Abstract
This study tested the hypothesis that combination of antiangiogenic therapy and tumor immunotherapy of cancer is synergistic. To inhibit angiogenesis, mice were immunized with dendritic cells (DCs) transfected with mRNA that encode products that are preferentially expressed during neoangiogenesis: vascular endothelial growth factor receptor-2 (VEGFR-2) and Tie2 expressed in proliferating endothelial cells, and vascular endothelial growth factor (VEGF) expressed in the angiogenic stroma as well as the tumor cells used in this study. Immunization of mice against VEGF or VEGFR-2 stimulated cytotoxic T lymphocyte (CTL) responses and led to partial inhibition of angiogenesis. Antiangiogenic immunity was not associated with morbidity or mortality except for a transient impact on fertility seen in mice immunized against VEGFR-2, but not VEGF. Tumor growth was significantly inhibited in mice immunized against VEGF, VEGFR-2, and Tie2, either before tumor challenge or in the setting of pre-existing disease in murine B16/F10.9 melanoma and MBT-2 bladder tumor models. Coimmunization of mice against VEGFR-2 or Tie2 and total tumor RNA exhibited a synergistic antitumor effect. Synergism was also observed when mice were coimmunized with various combinations of defined tumor-expressed antigens, telomerase reverse transcriptase (TERT) or TRP-2, and VEGF or VEGFR-2. This study shows that coimmunizing mice against angiogenesis-associated and tumor-expressed antigens can deliver 2 compatible and synergistic cancer treatment modalities via a common treatment, namely immunization.
Introduction
Despite persistent efforts and extensive investment of resources, progress in combating cancer has been modest.1 The difficulties stem from the nature of cancer cells, which are heterogeneous in origin, resemble normal cells, and are genetically unstable. It is widely accepted that effective cancer therapy will therefore require a combination approach. Active immunotherapy of cancer, whereby patients are immunized against antigens presented by tumor cells, is a promising new modality to prevent cancer recurrence. Development of effective immunotherapeutic strategies is, however, facing numerous challenges such as breaking tolerance against self-antigens targeted on tumor cells, the emergence of immunologic escape variants, and the need to identify potent and broadly expressed antigenic targets.2-5 Another promising treatment modality is antiangiogenic therapy. Antiangiogenic therapy exploits the fact that all tumors beyond a minimal size (0.5-2.0 mm) require blood supply and depend on intratumor neoangiogenesis.6,7 A major limitation of antiangiogenic therapy stems from the fact that it is a cytostatic therapy requiring continuous or repeated treatments to prevent the neovascularization of small but persisting tumors. Since antiangiogenic therapy targets the tumor vasculature and prevents tumor growth beyond a certain size, whereas tumor immunotherapy targets the tumor cells and is capable of eliminating the remaining tumor mass, combination of antiangiogenic therapy and tumor immunotherapy could be highly synergistic. In this study we use an immunologic approach to combine tumor immunotherapy and antiangiogenic therapy and explore its potential in murine tumor models.
It was recently shown that active immunotherapy targeting endothelial products could inhibit tumor progression. Immunization of mice with paraformaldehyde-fixed xenogeneic endothelial cells,8 with vascular endothelial growth factor receptor 2 (VEGFR-2) protein-loaded dendritic cells (DCs),9 or with an attenuated Salmonella typhyimurium expressing VEGFR-2 cDNA10 inhibited tumor growth and led to the rejection of transplanted tumors. In the current study, mice were immunized with syngeneic DCs transfected with mRNA that encode angiogenesis-associated products and tumor antigens, and the impact of immunization on tumor growth was determined. We have previously shown that mRNA-transfected DCs are highly effective in stimulating cytotoxic T lymphocyte (CTL) responses and tumor immunity in mice11,12 and stimulating CTLs in vitro from the peripheral blood mononuclear cells (PBMCs) of human volunteers and cancer patients11,13,14 (reviewed in Nair and Boczkowski15 ). Here we show that immunization of mice against the endothelial-specific products VEGFR-2 and Tie2 or the angiogenic factor vascular endothelial growth factor (VEGF) stimulates a potent antitumor response and that immunologic targeting of angiogenesis-associated products and tumor antigens results in a superior antitumor effect.
Materials and methods
Mice
C57BL/6 (H-2b) and C3H/HeN (H-2k) mice (4-6 weeks old) were obtained from the Jackson Laboratory (Bar Harbor, ME). In conducting the research described in this paper, the investigators adhered to the “Guide for the Care and Use of Laboratory Animals” as proposed by the committee on care of Laboratory Animal Resources Commission on Life Sciences, National Research Council. The facilities at the Duke vivarium are fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Murine cell lines
The F10.9 clone of the B16 melanoma of C57BL/6 origin is a highly metastatic, poorly immunogenic and a low class I–expressing cell line.16 EL4 is a thymoma cell line (C57BL/6, H-2b). The murine MBT-2 cell line, derived from a carcinogen-induced bladder tumor in C3H mice,17 was obtained from Dr T. Ratliff (Washington University, St Louis, MO). The SV40-transformed B6 fibroblast cell line, BLK.SV (TIB-88), was obtained from American Type Culture Collection (ATCC, Manassas, VA). Cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS), 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 2 mM l-glutamine, and 1 mM sodium pyruvate. Murine precursor-derived DCs were generated in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) supernatant harvested from F10.9 cells transfected with the GM-CSF cDNA. Actively growing F10.9/GM-CSF cells were cultured in RPMI 1640 supplemented with 5% FCS, 1 mM Na pyruvate,1 mM nonessential amino acids, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 5 × 10-5 M β-mercaptoethanol and 10 mM HEPES (complete RPMI) at 37°C and 5% CO2. GM-CSF–containing supernatant was harvested after 24 hours of capillary culture. The GM-CSF supernatant was used to generate murine DCs at a final dilution of 0.1%. The concentration of GM-CSF used was determined by enzyme-linked immunosorbent assay (ELISA).
Murine bone marrow precursor–derived DCs
Bone marrow precursor–derived dendritic cells (BMDCs) were generated from bone marrow progenitors as previously described.18 Briefly, marrow from tibias and femurs of C57BL/6 mice were harvested followed by treatment of the precursors with ammonium chloride Tris (tris(hydroxymethyl)aminomethane) buffer for 3 minutes at 37°C to deplete the red blood cells. The precursors were plated in RPMI–5% FCS with GM-CSF (15 ng/mL) and interleukin-4 (IL-4, 10 ng/mL; Peprotech, Rocky Hill, NJ). Cells were plated at 106/mL and incubated at 37°C and 5% CO2. Then, 3 days later the floating cells (mostly granulocytes) were removed and the adherent cells replenished with fresh GM-CSF– and IL-4–containing medium. At 4 days later, the nonadherent cells were harvested (immature day-7 DCs), washed, and replated at 106/mL in GM-CSF– and IL-4–containing medium. After one day the nonadherent cells were harvested, washed, and electroporated with RNA.
Preparation of VEGF, VEGFR-2, Tie2, tyrosinase-related protein 2 (TRP-2), telomerase, and actin RNA
Creation of pSP73-Sph/A64. Oligonucleotides containing 64 A-T base pair (bp) followed by an SpeI restriction site were placed between the EcoRI and NarI sites of pGEM4Z (Promega, Madison, WI) to create the plasmid pGEM4Z/A64. The HindIII-NdeI fragment of pGEM4Z/A64 was cloned into pSP73 (Promega) digested with HindIII and NdeI to create pSP73/A64. The plasmid pSP73-Sph was created by digesting pSP73/A64 with SphI, filling in the ends with T4 DNA polymerase and religating. pSP73-Sph/A64/Not contains a NotI restriction site adjacent to the SpeI site. C. Kontos (Duke University Medical Center, Durham, NC) generously provided plasmids containing murine VEGF, VEGFR-2, and Tie2. The cDNAs were amplified with Advantage DNA polymerase (Clontech, Palo Alto, CA) for cloning into pSP73-Sph/A64.
Cloning of SP73-Sph/VEGF/A64. The forward primer 5′-TATATATCTAGAGCCACCATGGCACCCACGACAGAAGGAGAGCAGAAG-3′ and reverse primer 5′-TATATAGAATTCTCACCGCCTTGGCTTGTCACATC-3′ were used to amplify a truncated version of the VEGF coding region, not including the signal sequence, from the plasmid and were cloned into the XbaI-EcoRI sites of pSP73-Sph/A64.
Cloning of pSP73-Sph/VEGFR-2/A64. VEGFR-2 was amplified in 3 reactions using the following primers: for bases 1 to 1420, 5′-TATATACTCGAGGCCACCATGGAGAGCAAGGCGATGCTAGCTG-3′ and 5′-AATTAATCTAGACTAGTTGGACTCAATGGGGCCTTC-3′; for bases 1420 to 2730, 5′-AATTAACTCGAGCCACCATGGAAGTGACTGAAAGAGATGCAG-3′ and 5′-AAAAAATCTAGATCAGCGCTCATCCAATTCATC-3′; and for bases 2695 to 4390, 5′-ATATATCTCGAGCCACCATGGATCCAGATGAATTGGATGAGCG-3′ and 5′-TATATATCTAGACTAAGCAGCACCTCTCTCGTGATTTC-3′. The fragments were cloned separately into the XhoI-XbaI sites of pSP73-Sph/A64.
Cloning of pSP73/Tie2/A64/Not. The forward primer 5′TATATATCTAGAGCCACCATGGACTCTTTAGCCGGCTTAGTTC-3′ and reverse primer 5′-TATATAGAATTCCTAGGCTGCTTCTTCCGCAGAGCAG-3′ were used to amplify the Tie2 coding sequence from plasmid DNA. The fragment was cloned into the XbaI-EcoRI sites of pSP73/A64/Not.
Cloning of pSP73-Sph/TRP-2/A64. Total RNA was isolated from actively growing B16/F10.9 cells. Reverse transcription was primed with an anchored oligo dT primer and the TRP-2 cDNA was amplified from the first stand using the forward primer 5′-GATGGATCCAAGCTTGCCACCATGGGCCTTGTGGGATGG-3′ and the reverse primer 5′-GTTAGATCTGCGGCCGCTAGGCTTCCTCCGTGTATC-3′. The resulting product was digested with BglII and BamHI and cloned into the BamHI site of pSP73-Sph/A64.
Cloning of pGEM4Z/murineTERT/A64. The EcoRI fragment of pGRN188 (Geron, Menlo Park, CA) was cloned into the EcoRI site of pGEM4Z/A64/Not. Linearization with NotI followed by in vitro transcription (Ambion mMessage mMachine kit; Ambion, Austin, TX) yields a transcript containing 61 nucleotides (nt) of the polylinker of pGEM4Z, followed by 34 nt of 5′ untranslated region (UTR) of murine telomerase reverse transcriptase (mTERT), 3366 nucleotide (nt) mTERT open reading frame (ORF), 36 nt of 3′ UTR of mTERT, 64 A residues, an SpeI site, and a NotI half-site.
Cloning of pGEM4Z/murine actin/A64. Reverse transcription of total RNA from F10.9 cells was primed by oligo dT and carried out by PowerScript reverse transcriptase (Clontech). The forward primer 5′-TATATAAGCTTCTTTGCAGCTCCTTCGTTG-3′ and reverse primer 5′-TTTATGGATCCAAGCAATGCTGTCACCTTCCC-3′ were used to amplify the actin coding sequence from the first-strand cDNA. The polymerase chain reaction (PCR) fragment was cloned into the HindIII-BamHI sites of pGEM4Z/A64.
Isolation of total RNA from cell lines
Total RNA was isolated from actively growing tumor cell lines using the RNeasy kits (Qiagen, Valencia, CA) following the manufacturer's protocols.
Electroporating murine DCs with RNA
Electroporation was performed as previously described for human DCs,19,20 with small modifications. Briefly, DCs were harvested on day 8, washed, and gently resuspended in Opti-MEM (GIBCO, Grand Island, NY) at 2.5 × 107/mL. The used DC culture media were saved as conditioned media for later use. Cells were electroporated in 2-mm cuvettes (200 μLof DCs [5 × 106 cells] at 300 V for 500 μs using an Electro Square Porator ECM 830 [BTX, San Diego, CA]). The amount of in vitro transcribed (IVT) RNA used was 2 μg and total tumor RNA was 10 μg, per 106 DCs. Cells were immediately transferred to 60-mm tissue culture petri dishes containing a 1:1 combination of conditioned DC growth media and fresh RPMI–5% FCS with GM-CSF and IL-4. Transfected cells were incubated at 37°C, 5% CO2 overnight, washed 2 times in phosphate-buffered saline (PBS), and then injected into mice.
CTL induction in vivo
BMDCs were generated and transfected with RNA as described. Naive, syngeneic mice were immunized intravenously with 5 × 105 precursor-derived DCs per mouse in 200 μL PBS, 3 times at 7-day intervals. Splenocytes were harvested 8 to 10 days after the final immunization and depleted of red blood cells with ammonium chloride Tris buffer. Splenocytes (107) were cultured with 2 × 105 stimulator cells (DCs electroporated with RNA) in 5 mL Iscoves modified Dulbecco medium (IMDM) with 10% FCS, 1 mM sodium pyruvate, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 5 × 10-5 M β-mercaptoethanol per well in a 6-well tissue culture plate. The responders were stimulated with the same antigen as used for the immunization. Cells were cultured for 5 days at 37°C and 5% CO2. Effectors were harvested on day 5 on Histopaque 1083 gradient (Sigma, St Louis, MO) prior to use in a CTL assay.
Tumor challenge
B16/F10.9 melanoma model. DCs were transfected with the various RNA preparations and naive, syngeneic mice were immunized intravenously with 5 × 105 precursor-derived DCs per mouse in 200 μL PBS, 3 times at 7-day intervals. Mice were challenged with 5 × 104 F10.9 cells intravenously 8 to 10 days after the final immunization. Mice were killed based on the metastatic death in the control groups. Metastatic loads were assayed by weighing the lungs.
MBT-2 murine bladder tumor model. DCs were transfected with the various RNA preparations, and naive, syngeneic mice were immunized intravenously with 5 × 105 precursor-derived DCs per mouse in 200 μL PBS, 3 times at 7-day intervals. Mice were challenged with 2 to 5 × 105 MBT-2 cells subcutaneously (in the flank) 8 to 10 days after the final immunization. Tumor growth was evaluated every other day starting on day 6. Mice were killed once the tumor size reached 20 mm.
For experiments testing synergy between different antigens, mice were immunized 2 times with 3 × 105 DCs in 100 μL for each antigen for a combined 6 × 105 DCs in 200 μL per mouse.
Tumor immunotherapy
Mice were challenged with 1 × 104 F10.9 cells subcutaneously (in the flank). At 3 days after tumor implantation mice were immunized intravenously with 5 × 105 precursor-derived DCs per mouse in 200 μL PBS, 3 times at 7-day intervals. Tumor growth was evaluated every other day starting on day 10. Mice were killed once the tumor size reached 20 mm.
For experiments testing synergy between different antigens, mice were immunized with 3 × 105 DCs in 100 μL for each antigen for a combined 6 × 105 DCs in 200 μL per mouse.
In vitro cytotoxicity assay
Target cells (5-10 × 106) were labeled with europium for 20 minutes at 4°C. Europium-labeled targets (104) and serial dilutions of effector cells at varying effector-target (E/T) ratios were incubated in 200 μL complete RPMI 1640. The plates were centrifuged at 500g for 3 minutes and incubated at 37°C for 4 hours. Supernatant (50 μL) was harvested and europium release was measured by time-resolved fluorescence.21 Specific cytotoxic activity was determined using the following formula: % specific release = [(experimental release - spontaneous release)/(total release - spontaneous release)] × 100. Spontaneous release of the target cells was less than 25% of total release by detergent in all assays. Standard errors of the means of triplicate cultures were less than 5%.
Dorsal skin-fold window-chamber assay
Details of the design and surgical technique used for the mouse dorsal skin-fold window-chamber assay are described elsewhere.22,23 Briefly, mice immunized with DCs transfected with VEGF or VEGFR-2 or PBS were randomly divided into 3 groups. An investigator who was unaware of the experimental details carried out all remaining procedures and measurements. At 5 days following the surgery for placing the window chambers, the mice were then implanted with tumor cells (B16/F10.9 cells expressing green fluorescent protein [GFP]). This approach ensured that there would not be any interference in interpretation from the vascular changes caused by surgery. Starting on day 4 after tumor implantation the mice were evaluated for the effect of immunization on tumor growth and vascularization. Tumor areas were measured with low magnification images of the whole tumor. Tumor vasculature was evaluated based on 4 random tumor areas, using higher magnification (objective, × 20). Image analysis software was used to measure the cumulative length of all vessels in focus in each image. The vascular length density was calculated by dividing the total vessel length density in the frame by the area of the frame. All images were calibrated against micrometer images at the same magnification.
Mouse pregnancy experiments
Mice were immunized with DCs electroporated with VEGF, VEGFR-2, or actin RNA 3 times at weekly intervals. Mice were mated with nonimmunized male mice 1 week and 8 weeks after the final immunization. This was done in triads (2 females to a male per cage). Number of pups delivered was recorded and the pups were examined for signs of sickness and abnormality and their weight after weaning was recorded.
Statistics
The different experimental groups within the study were compared using the Kruskal-Wallis test. The Mann-Whitney U test was used to determine significance in differences in lung weights between 2 groups. A probability of less than .05 (P < .05) was used for statistical significance. To determine the significance of combination therapy between tumor antigen and angiogenesis-related antigens we determined time to tumor onset (appearance of palpable tumors) for the various groups. Comparison between 2 groups was done using the log-rank test (Mantel-Haenszel test). Additional comparisons between 2 groups were done by determining the median time to tumor onset for each group.
Results
Angiogenesis-associated antigens: VEGFR-2, Tie2, and VEGF
VEGF and VEGFR play a critical role during angiogenesis, and thereby represent excellent targets for therapeutic interventions.24,25 VEGFR-2 is expressed exclusively in endothelial cells during angiogenesis and is the major transducer of VEGF-mediated signals in endothelial cells leading to cell proliferation and migration. The importance of VEGFR-2 signaling for tumor angiogenesis was suggested by the observation that a dominant-negative mutant of VEGFR-2 prevented tumor growth in mice.26 VEGFR-2 is up-regulated in tumor-associated endothelial cells but not in the vasculature of the surrounding tissue.27-30 In view of the specificity of VEGFR-2 expression in proliferating endothelial cells at sites of angiogenesis and the key role of VEGFR-2 signaling during angiogenesis, interference with VEGFR-2 signaling represents a logical target for the development and clinical testing of antiangiogenic therapies.31-34 Tie2, like VEGFR-2, is a receptor tyrosine kinase up-regulated on proliferating endothelial cells and following engagement with its ligand angiopoietin-1, transmits a proangiogenesis signal.25,34 Gene knock-out and inhibition studies have shown that Tie2 function is essential during embryogenesis35 and tumor neoangiogenesis.36-38 VEGF, the ligand for VEGFR-2, is an endothelial-specific growth factor and is essential for angiogenesis.24,31 Targeted inactivation of the VEGF gene in mice causes abnormal blood vessel development and lethality in embryos.39,40 Unlike VEGFR-2 or Tie2, VEGF is expressed in stromal cells during angiogenesis.24,31 VEGF also plays an essential role during tumor angiogenesis as shown by the fact that inhibition of VEGF function suppresses tumor growth in mice.41 Most human and murine tumors induce the expression of VEGF24,31 in response to the progressively hypoxic conditions in the growing tumor.42 Indeed, tumors are the main source of VEGF during tumor angiogenesis.24,31 Thus VEGF can serve a dual role as an antigen to target both the tumor and its vasculature.
Immunization against angiogenesis-associated products stimulates CTLs and inhibits angiogenesis
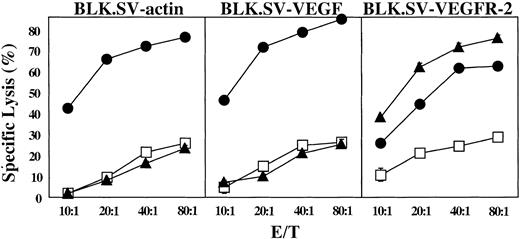
To determine whether immunization can break tolerance against angiogenesis-associated products, C57BL/6 mice were immunized with VEGFR-2, or VEGF mRNA–transfected syngeneic DCs, and CTL responses were measured in the splenocytic population following in vitro stimulation as described in “Materials and methods.” Targets used for CTL detection were syngeneic BLK.SV tumor cells (H-2b) transfected with actin mRNA, VEGF mRNA, or VEGFR-2 mRNA. BLK.SV cells, like most tumor cells, express VEGF as determined by reverse transcriptase (RT)–PCR (data not shown). As shown in Figure 1, immunization of mice with VEGF mRNA–transfected DCs stimulated CTLs, which recognized all BLK.SV targets. It is tempting to speculate that the slightly increased killing of VEGF mRNA–transfected targets compared with actin mRNA–transfected targets reflects the increased expression of VEGF in the VEGF mRNA–transfected cells, whereas the reduced killing of targets transfected with VEGFR-2 mRNA is due to competition by the ectopically expressed VEGFR-2 that leads to reduced presentation of VEGF epitopes on the cell surface. Figure 1 also shows that only targets transfected with VEGFR-2 mRNA were recognized by CTLs generated from mice immunized against VEGFR-2. This is consistent with the fact that BLK.SV tumor cells do not express VEGFR-2 (data not shown). In contrast, BLK.SV cells transfected with actin mRNA, or other mRNAs, were not recognized by CTLs generated from mice immunized against actin. This experiment, therefore, shows that it is possible to break tolerance against VEGF or VEGFR-2, but not actin, despite the fact that they represent normal gene products. Presumably, this is due to the fact that VEGF and VEGFR-2, as well as many other angiogenesis-associated products, exhibit a restricted tissue-specific pattern of expression.
Induction of CTLs in mice immunized with DCs transfected with VEGF and VEGFR-2 mRNA. DCs generated from C57BL/6 mice were electroporated with VEGF (▪), VEGFR-2 (▴), or actin (□) mRNA, and naive, syngeneic mice were immunized with 5 × 105 cells 3 times intravenously. Splenocytes were harvested, restimulated with DCs transfected with RNA (same as used in the immunization) for 5 days, and tested for the presence of CTLs. BLK.SV cells (BL/6 fibroblasts transformed with SV40) transfected with mRNA as indicated were used as targets. Data are expressed as mean percent specific lysis of triplicate samples ± SD.
Induction of CTLs in mice immunized with DCs transfected with VEGF and VEGFR-2 mRNA. DCs generated from C57BL/6 mice were electroporated with VEGF (▪), VEGFR-2 (▴), or actin (□) mRNA, and naive, syngeneic mice were immunized with 5 × 105 cells 3 times intravenously. Splenocytes were harvested, restimulated with DCs transfected with RNA (same as used in the immunization) for 5 days, and tested for the presence of CTLs. BLK.SV cells (BL/6 fibroblasts transformed with SV40) transfected with mRNA as indicated were used as targets. Data are expressed as mean percent specific lysis of triplicate samples ± SD.
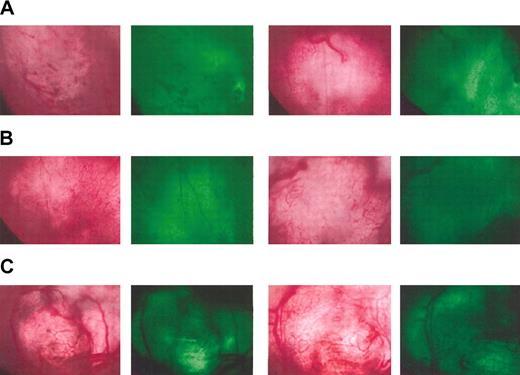
To determine whether angiogenesis is inhibited in mice immunized against either VEGFR-2 or VEGF, the development of neovasculature in a small tumor implant was followed in real time using the skin-flap window-chamber model.22,23 Mice were either injected with PBS or immunized with VEGFR-2 or VEGF mRNA–transfected DCs 3 times at weekly intervals. At 4 weeks following the last immunization a window chamber was surgically implanted. Then 5 days later, B16/F10.9 melanoma cells expressing GFP (to facilitate subsequent analysis) were implanted into the window chamber. Invasion of blood vessels into the tumor area was monitored daily and quantitated by image analysis as previously described.23 Figure 2 shows the invasion of blood vessels into the implanted GFP-expressing (green) tumor mass. Mice injected with PBS exhibit a typical pattern of microvessel invasion into the implanted tumor, illustrative of normal angiogenesis. By contrast, a significant paucity of microvasculature was seen in the implanted tumors of mice immunized against either VEGFR-2 or VEGF, showing that immunization against these antigens was associated with a partial inhibition of angiogenesis. The difference between control mice injected with PBS and mice immunized against the angiogenic products was confirmed using image analysis measuring time to microvessel invasion and microvasculature density (data not shown). The data shown in Figure 2 are representative of each group and of observations taken over time.
Inhibition of angiogenesis in mice immunized against angiogenesis-associated products. Mice were immunized 3 times with DCs transfected with VEGF (A) or VEGFR-2 (B) mRNA or injected with PBS (C). The angiogenic potential was determined using the dorsal skin-fold window-chamber assay. At 4 weeks following immunization, mice were implanted with window chambers and 5 days later inoculated with GFP-expressing B16 melanoma cells. Tumor growth and neovascularization were followed by light and fluorescent microscopy starting 4 days after tumor implantation. Representative pictures taken by light microscopy (left) and fluorescent microscopy to localize the implanted GFP-expressing tumor (right) from 2 mice per group are shown 14 days after tumor implantation.
Inhibition of angiogenesis in mice immunized against angiogenesis-associated products. Mice were immunized 3 times with DCs transfected with VEGF (A) or VEGFR-2 (B) mRNA or injected with PBS (C). The angiogenic potential was determined using the dorsal skin-fold window-chamber assay. At 4 weeks following immunization, mice were implanted with window chambers and 5 days later inoculated with GFP-expressing B16 melanoma cells. Tumor growth and neovascularization were followed by light and fluorescent microscopy starting 4 days after tumor implantation. Representative pictures taken by light microscopy (left) and fluorescent microscopy to localize the implanted GFP-expressing tumor (right) from 2 mice per group are shown 14 days after tumor implantation.
Immunization against endothelial products and tumor antigens is synergistic
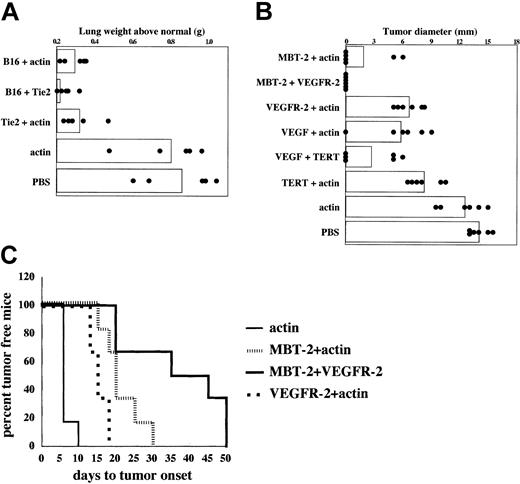
To determine whether the reduced rate of angiogenesis seen in mice immunized against angiogenesis-associated product (Figure 2) affects tumor progression, inhibition of tumor growth in mice immunized against VEGFR-2, Tie2, or VEGF was tested in the B16/F10.9 melanoma experimental metastasis model16 and the subcutaneously implanted MBT-2 bladder tumor model.11,17 RT-PCR analysis confirmed that VEGF was expressed in both B16/F10.9 and MBT-2 tumor cells, whereas neither VEGFR-2 nor Tie2 were expressed in either tumor (data not shown). In the experiment shown in Figure 3A, the B16/F10.9 experimental metastasis model was used to measure the impact of immunization on lung metastasis. The mRNAs corresponding to VEGF, VEGFR-2, or Tie2 were transfected into syngeneic bone marrow–derived DCs and used to immunize C57BL/6 mice 3 times at weekly intervals. At 8 days following the last immunization, mice were challenged intravenously with B16/F10.9 tumor cells, and lung metastasis was determined 35 days later. Mice injected with PBS or immunized with DCs transfected with murine actin mRNA were used as controls. As previously seen in this experimental system,11 immunization with B16/F10.9 tumor RNA-transfected DCs inhibited, although not completely, the development of lung metastasis (Figure 3A). Immunization with VEGFR-2 mRNA–transfected DCs had a comparable antimetastatic effect, whereas the impact of immunization with either Tie2- or VEGF mRNA–transfected DCs was more pronounced. A similar pattern of tumor inhibition was seen in the MBT-2 bladder tumor model (Figure 3B).
Inhibition of tumor growth in mice immunized with DCs transfected with VEGF, VEGFR-2, and Tie2 mRNA. (A) B16/F10.9 melanoma model: mice were immunized with 5 × 105 mRNA-transfected DCs 3 times at 7-day intervals and challenged intravenously with 5 × 104 B16/F10.9 tumor cells 8 days after immunization. Mice were killed based on the metastatic death in the control group (25-35 days after challenge with F10.9 cells). Metastatic loads were determined by measuring lung weights. Columns represent mean lung weight and circles represent individual lung weight (5 mice per group). (B) MBT-2 bladder tumor model: mice were immunized with 5 × 105 mRNA-transfected DCs for a total of 3 times at 7-day intervals and challenged subcutaneously with 5 × 105 MBT-2 cells 8 days after immunization. Day-18 measurements are shown. Columns represent mean tumor diameter and circles represent individual tumor diameter (5 mice per group).
Inhibition of tumor growth in mice immunized with DCs transfected with VEGF, VEGFR-2, and Tie2 mRNA. (A) B16/F10.9 melanoma model: mice were immunized with 5 × 105 mRNA-transfected DCs 3 times at 7-day intervals and challenged intravenously with 5 × 104 B16/F10.9 tumor cells 8 days after immunization. Mice were killed based on the metastatic death in the control group (25-35 days after challenge with F10.9 cells). Metastatic loads were determined by measuring lung weights. Columns represent mean lung weight and circles represent individual lung weight (5 mice per group). (B) MBT-2 bladder tumor model: mice were immunized with 5 × 105 mRNA-transfected DCs for a total of 3 times at 7-day intervals and challenged subcutaneously with 5 × 105 MBT-2 cells 8 days after immunization. Day-18 measurements are shown. Columns represent mean tumor diameter and circles represent individual tumor diameter (5 mice per group).
Since VEGFR-2 or Tie2 is expressed in proliferating endothelial cells and is not expressed in either MBT-2 or B16/F10.9 tumor cells, yet tumor growth is inhibited in mice immunized against each product (Figure 3), the observed inhibition of tumor growth must have been mediated via inhibition of tumor angiogenesis. This conclusion is also supported by the observation that immunization against VEGFR-2 is accompanied by a reduced state of angiogenesis in the immunized animal (Figure 2). We, therefore, tested whether targeting the tumor for immunologic destruction and simultaneously preventing tumor vasculature formation will exert a synergistic antitumor effect. B16/F10.9 and MBT-2 tumor RNA-transfected DCs were used to stimulate an immune response directed against antigens expressed by the tumor cells (the source of tumor RNA was tissue-cultured tumor cell lines devoid of normal cells such as endothelial cells). It also should be noted that the immune response elicited in mice immunized with tumor RNA–transfected DCs is directed to unique, and not shared, tumor antigens as judged by the fact that no cross-reactivity between the tumors has been observed.11 Figure 4A shows that in the B16/F10.9 tumor model coimmunization with B16/F10.9 tumor RNA and Tie2 mRNA is superior to immunization with either RNA alone. Similarly, Figure 4B-C show that in the MBT-2 model coimmunization with MBT-2 RNA– and VEGFR-2 mRNA–transfected DCs was superior to using either antigen alone, leading to a significant delay in tumor onset. Thus, these experiments demonstrate the potential value of combined immunization against tumor and its vasculature. The polypeptide component of telomerase (TERT), which is silent in normal tissues but reactivated in more than 85% of cancers,43 can serve as a broadly useful antigen in cancer vaccination.11,44,45 We have previously shown that immunization against TERT can elicit CTLs and protective tumor immunity against several tumors of unrelated origin.11 Figure 4B also suggests that immunization of mice against both VEGF and TERT is superior to immunization against either VEGF or TERT alone, suggesting that targeting the 2 broadly expressed prototype “universal” tumor antigens could improve the efficacy of antitumor vaccination. However, as noted, since VEGF is also expressed by tumor cells, including the B16/F10.9 and MBT-2 tumor cells used in this study, it is not clear whether the antitumor effects of immunizing against VEGF reflect a direct effect on the tumor, its vasculature, or both.
Combination immunotherapy targeting angiogenesis-associated products and tumor antigens. Mice were immunized twice weekly with 3 × 105 DCs for each antigen for a combined 6 × 105 DCs per mouse. (A) B16/F10.9 melanoma model as described in Figure 3A. Syngeneic tumor RNA (B16) was used as source of tumor antigens and actin mRNA was used as control antigen. Relative to the control group (PBS), P values were less than .05 for all groups except actin (P = .4206). The overall significance of the study as determined by the Kruskal-Wallis test was P = .001. (B) MBT-2 bladder tumor model as described in Figure 3B. Syngeneic tumor RNA (MBT-2) and TERT mRNA were used as source of tumor antigens and actin mRNA as control antigen. Day-18 measurements are shown. Relative to the control group (PBS), P values were less than .05 for all groups except actin (P=.13). The overall significance of the study as determined by the Kruskal-Wallis test was P<.0001. (C) Time to appearance of palpable tumors in the experiment shown in panel B. The log-rank test (Mantel-Haenszel test) was used to determine the differences between individual groups. Relative to the combination group (MBT-2 + VEGFR-2), P values were .02 and .001 for groups immunized with MBT-2 + actin and VEGFR-2 + actin, respectively. The median time to tumor onset for MBT-2 + actin was 20 days, VEGFR-2 + actin was 15 days, and MBT-2 + VEGFR-2 was 40 days.
Combination immunotherapy targeting angiogenesis-associated products and tumor antigens. Mice were immunized twice weekly with 3 × 105 DCs for each antigen for a combined 6 × 105 DCs per mouse. (A) B16/F10.9 melanoma model as described in Figure 3A. Syngeneic tumor RNA (B16) was used as source of tumor antigens and actin mRNA was used as control antigen. Relative to the control group (PBS), P values were less than .05 for all groups except actin (P = .4206). The overall significance of the study as determined by the Kruskal-Wallis test was P = .001. (B) MBT-2 bladder tumor model as described in Figure 3B. Syngeneic tumor RNA (MBT-2) and TERT mRNA were used as source of tumor antigens and actin mRNA as control antigen. Day-18 measurements are shown. Relative to the control group (PBS), P values were less than .05 for all groups except actin (P=.13). The overall significance of the study as determined by the Kruskal-Wallis test was P<.0001. (C) Time to appearance of palpable tumors in the experiment shown in panel B. The log-rank test (Mantel-Haenszel test) was used to determine the differences between individual groups. Relative to the combination group (MBT-2 + VEGFR-2), P values were .02 and .001 for groups immunized with MBT-2 + actin and VEGFR-2 + actin, respectively. The median time to tumor onset for MBT-2 + actin was 20 days, VEGFR-2 + actin was 15 days, and MBT-2 + VEGFR-2 was 40 days.
In the experiments shown in Figures 3 and 4 mice were immunized first and then challenged with tumor. To determine the impact of antitumor and antiangiogenic immunotherapy in the setting of pre-existing disease, mice were first implanted with B16/F10.9 tumor cells followed by the immunization protocol starting 3 days after tumor implantation. Figure 5A shows that in this setting, immunization against TERT or VEGFR-2 had a small antitumor effect, whereas the effect of anti-VEGF immunotherapy was more pronounced. Coimmunizing the mice against TERT and VEGFR-2 or VEGF and VEGFR-2 was synergistic, exhibiting an enhanced antitumor effect. Similarly, Figure 5B-D show that coimmunization against another tumor-expressed antigen TRP-2, a dominant antigen in B16 melanoma,46 and VEGF or VEGFR-2 is synergistic, leading to a significant delay in tumor growth.
Immunotherapy of tumor-bearing mice with DCs transfected with angiogenesis-associated and tumor antigens. (A-B) B16/F10.9 melanoma model: C57BL/6 mice were implanted subcutaneously with 1 × 104 B16/F10.9 tumor cells and 3 days later immunized with mRNA-transfected DCs 3 times at 7-day intervals. Mice were immunized with 3 × 105 DCs for each antigen for a combined 6 × 105 DCs per mouse. Data in panel A are 18 days after tumor implantation and in panel B are 25 days after tumor implantation. Columns represent mean lung weight and dots represent individual lung weight (7 mice per group). (A) Relative to the control group (PBS), P values were less than .05 for all groups except actin (P = .4206). The overall significance of the study as determined by the Kruskal-Wallis test was P < .0001. (B) Relative to the control group (PBS), P values were less than .05 for all groups except actin (P = .4206). The overall significance of the study as determined by the Kruskal-Wallis test was P = .0011. (C-D) Figure shows time to appearance of palpable tumors in the experiment shown in panel B. The log-rank test (Mantel-Haenszel test) was used to determine the differences between individual groups. (C) Relative to the combination group (TRP-2 + VEGFR-2), P values were .03 and .002 for groups immunized with TRP-2 + actin and VEGFR-2 + actin, respectively. The median time to tumor onset for TRP-2 + actin was 18 days, VEGFR-2 + actin was 15 days, and TRP-2 + VEGFR-2 was 25 days. (D) Relative to the combination group (VEGF + TRP-2), P values were .03 and .02 for groups immunized with VEGF + actin and TRP-2 + actin, respectively. The median time to tumor onset for TRP-2 + actin was 18 days, VEGF + actin was 18 days, and VEGF + TRP-2 was 37 days.
Immunotherapy of tumor-bearing mice with DCs transfected with angiogenesis-associated and tumor antigens. (A-B) B16/F10.9 melanoma model: C57BL/6 mice were implanted subcutaneously with 1 × 104 B16/F10.9 tumor cells and 3 days later immunized with mRNA-transfected DCs 3 times at 7-day intervals. Mice were immunized with 3 × 105 DCs for each antigen for a combined 6 × 105 DCs per mouse. Data in panel A are 18 days after tumor implantation and in panel B are 25 days after tumor implantation. Columns represent mean lung weight and dots represent individual lung weight (7 mice per group). (A) Relative to the control group (PBS), P values were less than .05 for all groups except actin (P = .4206). The overall significance of the study as determined by the Kruskal-Wallis test was P < .0001. (B) Relative to the control group (PBS), P values were less than .05 for all groups except actin (P = .4206). The overall significance of the study as determined by the Kruskal-Wallis test was P = .0011. (C-D) Figure shows time to appearance of palpable tumors in the experiment shown in panel B. The log-rank test (Mantel-Haenszel test) was used to determine the differences between individual groups. (C) Relative to the combination group (TRP-2 + VEGFR-2), P values were .03 and .002 for groups immunized with TRP-2 + actin and VEGFR-2 + actin, respectively. The median time to tumor onset for TRP-2 + actin was 18 days, VEGFR-2 + actin was 15 days, and TRP-2 + VEGFR-2 was 25 days. (D) Relative to the combination group (VEGF + TRP-2), P values were .03 and .02 for groups immunized with VEGF + actin and TRP-2 + actin, respectively. The median time to tumor onset for TRP-2 + actin was 18 days, VEGF + actin was 18 days, and VEGF + TRP-2 was 37 days.
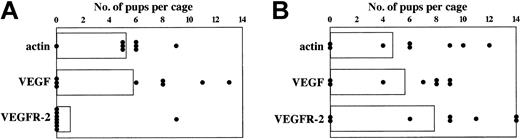
In 2 previous studies mice mated 10 days following immunization with VEGFR-2 protein-loaded DCs failed to become pregnant,9 whereas mice immunized with an attenuated Salmonella vector encoding a VEGFR-2 cDNA exhibited a slight delay in wound healing but no impact on fertility.10 In this study, despite reduced rate of angiogenesis seen in mice immunized against VEGFR-2 or VEGF no signs of morbidity or mortality were seen over an extended period of observation exceeding 6 months. However, a significant, albeit transient, impact on fertility of mice vaccinated against VEGFR-2, but not VEGF, was noted. As shown in Figure 6, mice vaccinated against VEGFR-2 and mated one week later failed to become pregnant, whereas if mating was delayed for 8 weeks the VEGFR-2–immunized mice were fertile, with litter sizes and average weights of offspring comparable with nonimmunized mice. These observations suggest that vaccination against angiogenesis-associated products can have transient adverse effects, presumably reflecting the limited persistence of an active antivascular immune response. The reason for the differential effects of anti-VEGF and –VEGFR-2 immunization on fertility (Figure 6A), despite a comparable inhibitory effect on angiogenesis (Figure 2), is unclear and will require additional studies. If confirmed, it suggests that in the setting of immunotherapy angiogenesis-associated products/antigens will exhibit a differential toxicity profile and that it may be possible to identify angiogenic targets, which exhibit significant antitumor activity, yet low toxicity.
Transient inhibition of fertility in mice immunized with VEGFR-2 mRNA–transfected DCs. Mice were immunized with DCs electroporated with actin or VEGF or VEGFR-2 mRNA 3 times at weekly intervals as described in Figure 3. One week (A) and 8 weeks (B) after the final immunization, mice were mated with nonimmunized male mice. This was done in triads (2 females to 1 male per cage). Number of pups delivered by each female mouse was recorded by the date of birth, and the pups were examined for signs of sickness and abnormality and their weight after weaning was recorded. Data shown represent pups born per cage (2 female mice per cage) over a period of 4 months. (A) Relative to the control actin immunized group, P values were .65 and .03 for DC + VEGF mRNA and DC + VEGFR-2 mRNA, respectively. There were no significant differences in the weights of the pups born. Average weight of 5 pups for the DC + actin mRNA group was 75 to 95 g, DC + VEGF mRNA group was 79 to 92 g, and DC + VEGFR-2 mRNA group was 100 g. (B) Relative to the control actin immunized group, P values were .88 and .44 for DC + VEGF mRNA and DC + VEGFR-2 mRNA, respectively. Average weight of 5 pups for the DC + actin mRNA group was 75 to 95 g, DC + VEGF mRNA group was 85 to 92 g, and DC + VEGFR-2 mRNA group was 85 to 100 g. Columns represent average number of pups per cage; each circle represents number of pups per cage.
Transient inhibition of fertility in mice immunized with VEGFR-2 mRNA–transfected DCs. Mice were immunized with DCs electroporated with actin or VEGF or VEGFR-2 mRNA 3 times at weekly intervals as described in Figure 3. One week (A) and 8 weeks (B) after the final immunization, mice were mated with nonimmunized male mice. This was done in triads (2 females to 1 male per cage). Number of pups delivered by each female mouse was recorded by the date of birth, and the pups were examined for signs of sickness and abnormality and their weight after weaning was recorded. Data shown represent pups born per cage (2 female mice per cage) over a period of 4 months. (A) Relative to the control actin immunized group, P values were .65 and .03 for DC + VEGF mRNA and DC + VEGFR-2 mRNA, respectively. There were no significant differences in the weights of the pups born. Average weight of 5 pups for the DC + actin mRNA group was 75 to 95 g, DC + VEGF mRNA group was 79 to 92 g, and DC + VEGFR-2 mRNA group was 100 g. (B) Relative to the control actin immunized group, P values were .88 and .44 for DC + VEGF mRNA and DC + VEGFR-2 mRNA, respectively. Average weight of 5 pups for the DC + actin mRNA group was 75 to 95 g, DC + VEGF mRNA group was 85 to 92 g, and DC + VEGFR-2 mRNA group was 85 to 100 g. Columns represent average number of pups per cage; each circle represents number of pups per cage.
Discussion
This study tested the hypothesis that combination of antiangiogenic therapy and tumor immunotherapy of cancer is synergistic. Recent studies have shown that immunizing mice with xenogeneic endothelial cells8 or against VEGFR-29,10 can stimulate antitumor immunity by interfering with the tumor vascularization process. This study has confirmed and extended these observations by targeting 3 angiogenesis-associated products, VEGFR-2 and Tie2, which are expressed in proliferating endothelial cells, and VEGF, which is expressed in the angiogenic stroma. Since VEGF is an essential angiogenic growth factor, which is also expressed by many tumor cells, including the tumor cells used in this study, immunizing against VEGF could target both the tumor and its vasculature and conceivably lead to an improved antitumor effect. Inhibition of angiogenesis by active immunotherapy to control tumor growth offers several attractive features: (1) Active immunotherapy can induce a prolonged state of reduced angiogenic activity decreasing the frequency of treatments, albeit increasing the risk of interfering with normal angiogenesis. (2) Immunotherapy, like other antiangiogenic strategies, provides multiple common targets, “universal” antigens, to inhibit tumor angiogenesis. (3) Due to the genetically stable nature and limited proliferative capacity of endothelial and stromal cells, emergence of antigen-loss or antigen processing–loss variants should be significantly reduced compared with that of tumor cells.47 (4) As will be discussed, an especially attractive feature of antiangiogenic immunotherapy is that it can be combined with tumor immunotherapy to deliver 2 distinct and potentially synergistic treatment modalities using a common procedure, immunization.
Immunization with mRNA-transfected DCs is emerging as an efficient strategy to stimulate cellular immunity,15 and this study extends the use of this approach to angiogenesis-associated targets. A particularly useful feature of using mRNA-encoded antigens is the ease of isolating and generating mRNAs. cDNAs can be isolated from cells expressing the desired antigen by simple RT-PCR techniques, and mRNA can be generated in pure form and large quantities using cell-free enzymatic reactions (“Materials and methods”). For example, this study has used the mRNA technology to study 3 angiogenic targets, VEGFR-2, Tie2, and VEGF, and the list can be readily expanded with candidates provided by the genomic revolution. Lastly, for clinical investigations, the generation of mRNA-encoded antigens is comparatively simple and inexpensive, and the regulatory requirements are straightforward.
Induction of CTL responses against VEGF and VEGFR-2 shows that it is possible to break tolerance against angiogenesis-associated targets (Figure 1), leading to reduced angiogenic activity in the immunized animals (Figure 2). Immunization against the angiogenesis-associated products VEGFR-2, Tie2, or VEGF was accompanied by inhibition of tumor growth in the B16/F10.9 melanoma metastasis and the MBT-2 bladder cancer models. Tumor inhibition was seen when mice were immunized before tumor challenge (Figures 3-4) or in the setting of pre-existing tumor burden (Figure 5). Since VEGFR-2 or Tie2 is expressed in proliferating endothelial cells,25 but not in MBT-2 or B16/F10.9 tumor cells, the observed tumor inhibition was an indirect consequence of interfering with the tumor neovascularization process. This conclusion is also consistent with the observation that immunization against VEGFR-2 is accompanied by a reduced state of angiogenesis in the immunized animal (Figure 2). Unlike VEGFR-2 or Tie2, VEGF is expressed by stromal cells and tumor cells, including the B16/F10.9 and MBT-2 tumor cells used in this study. It is therefore not clear whether the antitumor effect seen by immunizing against VEGF was mediated via inhibition of angiogenesis or direct antitumor immunity. It is not inconceivable that both mechanisms have contributed to tumor inhibition, in which case antigens belonging to this group may be particularly useful.
The experiments shown in Figures 4 and 5 establish the potential value of combining antiangiogenic therapy and tumor immunotherapy. Immunization with syngeneic tumor RNA (B16/F10.9 or MBT-2) stimulates tumor-specific non–cross-reactive protective immunity and thereby targets the tumor directly, whereas immunization with VEGFR-2 or Tie2 mRNA targets the tumor vascularization process. As shown in Figure 4, mice immunized with both syngeneic tumor RNA and endothelial-specific mRNA (VEGFR-2 or Tie2) exhibited a superior antitumor effect compared with mice immunized with either RNA alone. Furthermore, in the setting of pre-existing disease, under conditions that immunization with TERT, TRP-2, or VEGFR-2 had small or negligible effects, coimmunization against tumor (TERT or TRP-2) and angiogenesis-specific (VEGFR-2) targets exhibited a more pronounced inhibitory effect on tumor growth (Figure 5). These experiments also illustrate another key feature of inhibiting angiogenesis via active immunotherapy, namely the ability to deliver 2 compatible and synergistic cancer treatment modalities by a single protocol, immunization. Combination immunotherapy against VEGF and either TERT (Figure 4B), VEGFR-2 (Figure 5A), or TRP-2 (Figure 5B,D) was also synergistic, underscoring the value of targeting 2 defined and broadly expressed (“universal”) antigens. Although, in this instance it was not clear whether the contribution of VEGF was inhibition of angiogenesis, direct antitumor immunity, or a combination of both.
A primary concern of immunizing against angiogenesis-associated products is interference with normal angiogenesis, especially if the effect is sustained. No significant adverse effects were seen in mice immunized against angiogenesis-associated products in this and previous studies under conditions that significant antitumor effects were seen. In this study, no signs of morbidity or mortality were seen in the immunized animals except for a transient impairment of fertility in mice immunized against VEGFR-2, but not VEGF (Figure 6). These observations are consistent with previous studies that have shown that antiangiogenic therapy exhibits differential susceptibility on tumor growth and wound healing,48,49 suggesting that a partial and transient reduction in angiogenic activity could suffice to impact on tumor growth without eliciting serious adverse effects. Furthermore, since functional immunologic memory will require repeated immunizations,50,51 the persistence of an active antiangiogenic immune response can be controlled simply by terminating vaccination. Notwithstanding these and previous observations,9,10 the potential adverse effects of antiangiogenic immunotherapy have to be carefully considered in future experiments.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2002-12-3738.
Supported by grants 1RO1 CA 85307 and 1RO1 CA 89536 from the National Institutes of Health and the National Cancer Institute.
One of the authors (E.G.) has declared a financial interest in a company (Merix Bioscience) whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Catherine McLaughlin for generating murine bone marrow precursor–derived DCs.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal