Abstract
Establishment of an assay capable of generating all classes of human lymphocytes from hematopoietic stem cells (HSCs) will provide new insight into the mechanism of human lymphopoiesis. We report ontogenic, functional, and histologic examination results of reconstituted human lymphocytes in NOD/SCID/ γcnull mice after the transplantation of human cord blood (CB) CD34+ cells. After transplantation, human B, natural killer (NK), and T cells were invariably identified in these mice, even though no human tissues were cotransplanted. Immature B cells resided mainly in bone marrow (BM), whereas mature B cells with surface immunoglobulins were preferentially found in spleen. NK cells were identified in BM and spleen. T cells were observed in various lymphoid organs, but serial examinations after transplantation confirmed human T lymphopoiesis occurring in the thymus. These human lymphocytes were also functionally competent. Human immunoglobulin M (IgM), IgA, and IgG were detected in the sera of these mice. T cells showed a diverse repertoire of T-cell–receptor Vβ (TCR Vβ) chains, proliferated in response to phytohemagglutinin, and were cytotoxic against cell lines. NK activity was demonstrated using the K562 cell line. Immunohistochemical analysis revealed that human lymphocytes formed organized structures in spleen and thymus that were analogous to those seen in humans. In the thymus, CD4 and CD8 double-positive T cells were predominant and coexpressed CD1a and Ki-67, thereby supporting the notion that T lymphopoiesis was taking place. NOD/SCID/ γcnull mice provide a unique model to investigate human lymphopoiesis without the cotransplantation of human tissues.
Introduction
Reconstitution of functional human lymphocytes in experimental animals has been intensely explored because this approach could provide a valuable means of assessing human immunity or of preclinical testing of vaccines, pathogens, and new therapeutic strategies. In 1988, the first reports on engraftment of human hematopoietic cells in homozygous severe combined immunodeficiency disease (scid) mice were published.1,2 McCune et al1 reported that the simultaneous transplantation of fetal liver hematopoietic cells, fetal thymus, and fetal lymph node resulted in the differentiation of mature human T and B cells (SCID-hu mice model). Mosier et al2 reported that the transplantation of human peripheral blood (PB) mononuclear cells into scid mice resulted in the successful transfer of a functional human immune system (hu-PBL-SCID mice model). These initial models were soon followed by experimental human bone marrow (BM) transplantation into scid mice models,3-9 with modification—the cotransplantation of other human tissues, such as bone, or treating recipient mice with a combination of human cytokines. These modified models showed the multilineage differentiation of human hematopoietic cells to varying degrees. In some experimental settings, SCID-hu mice generated all classes of human immunoglobulins, thereby indicating the presence of human lymphocytes that interacted in response to environmental antigens and that induced B cells to undergo immunoglobulin switching. Although the use of these systems is feasible, they require various kinds of fetal human tissues or human cytokines. Moreover, the procedure is laborious, and engraftment of human hematopoietic cells is highly variable.
To overcome these problems, systematic approaches have been explored. One major approach involves suppressing the innate immunity of scid mice, based on the hypothesis that an efficient engraftment level can be achieved by eliminating residual innate immunity in the host. The NOD/SCID mouse strain was found to be an efficient recipient for the reconstitution of human hematopoietic cells.10-13 However, complete multilineage differentiation, including T cells, has not been achieved using this strain. To suppress residual natural killer (NK) activity in recipient mice, the β2 microglobulin-null (β2m-/-) allele was backcrossed onto the NOD/LtSz-scid background, and a new strain of NOD/SCID-β2mnull mice was established. In these mice there was an efficient engraftment for human hematopoietic stem cells (HSCs),14,15 but human T cells were not detected. To improve engraftment efficiency, we established a strain of NOD/Shi-scid mice that have a defective common cytokine receptor, γc (NOD/SCID/γcnull).16 Mutation in the common cytokine receptor γ chain led to a life-threatening, X-linked, severe combined immunodeficiency disease (XSCID) in humans, characterized by an extremely low number of T and NK cells.17,18 We observed that NOD/SCID/γcnull mice had no lymphocytes, no NK activity, and impaired dendritic cell function, thought to lead to high engraftment efficiency and full-lineage differentiation, including those of T cells.16 Following reports of the generation of functional T cells in these mice,19 we describe here the results of a comprehensive analysis of human T, B, and NK cells generated in NOD/SCID/γcnull mice, with regard to developmental process and functional maturation. Human T cells developed in the thymi of these mice, moved to the periphery, and functionally matured to produce cytokines and to have cytotoxicity. Human B cells matured to produce not only human IgM but also IgG and IgA. As in T cells, NK cells matured and had cytotoxicity against K562 cells. Histologic examinations revealed the formation of organized structures of lymphoid organs. This new mouse model is expected to pave the way toward the reconstitution of a human immune system within the body of a laboratory animal.
Materials and methods
Mice
NOD/Shi-scid, NOD/SCIDβ2mnull, and NOD/SCID/γcnull mice were used in this study. The NOD/Shi-scid strain was established, as reported.20 NOD/SCID-β2mnull mice were purchased from The Jackson Laboratory (Bar Harbor, ME). NOD/SCID/γcnull mice were developed at the Central Institute of Experimental Animals (Kawasaki, Japan) by crossing NOD/Shi-scid mice with C57B6/J-IL-2Rγnull mice. All mice were bred as a homozygous line, shipped to the animal facility of Kyoto University (Kyoto, Japan), and kept under specific pathogen-free conditions in accordance with the guidelines of the facility.
Purification of cell populations
Human CB was collected during normal full-term deliveries, after obtaining informed consent. Mononuclear cells (MNCs) were separated by Ficoll-Hypaque density gradient centrifugation after the depletion of phagocytes with Silica (Immuno Biological Laboratories, Fujioka, Japan). CD34+ cell fractions were isolated using AutoMACS (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer's protocol. Purity was evaluated using flow cytometry. By using Silica and AutoMACS with the most sensitive “Possel d2” protocol, no less than 95% (typically 98%) of the positively selected cells were CD34+. In experiments using lineage-depleted CD34+ cells (Lin- CD34+), CB MNCs were depleted of lineage-positive cells using StemSep (Stem Cell Technologies, Vancouver, BC, Canada) followed by CD34+ selection with AutoMACS. StemSep contains a cocktail of monoclonal antibodies of antihuman CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, and antihuman glycophorin A. After combined cell selection with StemSep and AutoMACS, almost all the lineage-positive cell fraction was removed. Depletion of CD56+ cells was carried out with AutoMACS and CD56 microbeads (Miltenyi) according to manufacturer's protocol. By choosing the “deplete 025” protocol, almost all CD56+ cells were successfully eliminated.
Transplantation of CB CD34+ cells into mice
Xenotransplantation of purified CB CD34+ cells was performed using a modification of a previously reported method.11,12 Briefly, 8- to 12-weekold NOD/Shi-scid, NOD/SCID-β2mnull, and NOD/SCID/γcnull mice received 240 cGy radiation. The indicated dose of CB CD34+ cells was injected through the tail vein. Only NOD/Shi-scid mice were treated with 400 μL phosphate-buffered saline (PBS) containing 20 μL antiasialo GM1 antiserum (Wako, Osaka, Japan) shortly before cell transplantation and every 11th day thereafter. After transplantation, mice were prophylactically provided sterile water with added neomycin sulfate (Gibco BRL, Grand Island, NY).
Flow cytometric analysis of mice with transplanted human cells
PB was taken from the retro-orbital venous plexus at the indicated times after transplantation. Blood was collected through heparinized calibrated pipettes and transferred to EDTA (ethylenediaminetetraacetic acid)–2 Na containing CAPIJECT (Terumo Medical, Somerset, NJ). Lineage analysis of human hematopoietic cells was made using flow cytometry (FACScalibur; BD PharMingen, San Diego, CA) according to the manufacturer's protocol. Mice were killed by cervical dislocation more than 5 months after cell transplantation. BM, spleens, and thymi were analyzed using flow cytometry. Antibodies used for flow cytometric analysis were antihuman CD45-fluorescein isothiocyanate (CD45-FITC), CD3-FITC, TdT-FITC, CD34-phycoerythrin (CD34-PE), CD10-PE, CD3-PE, T-cell receptor (TCR) γδ-PE, CD158a-PE, KIR p70-PE, antihuman surface IgD-PE, IgM-PE, IgG-PE, IgA-PE, CD8-phycoerythrin 5-succinimidylester (PC5), CD19-PC5, TCR αβ-PC5, and antimouse CD45-allophycocyanin (CD45-APC). Antibodies conjugated with FITC, PE, or APC were purchased from BD PharMingen except anti–CD158a-PE and anti–KIR p70-PE. Antibodies conjugated with PC5, anti-CD158a-PE, and anti-KIR p70-PE were purchased from Immunotech (Marseilles, France). The Vβ repertoire of TCR was analyzed using IOTest Beta Mark TCRVβ Repertoire kits (Immunotech).
Reverse transcription–polymerase chain reaction
RNA was isolated from the spleen and BM of NOD/SCID/γcnull mice with or without transplanted CD34+ cell using TRIzol Reagent (Invitrogen, Carlsbad, CA). Total RNA (1 μg) was taken from each sample and subjected to reverse transcription using SuperScript (Invitrogen). Using one-twentieth synthesized cDNA, PCR amplification was carried out with Takara LA Taq (Takara Bio, Ohtsu, Japan). Samples were denatured at 94°C for 5 minutes, then amplified by rounds consisting of 94°C for 30 seconds (denaturing), 60°C for 30 seconds (annealing), and 72°C for 30 seconds (extension) for 31 cycles using primer sets as follows: interleukin-2 (IL-2), 5′-CTTCAGTGTCTAGAAGAAGAACTCAA-3′ and 5′-GGTAAACCATTTTAGAGCCCC-3′; IL-15, 5′-CTGACTCTCAGTTCAGTTTTACTCT-3′ and 5′-TCTAAGCAGCAGAGTGATGTTTG-3′; human hypoxanthine phosphoribosyltransferase (HPRT) 5′-AATTATGGACAGGACTGAACGTC-3′ and 5′-CGTGGGGTCCTTTTCACCAGCAAG-3′; mouse HPRT 5′-GCTGGTGAAAAGGACCTCT-3′ and 5′-CACAGGACTAGAACACCTGC-3′. PCR products were separated on 2.0% agarose gel, stained with ethidium bromide, and photographed.
Functional analysis of human lymphocytes generated in NOD/SCID/γcnull mice
Anti–CD3-dependent cytotoxic T lymphocyte (CTL) activity was tested using a calcine release assay, with minor modification.21 Briefly, human MNCs in the spleen of a mouse that underwent cell transplantation were separated by Ficoll-Hypaque density gradient centrifugation. MNCs were cultured at 1 × 106/mL in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) with phytohemagglutinin (PHA; 1 μg/mL) and human IL-2 (50 IU/mL) for 48 hours. After taking the supernatant to analyze human cytokine production, culture was continued without PHA for 8 additional days. Expanded lymphocytes were suspended in Hanks balanced salt solution supplemented with 5% FBS. Target cells labeled with calcine-AM (Molecular Probes, Eugene, OR) were mixed with T blasts at the indicated effector-target (E/T) ratios in the presence of anti-CD3 monoclonal antibody (mAb). Cells with isotype-control mAb or without mAb were also prepared as negative controls. After 3 hours of incubation, the release of calcine into the supernatant was measured with an automated fluorescence scanner (Wallac 1420ARVOsx; Perkin Elmer, Fremont, CA). NK cell activity was measured in a similar way. In analyzing NK cell activity, expanded lymphocytes were mixed with calcine-labeled K562 cells without antibodies. The CD56+ NK cell population comprised less than 10% expanded lymphocytes. To measure the total release of calcine, 100 μL lysis buffer (50 mM sodium-borate, 0.1% Triton X-100, pH 9.0) was added to the wells with only labeled target cells after aliquots of supernatant had been taken to measure spontaneous release. Percentage cytotoxicity was determined using the following equation: (F CTL assay - F spontaneous release)/(F total lysis - F spontaneous release) × 100 = % cytotoxicity, where F represents fluorescence. All assays were performed in quadruplicate, and PB MNCs of a healthy adult donor served as a positive control. Human cytokine analysis was made using the Cytometric Bead Array Kit for human cytokines according to the manufacturer's protocol (BD PharMingen). Intracellular cytokine staining was performed as follows: PHA-stimulated splenocytes were further stimulated with 10 ng/mL phorbol ester (PMA; Sigma, St Louis, MO) and 1 μg/mL Ca2+ ionophore (ionomycin; Sigma) for 6 hours. Brefeldin A (Sigma) was added at final concentration of 10 μg/mL during the last 2 hours. Cells were washed with PBS with 2% FCS and stained with antihuman CD56-PC5 (Immunotech). Then the cells were washed with PBS with 2% FCS, fixed, and permeabilized with Cytofix/Cytoperm (BD PharMingen) for 20 minutes. After washing with Perm/Wash (BD PharMingen) twice, the cells were incubated with FITC- or PE-conjugated isotype control mAb (BD PharMingen), anti–IFN-γ (BD PharMingen), anti–IL-4 (BD PharMingen), and anti–tumor necrosis factor-α (anti–TNF-α) (Immunotech) for 30 minutes at 4°C. After washing with Perm/Wash, the cells were analyzed by flow cytometry.
Immunoglobulin analysis
Human IgA, IgM, and IgG in plasma of NOD/SCID/γcnull or NOD/SCID-β2mnull mice were measured using human immunoglobulin assay kits (Bethyl, Montgomery, TX). Cross-reactivity against mouse IgG, IgA, and IgM checked with mouse serum standard (Bethyl) were 0.1% for IgM and less than 0.01% for IgG and IgM.
Immunohistochemical and immunofluorescence analysis
Frozen blocks were cut at 4 to 6 μm, and, after air-drying, sections were fixed in cold acetone. After blocking with 0.5% casein and 5% goat serum, the following primary antibodies were incubated for 1 hour at room temperature: mouse antihuman CD3 (UCHT-1), mouse antihuman CD20-EPOS (2L6), mouse antihuman CD1a (BL6), and mouse antihuman Ki-67 (MIB-1). An appropriate secondary antibody was incubated for 30 minutes, and either DAB or VECTOR Blue (Vector Laboratories, Burlingame, CA) was used for visualization. For confocal microscopy, each tissue was stained sequentially with mouse antihuman CD4 (NU-TH/I) followed by a Cy3-labeled F(ab′)2 fragment of donkey antimouse IgG and with biotinylated mouse antihuman CD8 (HIT8α) followed by streptavidin Alexa 488.
Statistical analysis
Data are presented as the mean ± SEM. Statistical significance was determined using the Mann-Whitney U test. P < .05.
Results
Efficient engraftment and full lineage lymphoid differentiation from CB CD34+ cells in NOD/SCID/γcnull mice
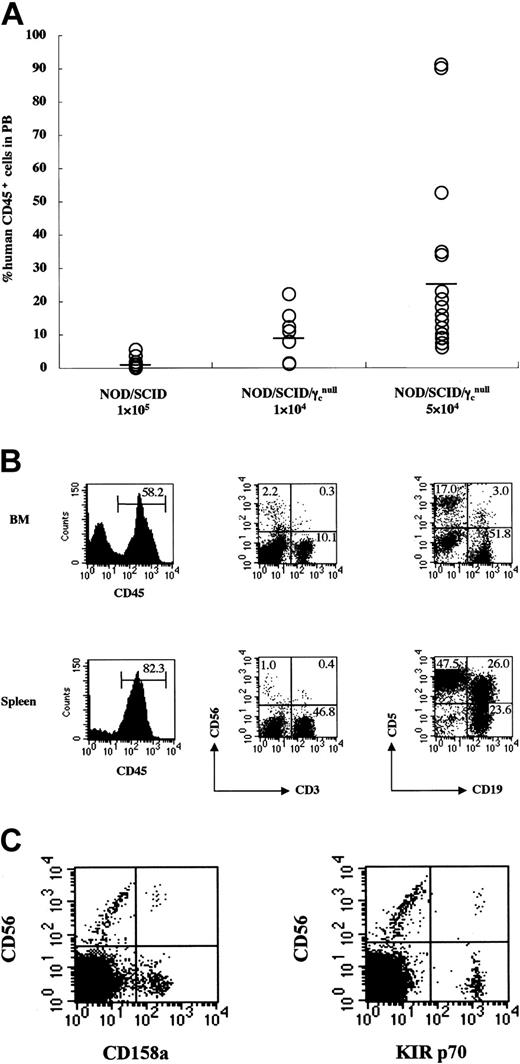
To evaluate the efficiency of engraftment of human hematopoietic cells in NOD/SCID/γcnull mice, 1 × 104 or 5 × 104 CB CD34+ cells were transplanted into NOD/SCID/γcnull mice, and 1 × 105 CB CD34+ cells were transplanted into NOD/Shi-scid (NOD/ SCID) mice; the engrafted human hematopoietic cells were then analyzed using flow cytometry. Three months after transplantation, the percentage of human CD45+ cells in the peripheral blood was much higher in NOD/SCID/γcnull mice, even though the transplanted cell dose was only one half or one tenth that for NOD/SCID mice (Figure 1A). Four months after transplantation of 1 × 105 CB CD34+ cells, the percentage of human CD45+ cells in the BM of NOD/SCID/γcnull mice was much higher than that in NOD/SCID (72.6% ± 6.3% vs 10.6% ± 4.4%; n = 8; P < .01).16
Efficient engraftment and complete reconstitution of human lymphocytes in NOD/SCID/γcnull mice that underwent transplantation with CB CD34+ cells. 1 × 104 (n = 7) or 5 × 104 (n = 17) CB CD34+ cells were transplanted into NOD/SCID/γcnull mice, and 1 × 105 (n = 15) CB CD34+ cells were transplanted into NOD/SCID mice. (A) Percentages of human CD45+cells in PB of mice were analyzed 3 months after transplantation using flow cytometry. Percentages of human CD45+cells were calculated as: % human CD45+ = human CD45+cells/(human CD45+cells +mouse CD45+cells) × 100. Results of 7 independent experiments are shown. (B) Representative FACS analysis of human lymphocytes in BM and spleen of NOD/SCID/γcnull mice that underwent transplantation with CB CD34+ cells. (C) Representative FACS analysis of KIR antigens is shown. CD158a or KIR p70 were detected on 7.7% or 3.8% of CD56+cells, respectively.
Efficient engraftment and complete reconstitution of human lymphocytes in NOD/SCID/γcnull mice that underwent transplantation with CB CD34+ cells. 1 × 104 (n = 7) or 5 × 104 (n = 17) CB CD34+ cells were transplanted into NOD/SCID/γcnull mice, and 1 × 105 (n = 15) CB CD34+ cells were transplanted into NOD/SCID mice. (A) Percentages of human CD45+cells in PB of mice were analyzed 3 months after transplantation using flow cytometry. Percentages of human CD45+cells were calculated as: % human CD45+ = human CD45+cells/(human CD45+cells +mouse CD45+cells) × 100. Results of 7 independent experiments are shown. (B) Representative FACS analysis of human lymphocytes in BM and spleen of NOD/SCID/γcnull mice that underwent transplantation with CB CD34+ cells. (C) Representative FACS analysis of KIR antigens is shown. CD158a or KIR p70 were detected on 7.7% or 3.8% of CD56+cells, respectively.
Analysis of BM and spleen revealed that the NOD/SCID/γcnull mice carried all classes of human lymphocytes—human CD3+ T cells, CD3-CD56+ NK cells, and CD19+ B cells. CD5+ B cells were considered to constitute an ontogenically unique subpopulation (Figure 1B). Expression of killer inhibitory receptor (KIR) antigens, such as CD158a and KIR p70, was also identified on a population of CD56+ NK cells (Figure 1C). Human B cells and NK cells were detected in NOD/SCID mice, but the percentage of these cells was low and human CD3+ T cells were never identified.
Flow cytometric analysis of human T cells in NOD/SCID/γcnull mice after transplantation of CB CD34+ cells
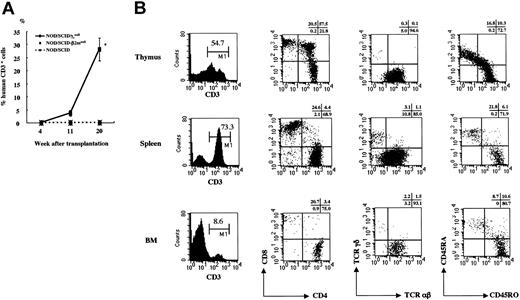
Next, we focused on human T cells in the NOD/SCID/γcnull mice. After the transplantation of 4 × 104 CB CD34+ cells into NOD/ SCID/γcnull, NOD/SCID-β2mnull, and NOD/SCID mice, human CD3+ T cells in PB were measured sequentially using flow cytometry. Human T cells were identified only in NOD/SCID/γcnull mice, and the percentage of T cells was gradually increased with time (Figure 2A).
Human CD3+cells in PB of NOD/SCID/γcnull, NOD/SCID-β2mnull, and NOD/SCID mice that underwent transplantation with CB CD34+cells. (A) Percentage of human CD3+cells among human CD45+cells in PB was analyzed sequentially after the transplantation of 4 × 104 CB CD34+cells. Results of 2 independent experiments (n = 3 each) are shown. Error bars represent SDs. (B) Surface phenotypes of human CD3+T cells in thymus, spleen, and BM of NOD/SCID/γcnull mice were evaluated 5 months after the transplantation of 2 to 4 × 104 CB CD34+cells. Representative data from 5 independent analyses of similar results are shown.
Human CD3+cells in PB of NOD/SCID/γcnull, NOD/SCID-β2mnull, and NOD/SCID mice that underwent transplantation with CB CD34+cells. (A) Percentage of human CD3+cells among human CD45+cells in PB was analyzed sequentially after the transplantation of 4 × 104 CB CD34+cells. Results of 2 independent experiments (n = 3 each) are shown. Error bars represent SDs. (B) Surface phenotypes of human CD3+T cells in thymus, spleen, and BM of NOD/SCID/γcnull mice were evaluated 5 months after the transplantation of 2 to 4 × 104 CB CD34+cells. Representative data from 5 independent analyses of similar results are shown.
The surface phenotype of human T cells in the thymus, spleen, and BM was analyzed 5 months after transplantation using flow cytometry (Figure 2B). The thymi were always highly atrophic at this time, and approximately 2 to 5 × 105 human nucleated cells were identified.
Two subpopulations of CD3+ T cells, CD3high and CD3low, were identified in the thymus, but almost all the T cells in the spleen and BM had the CD3high phenotype. Simultaneous staining of CD4 and CD8 revealed that CD4+CD8+ double-positive (DP) T cells were predominant in the thymus, whereas CD4+ or CD8+ single-positive (SP) T cells were predominant in the spleen and BM. Analysis of the TCR revealed that almost all T cells had αβ TCR in the thymus but that T cells with γδ TCR were also detected in the spleen and BM. Considerable numbers of naive T cells of the CD3+CD45RA+ phenotype were identified in all organs analyzed. NOD/SCID/γcnull mice with transplanted CB CD34+ cells showed little clinical evidence of graft-versus-host disease (GVHD), whereas transplantation of PB CD3+ cells evoked GVHD responses even shortly after the procedure (data not shown). Transplantation of CB CD3+CD45RA+ cells did not result in the maintenance of human T cells for more than 3 months (data not shown). These results are highly suggestive of the de novo generation of human T cells from HSCs in these mice.
Development of human T cells in the thymi of NOD/SCID/γcnull mice
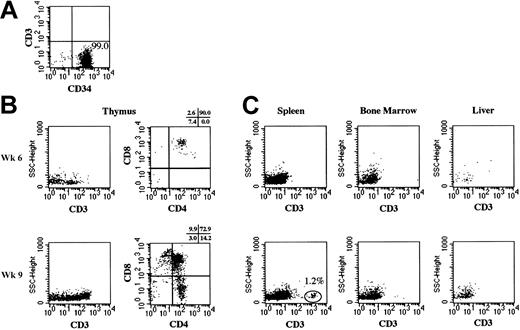
To confirm the de novo generation of human T cells from HSCs and to clarify the site(s) responsible, we transplanted further purified lineage-depleted (Lin-) CB CD34+ cells into NOD/SCID/γcnull mice. The purity of transplanted Lin- CD34+ was greater than 98%, and contamination of CD3+ cells was not identified by flow cytometry (Figure 3A). After the transplantation of 5 × 104 Lin- CD34+ cells, various organs were analyzed through flow cytometry (Figure 3B-C).
Human T cells in various organs of NOD/SCID/γcnull mice that underwent transplantation with CB Lin- CD34+cells. Human CD3+cells in thymus, spleen, bone marrow, and liver were analyzed using flow cytometry at 6 and 9 weeks after the transplantation of 5 × 104 CB Lin- CD34+cells. (A) The purity of Lin- CD34+cells was evaluated using flow cytometry. (B) Expressions of human CD3, CD4, and CD8 on human CD45+cells in thymus are shown. (C) Expressions of human CD3 on human CD45+cells in spleen, bone marrow, and liver are shown.
Human T cells in various organs of NOD/SCID/γcnull mice that underwent transplantation with CB Lin- CD34+cells. Human CD3+cells in thymus, spleen, bone marrow, and liver were analyzed using flow cytometry at 6 and 9 weeks after the transplantation of 5 × 104 CB Lin- CD34+cells. (A) The purity of Lin- CD34+cells was evaluated using flow cytometry. (B) Expressions of human CD3, CD4, and CD8 on human CD45+cells in thymus are shown. (C) Expressions of human CD3 on human CD45+cells in spleen, bone marrow, and liver are shown.
After the transplantation of CB Lin- CD34+ or CB CD34+ cells, human T cells became detectable in PB at approximately the 8th week. Therefore, we conducted our analysis shortly before (6th week after transplantation) and after (9th week after transplantation) the emergence of human T cells. At the 6th week, T cells were identified in the thymus, yet spleen, BM, and liver had no T cells. T cells in the thymus consisted mainly of DP T cells with barely detectable levels of double-negative (DN) T cells. At the 9th week, the thymus was further engrafted with DP T cells, and the spleen began to show a small number of T cells with the SP phenotype. These results clearly indicated that de novo generation of human T cells occurred in the thymus of NOD/SCID/γcnull mice, followed by seeding of these cells in peripheral organs such as the spleen.
Human B-cell development and immunoglobulin production in NOD/SCID/γcnull mice
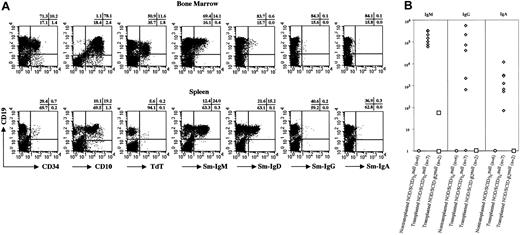
B-cell–specific markers of human hematopoietic cells in the BM and spleen of NOD/SCID/γcnull mice were evaluated using flow cytometry (Figure 4A). In the BM, phenotypically immature B cells were predominant. A large number of human CD19+ B cells simultaneously expressed human CD34 or CD10. Terminal deoxytransferase (TdT)+ B cells were also identified in BM and corresponded to premature pro-B cells. Human surface immunoglobulin expression showed immature B cells (IgM+) to be the major population. On the other hand, most human B cells in the spleen were mature. The expression of human CD34 or TdT was hardly detectable, and human surface IgM+ and IgD+ B cells were more abundant than in the BM. To determine whether these B cells were functionally mature, we measured plasma human immunoglobulin levels using enzyme-linked immunosorbent assay (ELISA) (Figure 4B).
B-cell development in NOD/SCID/γcnullmice that underwent transplantation with CB CD34+ cells. (A) Surface expressions of CD34, CD10, CD19, IgM, IgD, IgG, and IgA and intracellular expression of TdT were examined after setting a gate on the human CD45+population. (B) Human IgM, IgG, and IgA concentrations in plasma of NOD/SCID/γcnull and NOD/SCID-β2mnull mice that underwent transplantation with 4 × 104 CB CD34+ cells were measured by ELISA.
B-cell development in NOD/SCID/γcnullmice that underwent transplantation with CB CD34+ cells. (A) Surface expressions of CD34, CD10, CD19, IgM, IgD, IgG, and IgA and intracellular expression of TdT were examined after setting a gate on the human CD45+population. (B) Human IgM, IgG, and IgA concentrations in plasma of NOD/SCID/γcnull and NOD/SCID-β2mnull mice that underwent transplantation with 4 × 104 CB CD34+ cells were measured by ELISA.
All NOD/SCID/γcnull mice that underwent CB CD34+ cell transplantation produced not only human IgM but also IgG and IgA. For comparative purposes, NOD/SCID-β2mnull mice, which were reported to be better recipients for human HSCs,14 were also evaluated for the production of human immunoglobulin after transplantation with 4 × 104 CB CD34+ cells. Only one NOD/ SCID-β2mnull mouse had very low levels of IgM, and other types of immunoglobulin were not detected. Thus, the differentiation of B cells occurred mainly in the BM of NOD/SCID/γcnull mice then functionally maturated to produce human immunoglobulin with isotype switching from IgM to IgG and IgA.
Diverse Vβ repertoire of human T cells undergoing differentiation in NOD/SCID/γcnull mice
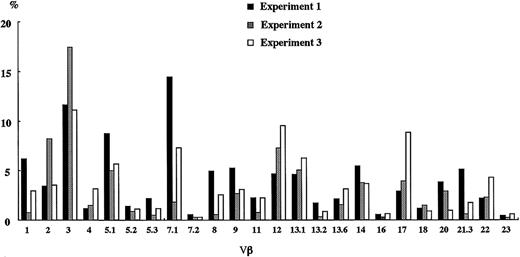
To determine whether the differentiation of human T cells in NOD/SCID/γcnull mice proceeded in a coordinated manner, recombination diversity of TCR was analyzed using flow cytometry. Five to 6 months after the transplantation of CB CD34+ cells, the TCR repertoire of human T cells in the spleen of NOD/SCID/γcnull mice was quantified using a panel of 24 different antibodies specific to each Vβ (Figure 5).
TCR Vβ repertoire analysis of human T cells in spleen. Four to 6 months after the transplantation of 2 × 104 to 5 × 104 CB CD34+cells, spleen cells were taken and the TCR Vβ repertoire was analyzed by flow cytometry using a panel of 24 different antibodies. The results of 3 independent experiments are shown.
TCR Vβ repertoire analysis of human T cells in spleen. Four to 6 months after the transplantation of 2 × 104 to 5 × 104 CB CD34+cells, spleen cells were taken and the TCR Vβ repertoire was analyzed by flow cytometry using a panel of 24 different antibodies. The results of 3 independent experiments are shown.
Surprisingly, a diverse T-cell repertoire was confirmed in each experiment. These results imply that in the NOD/SCID/γcnull mice with transplanted CB CD34+ cells, a tightly regulated program of sequential TCR gene expression occurred that altered the phenotypes of developing cells and finally promoted the generation of a diverse Vβ repertoire.
Functional maturation of human T cells and NK cells differentiated in NOD/SCID/γcnull mice
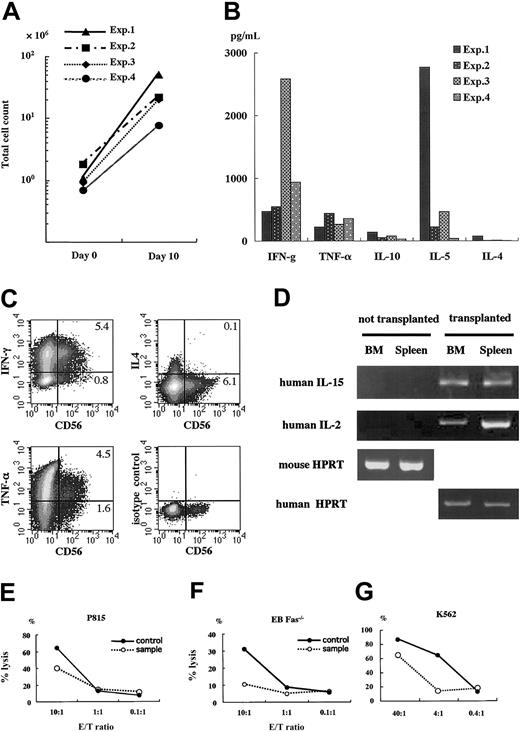
We further analyzed the functions of human T cells and NK cells developing in NOD/SCID/γcnull mice. Human mononuclear cells in spleens of mice that underwent transplantation were cultured in the presence of PHA and human IL-2 for 2 days, after which the supernatant was taken to assess human cytokine production. Cultures were continued without PHA for 8 additional days to yield enough cells to evaluate cytotoxicity. Human T cells in NOD/SCID/ γcnull mice responded to the stimulation of PHA and showed characteristics of T-cell blasts. The number of cells expanded more than 10-fold at day 10 (Figure 6A). These data suggest that the signals of PHA were transduced through the TCR of these T cells and resulted in cell expansion. Human interferon-γ (IFN-γ), TNF-α, IL-10, IL-5, and, though barely detectable, IL-4 was also identified in the supernatant of PHA-stimulated T cells (Figure 6B). To investigate the cytokine production of NK cells, we performed intracellular cytokine staining. Considerable populations of CD56+ NK cells produced IFN-γ or TNF-α, and IL-4 production was limited (Figure 6C). Concerning NK cell development, it has been reported that IL-2 and IL-15 are essential to the development and maintenance of NK cells.22,23 Therefore, we also examined the production of these cytokines by reverse transcription–polymerase chain reaction (RT-PCR) and identified them in the spleen and BM of NOD/SCID/γcnull mice with transplanted CD34+ (Figure 6D).
Functional analysis of human lymphocytes in spleen of NOD/SCID/γcnull mice. Four to 6 months after the transplantation of 2 × 104 to 5 × 104 CB CD34+cells, human lymphocytes in spleen were cultured and expanded for functional analyses. (A) Cell proliferation after 10-day culture; PHA (1 μg/mL) and hIL-2 (50 IU/mL) for the initial 48 hours followed by hIL-2 (50 IU/mL) only for 8 days. (B) Supernatants of the spleen cells after 48-hour stimulation with PHA and hIL-2 were taken, and the production of human cytokines was evaluated using Cytometric Bead Array Kit for human cytokine (BD PharMingen). (C) PHA-stimulated human lymphocytes were further stimulated with 10 ng/mL PMA and 1 μg/mL ionomycin for 6 hours. Brefeldin A was added during the last 2 hours. Then the cells were stained with membrane CD56 and intracellular cytokines and were analyzed using flow cytometry. (D) RNA was isolated from the spleen and BM of NOD/SCID/γcnull mice with or without transplanted CD34+cells, and the expression of mRNA for human IL-2 and IL-15 was examined by RT-PCR. Human or mouse HPRT was used as a positive control. (E-G) Anti–CD3-dependent cytotoxic T-lymphocyte activity and NK activity were evaluated by measuring the release of calcine-AM into the supernatant after cytolysis of target cells labeled with calcine. Results are expressed as the percentage of specific lysis. PB MNCs of a healthy adult were used as a positive control. Representative data with similar results of 4 independent experiments are shown.
Functional analysis of human lymphocytes in spleen of NOD/SCID/γcnull mice. Four to 6 months after the transplantation of 2 × 104 to 5 × 104 CB CD34+cells, human lymphocytes in spleen were cultured and expanded for functional analyses. (A) Cell proliferation after 10-day culture; PHA (1 μg/mL) and hIL-2 (50 IU/mL) for the initial 48 hours followed by hIL-2 (50 IU/mL) only for 8 days. (B) Supernatants of the spleen cells after 48-hour stimulation with PHA and hIL-2 were taken, and the production of human cytokines was evaluated using Cytometric Bead Array Kit for human cytokine (BD PharMingen). (C) PHA-stimulated human lymphocytes were further stimulated with 10 ng/mL PMA and 1 μg/mL ionomycin for 6 hours. Brefeldin A was added during the last 2 hours. Then the cells were stained with membrane CD56 and intracellular cytokines and were analyzed using flow cytometry. (D) RNA was isolated from the spleen and BM of NOD/SCID/γcnull mice with or without transplanted CD34+cells, and the expression of mRNA for human IL-2 and IL-15 was examined by RT-PCR. Human or mouse HPRT was used as a positive control. (E-G) Anti–CD3-dependent cytotoxic T-lymphocyte activity and NK activity were evaluated by measuring the release of calcine-AM into the supernatant after cytolysis of target cells labeled with calcine. Results are expressed as the percentage of specific lysis. PB MNCs of a healthy adult were used as a positive control. Representative data with similar results of 4 independent experiments are shown.
Further, these T cells exerted cell-mediated cytotoxicity. Fas + perforin-dependent or perforin-dependent cytotoxicity was evaluated using P815 cells or the Fas-deficient Epstein-Barr virus–transformed B-cell line (Fas-/- EB) as target cells, respectively. Fas + perforin-dependent cytotoxicity was comparable but a little lower than in a healthy adult human (Figure 6E). Cytotoxic activity dependent solely on the perforin-mediated pathway was lower than in control (Figure 6F). Although the value was not enough to be statistically significant, the percentage lysis of Fas-/- EB cells by samples always surpassed those of negative controls (typically less than 5% and never more than 10%), which are the ones with isotype control mAb or without antibody. Further investigation will be needed to clarify the pathway involved in the killing by these cells. Natural killer activity was evaluated using K562 cells, which are commonly used for the assessment of NK cell activity.24 As shown in Figure 6G, human mononuclear cells in the spleens of NOD/SCID/γcnull mice showed substantial levels of cytotoxicity against K562 cells, suggesting that human NK cells functionally matured. However, to exclude the possibility that coexisting other cells, such as T cells in the test sample, might contribute to K562 cell killing, we performed the same experiments with human CD56-depleted splenocytes as effector cells. Human CD56 depletion abrogated K562 cell killing almost completely (99.8% ± 0.03% inhibition; n = 3), which clearly demonstrated that the lysis of K562 cells was attributable to NK cells.
Histologic evaluation of reconstituted lymphoid organs
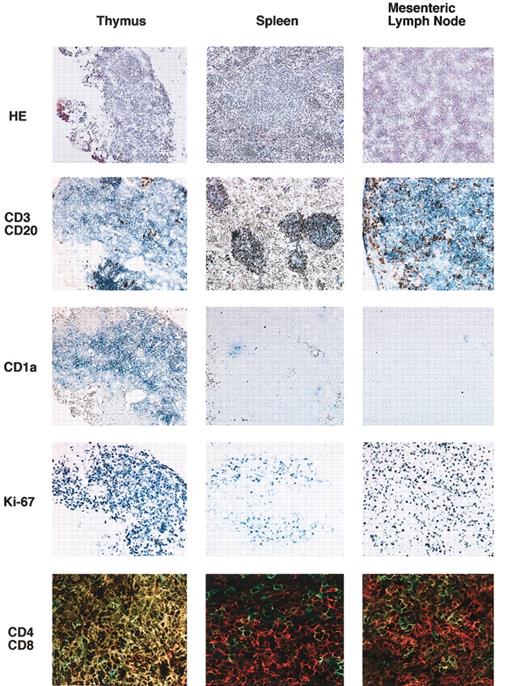
Lymphoid organs in NOD/SCID/γcnull mice that received transplanted human CD34+ CB cells were examined histologically 5 months after transplantation (Figure 7). Although the thymus of the recipient mouse was highly atrophic, human CD45+ cells were identified, usually as CD3+ T cells. These T cells were predominantly CD4+CD8+ DP T cells and were positive for human CD1a+, which is expressed on immature thymocytes. These cells were also positive for the cell-cycle–specific antigen Ki-67. Cytokeratin staining revealed the presence of an epithelial cell network inside the thymus, and these epithelial cells were positive for major histocompatibility complex class 2 (MHC class 2) of NOD mice (data not shown). These findings suggested that the immature human T cells that resided in the epithelial network were of mouse origin. In the spleen, a mononuclear cell-rich region similar to white pulp appeared after transplantation. This structure was never found in NOD/SCID/γcnull mice that did not undergo transplantation. Antihuman CD45 staining showed that the cell-rich region consisted mainly of human hematopoietic cells. Antihuman CD3 and CD20 double staining revealed that the structure consisted of human CD3+ cells surrounding a vessel and human CD20+ cells flanking the CD3+-cell–rich region. This structure resembled a periarteriolar lymphoid sheath (PALS) and primary follicles seen in humans. Mesenteric lymph nodes in NOD/SCID/γcnull mice with transplanted CB CD34+ cells also consisted mainly of human CD3+ cells. Double staining with CD3 and CD20 revealed that this tissue was densely engrafted with human T and B cells but devoid of typical follicular structures. These findings indicated that organized structures of the thymus, spleen, and mesenteric lymph nodes were reconstituted to a considerable extent by humanderived lymphocytes in NOD/SCIDγcnull mice.
Immunohistochemical staining of the thymus, spleen, and mesenteric lymph nodes. Six months after transplantation, various organs were taken, and frozen sections were prepared. Expressions of human CD3 (Vector Blue)/human CD20 (DAB), human CD1a (Vector Blue), human Ki-67 (Vector Blue), and human CD4 (Alexa 488)/human CD8 (Cy3) were evaluated. Original magnification is × 400 for CD4/CD8 images, × 100 for all others.
Immunohistochemical staining of the thymus, spleen, and mesenteric lymph nodes. Six months after transplantation, various organs were taken, and frozen sections were prepared. Expressions of human CD3 (Vector Blue)/human CD20 (DAB), human CD1a (Vector Blue), human Ki-67 (Vector Blue), and human CD4 (Alexa 488)/human CD8 (Cy3) were evaluated. Original magnification is × 400 for CD4/CD8 images, × 100 for all others.
Discussion
Development and functional maturation of human lymphocytes are achieved through complex networks in various organs, such as BM, thymus, spleen, and lymph node. Many attempts have been made to reconstitute a functional human immune system in mice with the scid phenotype but have resulted in limited success.1-3,25-27 We obtained evidence for the reconstitution of functional T cells, B cells, and NK cells from CB CD34+ cells in NOD/SCID/γcnull mice without the cotransplantation of human tissues. After the transplantation of CB CD34+ cells, all classes of human lymphocytes were invariably identified. T-cell development and maturation levels in these mice were similar to those in humans. The thymus contained immature T cells of DP and DN phenotypes, and the spleen was rich in mature SP T cells. CD3+CD45+ naive T cells were generated to an extent comparable to that in humans. The TCR of these T cells was predominantly αβ, but T cells with γδTCR were also observed in the spleen. Flow-cytometry–based quantification of TCR Vβ (TCRBV) of human CD3+ cells in the spleen showed a divergent repertoire, thus implying acquisition of the potential to respond to highly diverse molecules. Indeed, generated T cells proliferated with PHA stimulation and produced human cytokines. These T blasts showed cell-mediated cytotoxicity, thereby demonstrating the functional maturation of human T cells in a mouse environment. Like T cells, NK cells were mature enough to exert cytotoxicity against K562 cells. B-cell development and maturation levels were also comparable to those in humans. Immature B cells predominantly resided in the BM, and mature surface immunoglobulin-positive B cells were predominant in the spleen. Although human B-cell differentiation can be seen in NOD/SCID mice, only the NOD/SCID/γcnull mice model enabled human IgM, IgG, and IgA production in mouse serum, which means that the generated human T and B cells were functionally competent. These findings suggest that complicated developmental processes of T and B lymphocytes were reproduced in the NOD/SCID/ γcnull mice model.
In the SCID-hu mouse model, simultaneous implantation of fetal thymus and liver was indispensable for the generation of functionally mature human T cells.8 In the beige/nude/xid/human (bnx/hu) mice model, human T cells could develop from HSCs through the cotransplantation of genetically altered human BM stromal cells, which produced human cytokines.28 However, extrathymically developed T cells were functionally impaired. Systemic administration of human IL-7 restored function, but to a limited extent.9 Considering these findings, NOD/SCID/γcnull mice seemed to provide an excellent environment for human T-cell development. Contrary to previous models, the NOD/SCID/γcnull mice model did not require the cotransplantation of human tissue. The interesting question is whether these mice have unique properties for human lymphocyte development or a profound immunodeficiency that enables some kind of human tissue formation. Production of IL-2 and IL-15, which are essential for NK cell development, in these mice after transplantation may suggest that this model can reconstitute the environment required for lymphopoiesis. More detailed identification of engrafted human cells is under investigation. Organized clustering of lymphocytes in appropriate organs is required for the reconstitution of a functional immune system. In NOD/SCID/γcnull mice with transplanted human CB CD34+ cells, T- and B-cell clustering is seen in the thymus, spleen, and lymph nodes. The thymus was engrafted with immature T cells, whereas the spleen contained mature T cells that formed clusters with B cells. PALS-like structures consisting mainly of human hematopoietic cells were also identified, and these structures were absent before transplantation. In lymph nodes, many T and B cells were identified, but distinct follicular structures were missing. This may reflect that critical molecules for lymphocytes, such as adhesion molecules or chemokines, are not available for human cells in a mouse environment, which could prove an obstacle to analyzing the human immune system in detail.
One important question is how human T cells develop. Human CD3+ T cells with the CD4+CD8+ DP phenotype in the thymus were positive for human CD1a, which is expressed on immature thymocytes.29 These cells were also positive for TdT (data not shown) and the cell-cycle–specific antigen Ki-67. Furthermore, cytokeratin staining revealed the presence of an epithelial cell network derived mainly from mice (data not shown). These results strongly suggest that the generation of human T cells occurs in the thymus of NOD/SCID/γcnull mice. To exclude the possibility that contaminated T cells expanded in mice, we transplanted CB Lin- CD34+ cells into NOD/SCID/γcnull mice and performed sequential analysis. T cells first appeared only in the thymus with the phenotype of immature thymocytes, followed by seeding to peripheral organs. This finding supports the notion that human T cells were derived from HSCs, maturated in the mouse thymus, and seeded to the periphery. Another important observation is that as few as 100 CD34+ cells could engraft and give rise to multilineage blood cells in our mice, as we have already reported.16 Even after the transplantation of these few CD34+ cells, we confirmed the presence of CD3+ cells 5 months after transplantation using flow cytometry. Although our results strongly suggest the generation of human T cells in the mouse thymus, the possibility of extrathymic differentiation must be examined. To clarify the site(s) for T-cell development, the results of thymectomy experiments have to be analyzed. It is also noteworthy that NOD/SCID/γcnull mice with transplanted human CB CD34+ cells showed almost no appreciable evidence of GVHD responses despite a considerable number of human T cells in these mice. Transfer of human PB lymphocytes into scid mice induces a substantial immune response against mouse xenoantigens that skews the human TCR repertoire30 and induces GVHD.31,32 The mice died within a few weeks. Under physiological conditions, intrathymic T-cell differentiation is characterized by 2 selection events: positive and negative selection. Two types of molecules produced by nonlymphoid thymic cells—MHC molecules and cytokines—play important roles in T-cell maturation. MHC molecules are indispensable for positive and negative selection, serving to preserve useful cells and to eliminate potentially harmful ones. As for which MHC restriction orchestrates human lymphocyte development, at least 2 possibilities are likely. One is human MHC. Sanchez et al33 reported that human CB contains a cell population that supports the differentiation of CD34+ cells into CD4+ or CD8+ naive T cells in serum-deprived cultures. The other possibility is xenogenic mouse MHC. Robin et al34 and Weekx et al35 reported the generation of human T-lymphoid progenitor cells from CD34+CD38-, CD34+CD38low, and CD34+CD38+ subsets of human CB and BM cells in fetal thymus organ cultures from NOD/SCID or scid mice. Zhao et al36 also showed that normal immune functions and specific T-cell tolerance to discordant xenogenic donors were achieved by grafting fetal pig thymus and liver tissue to T-cell–and NK-cell–depleted thymectomized mice. These 2 possibilities—human MHC and xenogenic mouse MHC—must be examined further to elucidate the mechanism underlying the generation of human T cells in NOD/SCID/ γcnull mice.
Our mouse model is an excellent in vivo model in which to complete the reconstitution of human lymphocytes from HSCs without transplanting additional human tissues. Use of this model will be a unique way to investigate human lymphopoiesis, which has been analyzed in a combination of different assay systems. We conclude that NOD/SCID/ γcnull mice are a potent and versatile species that can be used to analyze human lymphopoiesis and human HSCs.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2002-09-2755.
Supported by the Program for the Promotion of Fundamental Studies in Health Science of the Organization for Pharmaceutical Safety and Research of Japan and by a Grant-in-Aid (13GS0009) for Creative Scientific Research, from the Ministry of Education, Science, Technology, Sports and Culture of Japan.
H.H. and R.N. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr M. Yasukawa for kindly providing the EB Fas-/-cell line, Dr K. Omori (Kyoto University Hospital, Kyoto, Japan) for helpful discussions, and M. Ohara (Fukuoka, Japan) for language assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal