinase assay rather than phosphorylation to measure Akt activity directly. Control experiments (data not shown) showed good correlation between the 2 assays. As shown in Figure 2A, 5 of 5 pheresis samples analyzed showed activation of Akt kinase (as noted above, sample no. 82, Figure 2A, lane 6, is from a patient with CML blast crisis and was not included in analysis). Level of activation was equivalent to that seen in U937 cells (data not shown). In contrast, CD34 cells from a healthy donor showed only low-level activation of Akt kinase. This experiment suggests that Akt is activated in purified leukemic blasts.
To demonstrate that activation of Akt is not restricted to blasts from patients with high white blood cell counts, we fractionated cells from patient samples with varying percentage of blasts (Figure 2B-C). The blast population can be identified by flow cytometry within the mononuclear cell fraction by the level of CD45 and side scatter (Figure 2B). Blasts are CD45+ (dim) and side scatter intermediate. Mononuclear cells from patient samples were thawed, labeled with CD45, and separated by flow cytometry into the blast population (labeled B in Figure 2C) and the nonblast population (labeled O in Figure 2C). Fractionated cells were lysed and analyzed for activation of Akt. As can be seen, Akt activation is seen in the blast population from 5 of 5 samples analyzed in this manner (Figure 2C, lanes 4, 6, 8, 10, 12). Level of activation was compared to a separate pheresis sample (no. 134), which was sorted in parallel to control for effects of sorting. Sample no. 134 showed similar levels of activation before and after sorting (compare Figure 2A, lane 3, to 2C, lane 2, and data not shown). These experiments confirm that leukemic blasts have constitutive activation of Akt kinase. Activation of Akt is not seen in all samples but is present in most samples. Overall, we have analyzed 22 patient samples that contain more than 70% blasts or were purified to high levels of purity; 20 (91%) of 22 showed activation of Akt by either phosphorylation or a positive kinase assay.
Survival of AML cells requires PI3 kinase activity
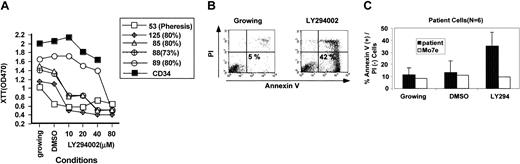
To assess the biologic role of PI3 kinase in the growth and survival of AML blasts, we used the PI3 kinase inhibitor LY294002 in a short-term culture system. LY294002 is a small molecule inhibitor that is specific for PI3 kinases when used in concentrations of less than 50 μM.32,33 For these assays, AML blasts were thawed and allowed to recover in serum-free medium for 2 hours. Samples that had a minimum amount of cell death after freeze/thawing and a percentage of blasts greater than 70% were selected. Cells were plated in serum-free medium containing IL-3, SCF, and IL-6, conditions known to support the growth and survival of AML blasts for up to 2 weeks. To assess the effects of LY294002, cells were plated in 96-well dishes and incubated in the absence or presence of varying concentrations of LY294002 (Figure 3). Relative cell survival was analyzed using an XTT assay. A total of 9 separate patient samples were tested. DMSO, the solvent for LY294002, inhibited 1 of the 9 samples, so the effects of LY294002 could not be assessed in this sample (Figure 3, patient no. 53). One of the 8 remaining samples showed decreased survival only at high doses of LY294002 (Figure 3, patient no. 89), at a concentration that may have nonspecific toxicity. However, 7 of 8 evaluable cases showed a consistent dose-response curve to LY294002 with inhibition of growth seen at 10 μM LY294002 and increased inhibition in doses up to 80 μM. Results for 3 of these samples are shown in Figure 3. The dose that gives 50% of the maximum effect (ED50) for this effect was less than 20 μM, a concentration consistent with a PI3 kinase–dependent effect. As a control, we again compared leukemic cells to normal CD34+ cells. C34+ cells showed decreased survival in LY294002 but only at high doses (Figure 3, closed squares), suggesting a nonspecific effect. Taken together, these results demonstrate that most leukemic samples, but not normal CD34 cells, require PI3 kinase activation for survival in vitro.
LY294002 inhibits survival of AML blasts. (A) AML blasts were thawed and rested for 2 hours in serum-free medium. Viable cells were counted and plated in serum-free medium containing IL-3 (5 ng/mL), SCF (50 ng/mL), and IL-6 (10 ng/mL). Cells were plated in 96-well dishes at 2 million cells per milliliter in medium lacking or containing LY294002 at the indicated concentrations. Cells were incubated for 48 hours and relative cell survival estimated using an XTT assay. For comparison, normal CD34 cells were incubated in similar conditions (▪). (B-C) AML blasts were thawed and rested for 2 hours in serum-free medium. Viable cells were counted and replated in serum-free medium containing IL-3 (5 ng/mL), SCF (50 ng/mL), and IL-6 (10 ng/mL) for 48 hours without or with LY294002 (25 μM) and then stained with propidium iodide and annexin V conjugated to FITC to analyze for apoptosis. Cells were analyzed by 2-color immunofluorescence and data analyzed. (B) A representative analysis of one patient sample. Apoptotic cells are in the bottom right quadrant (PI-, annexin V+). (C) A graphical summary of results for 6 patient samples treated as in panel B. Black speckled bars indicate results for patient samples; these results are compared to MO7e cells (open bars), which do not undergo apoptosis after treatment with LY294002, to show specificity of effect. Error bars for patient samples indicate SDs.
LY294002 inhibits survival of AML blasts. (A) AML blasts were thawed and rested for 2 hours in serum-free medium. Viable cells were counted and plated in serum-free medium containing IL-3 (5 ng/mL), SCF (50 ng/mL), and IL-6 (10 ng/mL). Cells were plated in 96-well dishes at 2 million cells per milliliter in medium lacking or containing LY294002 at the indicated concentrations. Cells were incubated for 48 hours and relative cell survival estimated using an XTT assay. For comparison, normal CD34 cells were incubated in similar conditions (▪). (B-C) AML blasts were thawed and rested for 2 hours in serum-free medium. Viable cells were counted and replated in serum-free medium containing IL-3 (5 ng/mL), SCF (50 ng/mL), and IL-6 (10 ng/mL) for 48 hours without or with LY294002 (25 μM) and then stained with propidium iodide and annexin V conjugated to FITC to analyze for apoptosis. Cells were analyzed by 2-color immunofluorescence and data analyzed. (B) A representative analysis of one patient sample. Apoptotic cells are in the bottom right quadrant (PI-, annexin V+). (C) A graphical summary of results for 6 patient samples treated as in panel B. Black speckled bars indicate results for patient samples; these results are compared to MO7e cells (open bars), which do not undergo apoptosis after treatment with LY294002, to show specificity of effect. Error bars for patient samples indicate SDs.
LY294002 induces apoptosis in AML blasts
In order to understand the mechanism of decreased cell number in AML blasts, we assessed cells for the presence of apoptosis after incubation with LY294002. A relative decrease in cell number may result either from apoptosis or a block of the cell cycle. Both mechanisms have been reported to be regulated by PI3 kinase in different cells.34,35 Preliminary experiments (data not shown) demonstrated no change in the percentage of cells in different parts of the cell cycle after treatment of cells with LY294002. Primary leukemic cells are usually in G0/G1, and this was not altered by the drug. In order to evaluate apoptosis, cells were incubated (Figure 3B-C) for 24 to 96 hours and assayed for the binding of annexin V (Figure 3B and data not shown). Annexin V is a calcium-dependent phospholipid-binding protein with a high affinity for phosphatidylserine, an inner cell membrane phospholipid that is expressed on the external surface of cells when they undergo apoptosis. As shown in Figure 3B, apoptotic cells in this assay are positive for annexin V, while necrotic cells are positive for both. Of 9 samples tested, 6 showed more than a 20% increase in apoptosis in LY294002-treated cells compared to DMSO-treated controls. Percent of apoptosis induction after treatment for 48 hours in 25 μM LY294002 for the 6 responders is shown in Figure 3C. In susceptible samples, apoptotic cells continued to accumulate for the duration of the experiment (up to 96 hours; data not shown). For comparison, the human leukemic cell line MO7e was analyzed in parallel (Figure 3C, open boxes). MO7e cells are not induced to undergo apoptosis at the same concentration of LY294002. Taken together these results demonstrate that LY294002 induces apoptosis in two thirds of AML samples. The remaining 3 samples showed evidence of LY294002-induced necrosis (PI+), but whether this was a specific effect of inhibition of PI3 kinase or a nonspecific effect is unclear. These data suggest that leukemic cells require PI3 kinase activation to inhibit apoptosis in vitro.
Leukemic stem cells may require PI3 kinase for survival
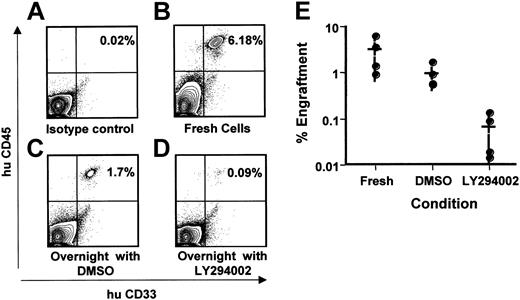
Work by several groups has defined the leukemic stem cell as a cell that is present in the CD34++, CD38- fraction of leukemic blasts and will engraft in a nonobese diabetic–severe combined immunodeficiency (NOD/SCID) animal.36,37 Such cells are defined as SCID leukemia-initiating cells (SL-ICs). To determine if SL-ICs require PI3 kinase for survival, we performed the following 2-phase experiment. Leukemic stem cells have a limited ability to survive in vitro. Therefore, initially, we incubated the total population of leukemic cells from one patient with DMSO or LY294002 (25 μM) for 16 hours. At this early time point, minimal apoptosis is seen in the cells, but we reasoned that perhaps the stem cell compartment would be impaired. In order to assay for the presence of leukemic stem cells, treated cells were then injected into sublethally irradiated NOD/SCID animals. Animals were monitored for 7 weeks and then killed for analysis. Bone marrow mononuclear cells were examined for the presence of human CD45+, CD33+ cells consistent with a leukemic phenotype. CD45+, CD19+ cells were not seen, demonstrating the absence of normal stem cells in the sample. As can be seen in Figure 4B, cells from this patient (no. 80), which were injected immediately after thawing, engrafted and 6.2% of marrow cells expressed the human CD45+/CD33+ phenotype. As expected, incubation overnight or in the presence of DMSO alone decreased engraftment quantitatively, but all animals injected after these conditions engrafted (n = 4), and the differences in the level of engraftment were not statistically significant (Figure 4E). In contrast, animals injected with cells treated with LY294002 engrafted at a low level in 2 animals and did not engraft in 2 other animals (Figure 4D-E), consistent with decreased survival of leukemic stem cells after treatment with LY294002. This experiment, although done on a single patient sample, suggests that leukemic stem cells also may require PI3 kinase for survival. Importantly, control experiments with normal CD34+ cells incubated in DMSO or LY294002 demonstrated that LY294002 decreased the level of engraftment by 2-fold on average (n = 4), but all animals engrafted.
Leukemic stem cells may require PI3 kinase for survival. Leukemic patient cells were thawed and rested for 2 hours in serum-free medium. Viable cells were counted and divided into 4 groups. One group of cells was injected immediately into NOD/SCID animals, with 5 × 106 mononuclear cells injected per animal (fresh cells). The 3 remaining groups were incubated overnight in serum-free medium containing cytokines and either nothing additional (not shown), 0.1% DMSO, or LY294002 at 25 μg/mL in DMSO. Cells from these 3 groups were injected into irradiated NOD/SCID animals after a 16-hour incubation. Animals were observed for 7 weeks, humanely killed, and analyzed for engraftment. Total bone marrow cells were harvested, red blood cells removed, and remaining cells stained with antibodies to human CD45 (y-axis) and human CD33 (x-axis). Corresponding stains for human CD19 were negative. (A-D) Contour plots of engraftment of CD45+/CD33+ cells in a representative experiment. Engrafted cells are in the upper right quadrant of each panel, and percentage of human cells is indicated by inset number. (E) Graph of individual results and averages (medians indicated by horizontal bars) from 4 separate animals in each group. Each point represents a single animal. Averages are average engraftment for the 4 animals in each cell treatment group.
Leukemic stem cells may require PI3 kinase for survival. Leukemic patient cells were thawed and rested for 2 hours in serum-free medium. Viable cells were counted and divided into 4 groups. One group of cells was injected immediately into NOD/SCID animals, with 5 × 106 mononuclear cells injected per animal (fresh cells). The 3 remaining groups were incubated overnight in serum-free medium containing cytokines and either nothing additional (not shown), 0.1% DMSO, or LY294002 at 25 μg/mL in DMSO. Cells from these 3 groups were injected into irradiated NOD/SCID animals after a 16-hour incubation. Animals were observed for 7 weeks, humanely killed, and analyzed for engraftment. Total bone marrow cells were harvested, red blood cells removed, and remaining cells stained with antibodies to human CD45 (y-axis) and human CD33 (x-axis). Corresponding stains for human CD19 were negative. (A-D) Contour plots of engraftment of CD45+/CD33+ cells in a representative experiment. Engrafted cells are in the upper right quadrant of each panel, and percentage of human cells is indicated by inset number. (E) Graph of individual results and averages (medians indicated by horizontal bars) from 4 separate animals in each group. Each point represents a single animal. Averages are average engraftment for the 4 animals in each cell treatment group.
Activation of other PI3 kinase–dependent signaling proteins
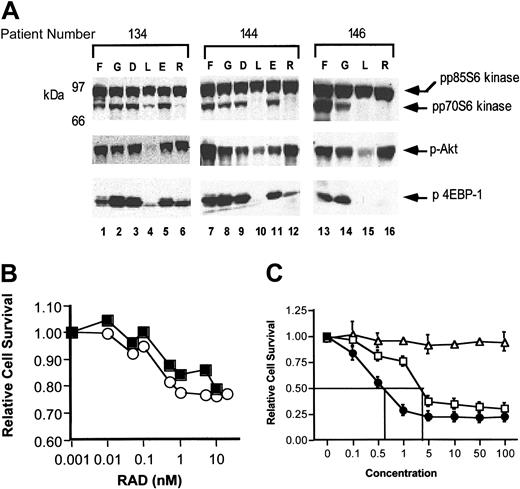
Akt is known to regulate several proteins, which in turn act on cell growth or survival pathways.26 We examined AML blasts for expression of the proapoptotic protein, Bcl-XL/Bcl-2–association death promoter (BAD), but found little expression of this protein in AML blasts (data not shown). Similarly, phosphorylation of the FKHD1 protein was detectable, but only in a small percentage of samples (data not shown). In contrast, phosphorylation of p70 S6 kinase and 4EBP-1, 2 protein substrates of mTOR, were phosphorylated on thawing in all samples analyzed (Figure 5A, lanes 1, 7, 13, and data not shown). Phosphorylation was generally stable while in culture, although we saw some differences in the level of phosphorylation of these proteins. Interestingly, these changes were not uniform. p70 S6 kinase was occasionally down-regulated in culture (see sample no. 146, Figure 5A, lanes 13 and 14, upper panel), whereas 4EBP-1 phosphorylation was occasionally increased in culture (see sample no. 134, Figure 5A, lanes 1 and 2, lower panel). The significance of this variation is unclear. These data confirm the activation of the PI3 kinase signaling pathway in AML.
Activation of other PI3 kinase–dependent signaling proteins. (A) Cells from patient collections were thawed, washed, and either immediately lysed in 1% Triton X-100 lysis buffer (lanes 1, 7, 13) or incubated in cytokine-free, serum-free medium for 4-16 hours in the absence of added compounds (lanes 2, 8, 14), in 0.1% DMSO (lanes 3 and 9), 0.1% ethanol (lanes 5 and 11), 25 μM LY294002 in DMSO (lanes 4, 10, 15), or 10 nM RAD001 in ethanol (lanes 6, 12, 16). After incubation, cells were lysed and 100 μg protein per lane analyzed using SDS-PAGE and Western blotting. Protein phosphorylation and expression were examined using indicated antibodies. In top panel, upper band is the p85 isoform of S6 kinase, which is constitutively expressed and phosphorylated in all samples. This band acts as a loading control. The blot was cut horizontally and the lower portion probed for phospho–4EBP-1 (lower panel). The upper panel was stripped and reprobed for phospho-Akt (middle panel). F indicates fresh; G, growing; D, DMSO 0.1%; L, LY294002 25 μM; E, ethanol 0.1%; and R, RAD001 10 nM. (B) Leukemic samples were thawed and incubated in serum-free medium with cytokines for 48 hours in varying concentrations of RAD001 as shown. Relative cell survival was measured using an XTT assay. ▪ indicates patient no. 111; ○, patient no. 88. (C) Cell line U937 cells were incubated in the presence of 10 nM RAD001 alone (▵),Ara-C alone (□), or Ara-C with 10 nM RAD001 (•). Relative cell survival was measured after 48 hours using an XTT assay. Error bars indicate SDs from 3 experiments.
Activation of other PI3 kinase–dependent signaling proteins. (A) Cells from patient collections were thawed, washed, and either immediately lysed in 1% Triton X-100 lysis buffer (lanes 1, 7, 13) or incubated in cytokine-free, serum-free medium for 4-16 hours in the absence of added compounds (lanes 2, 8, 14), in 0.1% DMSO (lanes 3 and 9), 0.1% ethanol (lanes 5 and 11), 25 μM LY294002 in DMSO (lanes 4, 10, 15), or 10 nM RAD001 in ethanol (lanes 6, 12, 16). After incubation, cells were lysed and 100 μg protein per lane analyzed using SDS-PAGE and Western blotting. Protein phosphorylation and expression were examined using indicated antibodies. In top panel, upper band is the p85 isoform of S6 kinase, which is constitutively expressed and phosphorylated in all samples. This band acts as a loading control. The blot was cut horizontally and the lower portion probed for phospho–4EBP-1 (lower panel). The upper panel was stripped and reprobed for phospho-Akt (middle panel). F indicates fresh; G, growing; D, DMSO 0.1%; L, LY294002 25 μM; E, ethanol 0.1%; and R, RAD001 10 nM. (B) Leukemic samples were thawed and incubated in serum-free medium with cytokines for 48 hours in varying concentrations of RAD001 as shown. Relative cell survival was measured using an XTT assay. ▪ indicates patient no. 111; ○, patient no. 88. (C) Cell line U937 cells were incubated in the presence of 10 nM RAD001 alone (▵),Ara-C alone (□), or Ara-C with 10 nM RAD001 (•). Relative cell survival was measured after 48 hours using an XTT assay. Error bars indicate SDs from 3 experiments.
LY294002 inhibits PI3 kinase signaling
In order to confirm that LY294002 was inhibiting the PI3 kinase pathway, phosphorylation of Akt, p70 S6 kinase, and 4EBP-1 was examined after treatment of cells with LY294002 (Figure 5A and data not shown). As can be seen, a 4-hour incubation of AML blasts with 20 μM LY294002 decreased phosphorylation of all 3 of these signaling proteins (Figure 5A, lanes 3 and 4, 9 and 10, 14 and 15). This decrease was not seen with the solvent DMSO. As noted, Akt activates mTOR, and, as suggested by its name, mTOR is inhibited by rapamycin and newer derivatives such as RAD001.38 Therefore, we also tested the sensitivity of these signaling proteins to RAD001 as shown (Figure 5A). Overall, RAD001 was efficacious in inhibiting phosphorylation of p70S6 kinase and 4EBP-1 (Figure 5A, lanes 6, 12, 16), although the inhibition of 4EBP-1 phosphorylation was incomplete in several samples. (Figure 5A, lanes 6 and 12, lower panel). Taken together, these data demonstrate that pharmacologic agents in AML cells can inhibit the PI3 kinase pathway. In addition, these results support the conclusion that the apoptosis induced by LY294002 (Figure 3) is associated with inhibition of PI3 kinase signaling.
Effects of RAD001 on survival of leukemic blasts
We were interested in the potential clinical implications of our findings. LY294002 is a toxic compound, but mTOR inhibitors have been clinically tested and shown to be efficacious in therapy of animal models of lymphoid malignancy.39-41 In order to determine if mTOR inhibitors would be toxic to leukemic blasts, we again performed a simple dose-response experiment (Figure 5B). For these experiments we used the inhibitor RAD001. Leukemic blasts were incubated in increasing doses of RAD001 as shown. However, only minimal induction of cell death was seen when RAD001 was used as a single agent (Figure 5B). We also examined the effect of RAD001 on the leukemic cell line U937 (Figure 5C). Although U937 cells have constitutive activation of Akt (Figure 1A, lane 3), RAD001 had no significant effect on survival of these cells when used as a single agent (Figure 5C, open triangles). However, interestingly, when U937 cells were incubated with RAD001 and Ara-C, a nucleoside analog that inhibits DNA synthesis and is used for the therapy of AML, the dose response to Ara-C was significantly enhanced (Figure 5C; compare closed circles to open squares). These results support our hypothesis that the PI3 kinase signaling pathway is important for survival of AML cells, particularly after genotoxic stress induced by Ara-C. It may be that combinations of RAD and Ara-C would be clinically useful for the treatment of AML.
Expression of negative regulators of PI3 kinase in AML
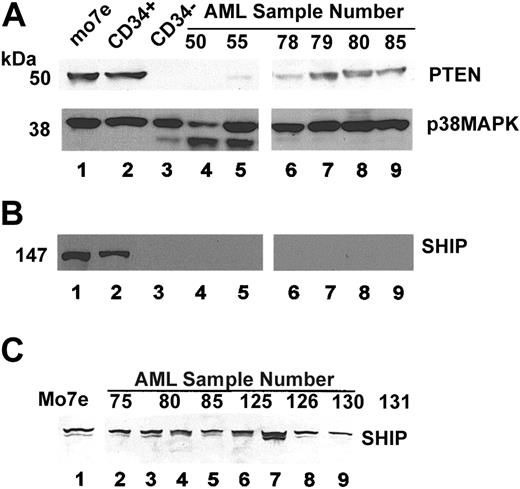
Activation of Akt is associated with an increase in the cellular concentration of PIP3. This may occur either because of an up-regulation of PI3 kinase activity (ie, as a result of cytokine stimulation) or because of down-regulation of the phosphatases SHIP and PTEN. SHIP and PTEN are constitutively expressed in most hematopoietic cells and remove phosphate from the 5′ or 3′ position of PIP3, respectively, thus acting as negative regulators of the PI3 kinase pathway.28,42 As our analysis was done in the absence of cytokines, we analyzed expression of the phosphatases PTEN and SHIP (Figure 6). Both proteins are strongly expressed in MO7e cells (Figure 6A-B, lane 1) and in purified CD34 cells (Figure 6A-B, lane 2). The CD34-depleted mononuclear cell (MNC) fraction did not express PTEN at a detectable level. Expression of PTEN in AML samples was either absent (Figure 6, lanes 4-5) or low (Figure 6, lanes 6-9). The level of PTEN expression did not correlate with the subtype of leukemia or degree of phosphorylation of Akt. Interestingly, Dahia et al have previously described mutations in the PTEN gene in 3 AML cell lines and 1 primary patient sample.41 These data suggest that the role of PTEN in regulating PI3 kinase activity in primary AML cells deserves further study.
AML cells express the SHIP and PTEN phosphatases. MO7e cells in log phase growth (lane 1), purified CD34+ cells (lane 2), or CD34-depleted MNCs (lane 3), and patient samples were lysed in 1% Triton X-100 lysis buffer (A,B). Protein lysates were quantitated and 100 μg protein loaded for analysis by SDS-PAGE. (A) Blot was probed for expression of PTEN (upper panel). Blot was separated and lower portion separately probed for expression of p38 MAP kinase as a loading control (lower panel). (B) The upper portion of the same blot as in A was probed for SHIP. (C) In a separate experiment, samples were thawed, pelleted by centrifugation, and immediately lysed by boiling in 10% SDS with 2-mercaptopurine present. Samples were equalized to 1 million cells per lane. Proteins were again analyzed by SDS-PAGE using the same antibody to probe for SHIP expression. In contrast to panel B, SHIP expression was present in all samples analyzed.
AML cells express the SHIP and PTEN phosphatases. MO7e cells in log phase growth (lane 1), purified CD34+ cells (lane 2), or CD34-depleted MNCs (lane 3), and patient samples were lysed in 1% Triton X-100 lysis buffer (A,B). Protein lysates were quantitated and 100 μg protein loaded for analysis by SDS-PAGE. (A) Blot was probed for expression of PTEN (upper panel). Blot was separated and lower portion separately probed for expression of p38 MAP kinase as a loading control (lower panel). (B) The upper portion of the same blot as in A was probed for SHIP. (C) In a separate experiment, samples were thawed, pelleted by centrifugation, and immediately lysed by boiling in 10% SDS with 2-mercaptopurine present. Samples were equalized to 1 million cells per lane. Proteins were again analyzed by SDS-PAGE using the same antibody to probe for SHIP expression. In contrast to panel B, SHIP expression was present in all samples analyzed.
Expression of SHIP during myeloid differentiation has been somewhat controversial. Geier et al, using a flow cytometric assay, originally reported down-regulation of SHIP coincident with expression of the myeloid lineage marker CD33.43 However, Horn et al recently demonstrated that SHIP is expressed in myeloid lineage cell lines and peripheral blood mononuclear cells but is susceptible to proteolytic cleavage if cells are prepared in a NP-40–based lysis buffer.44 Our analysis is consistent with the results of Horn et al. Initial analysis of the blot shown in Figure 6B showed no expression of SHIP in multiple AML samples (Figure 6B). However, when fresh lysates were prepared by boiling cells in SDS and a reducing agent, p145 SHIP was present in all samples analyzed (Figure 6C). Thus, we concur with the results of Horn et al that p145 SHIP is expressed in AML.
Discussion
We have studied the activation of the PI3 kinase signaling pathway in primary AML cells from patients with acute myeloid leukemia. These results demonstrate that the PI3 kinase signaling pathway is activated in most AML samples. Activation of this pathway is demonstrated by activation of the Akt kinase and phosphorylation of p70 S6 kinase and 4EBP-1. Furthermore, AML cells require PI3 kinase for survival as shown by a decrease in cell number, the induction of apoptosis, and the inhibition of engraftment of one patient sample in NOD/SCID animals after treatment with the PI3 kinase inhibitor LY294002. In contrast, LY294002 has little effect on the survival of CD34 cells in the same assay conditions. The mechanism of activation of PI3 kinase is unclear but may be related to the relatively low level of PTEN expressed in leukemic blasts compared to CD34 cells.
A number of comments are warranted about the methods used for these studies. Studies of signal transduction in primary cells are difficult because of the wide range of variables that can affect signaling pathways and the wide variability between patient samples. We have found that PI3 kinase activation appears to be fairly robust as judged by phosphorylation and activation of Akt. Akt activation was seen in fresh cells, freshly thawed cells, and cells thawed and then incubated for up to 24 hours (data not shown). Thus, it is unlikely that Akt activation is an artifact of handling. The level of rigor required to demonstrate that a signaling pathway is required for survival in primary cells is challenging, especially for AML cells. Conclusive evidence of the role of PI3 kinase in survival of AML blasts would require expression of a genetic inhibitor of the pathway (ie, PTEN or a dominant-negative Akt). Achievement of this goal is technically challenging because of the low efficiency of transfecting DNA into primary leukemic cells and will probably require efficient lentiviral delivery vectors expressing such constructs. The induction of apoptosis in the patient samples studied was variable and in no cases exceeded 50% at any given time point. Although, as noted, this number may underestimate the number of affected cells, these data are consistent with previous reports suggesting heterogeneity of leukemic cells. Bonnet and Dick have described a leukemic stem cell that phenotypically expresses the CD34 cell surface marker.36 As shown, we have examined the effect of LY294002 on survival of leukemic stem cells in one patient sample, but clearly further work will be required to determine if AML stem cells commonly require PI3 kinase for survival.
The finding that a high percentage of AML samples in our studies contains activated Akt is striking, although the mechanism of activation is unclear. Our analysis of Akt activation was performed in the absence of cytokines, thus this activation is not from exogenous cytokines. We have examined samples for the presence of Flt3 mutations (Table 1). We have both Akt activation data and mutational analysis on a total of 14 patient samples. All 5 samples that contain the Flt3 ITD have activation of Akt. However, 8 of 9 samples that did not have Flt3 ITDs also had activation of Akt. No Flt3 D835 mutations were identified in this small cohort, although they are present at the predicted frequency (7.4%) in our tissue bank overall (data not shown).30 Future experiments will test whether the Flt3 ITD mutation is involved in activation of Akt in these samples or whether Akt is activated by another mechanism in both Flt3 ITD-positive and -negative samples. Our finding that PTEN appears to be down-regulated in some patient samples suggests an alternative mechanism. In this regard, it is interesting that previous authors have reported mutations in PTEN in AML, although their analysis was limited to cell lines and 7 patient samples.41 Clearly, PTEN is a potent tumor suppressor, and if it is down-regulated in AML, this finding would have significant implications.45 Again, further studies will be needed to determine if this is true in a larger population of leukemic samples. Alternatively, either other known mutations (such as activation of Ras or c-kit) or undescribed mutations may act upstream of PI3 kinase to activate multiple signaling pathways.
Documentation that the PI3 kinase pathway is important for the survival of AML blasts may have significant clinical implications. Although LY294002 is toxic when given systemically, rapamycin derivatives such as RAD001 and other signal transduction inhibitors are being developed that are nontoxic and efficacious. The results presented here form the foundation for exploring use of these compounds, either alone or in combination with chemotherapy, in the treatment of AML. More important, these results provide an insight into the pathogenesis of acute myeloid leukemia. AML cells are characterized by a remarkable resistance to chemotherapy-induced apoptosis. The mechanism of this resistance has been largely unexplained. However, we have demonstrated that constitutive activation of signal transduction pathways and the PI3 kinase, in particular, may be a common mechanism contributing to the survival of AML blasts. Understanding how the PI3 kinase pathway is dysregulated should improve our understanding and treatment of this disease.
Prepublished online as Blood First Edition Paper, April 17, 2003; DOI 10.1182/blood-2002-11-3429.
Supported by the Leukemia and Lymphoma Society of America (A.B.) and the G & P Charitable Foundation for Cancer Research award (M.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Thanks to Heidi Lane and Terri Laufer for critical reading of the manuscript, to Treasa Smith for technical assistance, and to Soheil Meshinchi (Fred Hutchinson Cancer Research Center) for helpful discussions on the FLT3 genomic assays. Particular thanks to the patients who provided leukemic samples and to the nurses and fellows who assisted with identifying and collecting samples.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal