Abstract
We analyzed the impact of CD34+ cell dose on the outcome of 86 patients undergoing reduced-intensity conditioning (RIC) allogeneic peripheral blood stem cell transplantation. The RIC was based on fludarabine 150 mg/m2 and melphalan 140 mg/m2 or busulphan 10 mg/kg. A median of 5.68 × 106 CD34+ cells/kg and 2.86 × 108 CD3+ cells/kg were infused. All patients receiving more than percentile 75 (p75) of CD34+ cells reached complete chimerism in T lymphocytes by days 21 to 28, compared with 44% among those receiving p75 or fewer cells (P = .046). Overall, 30.3% patients developed grade 2 to 4 acute graft-versus-host disease (aGVHD). Among 83 evaluable patients, 55.8% developed chronic GVHD (cGVHD). The dose of CD34+ cells infused did influence the development of cGVHD, with a cumulative incidence of extensive cGVHD of 74% vs 47% (P = .02) among patients receiving more than p75 CD34+ cells vs those receiving p75 or fewer. Projected overall survival (OS) and event-free survival (EFS) at 43 months were 60% and 46%, respectively. Neither the dose of CD34+ cells nor the dose of CD3+ cells infused significantly influenced OS and EFS, although among patients categorized as high-risk, 36% of those receiving p75 or fewer CD34+ cells relapsed or progressed, compared with only 9% among those receiving more than p75 CD34+ cells (P = .07). Among patients receiving p75 or fewer CD34+ cells, 36% of high-risk patients relapsed, compared with 10% of low- and intermediate-risk patients (P = .004), while relapse rates were not significantly different between both subgroups when we infused more than p75 CD34+ cells, thus indicating that infusing high doses of CD34+ cells ameliorates the negative effect of advanced disease status at transplantation. cGVHD was associated with better EFS (63% vs 16% at 43 months for patients with and without cGVHD; P < .0001) and better OS (78% vs 28% for patients with and without cGHVD; P < .001). The number of CD34+ cells infused should be tailored to prevent extensive cGVHD among patients categorized as low-risk, while high-risk patients, in whom the graft-versus-leukemia effect may determine disease outcome, should receive high doses of CD34+ cells.
Introduction
While in autologous transplantation the studies published so far have shown that infusion of high doses of CD34+ cells leads to a faster hematopoietic engraftment and decreased transplantation-related morbidity,1-5 within the allogeneic setting information regarding the optimal dose of hematopoietic progenitor cells remains controversial. While some studies have reported a positive impact on outcome in terms of faster engraftment and fewer infectious episodes by infusing high numbers of CD34+ cells in patients undergoing bone marrow transplantation,6-8 other authors have shown an increased risk of acute or chronic graft-versus-host disease (GVHD) in patients receiving high doses of unmanipulated peripheral blood stem cells (PBSCs).9,10 Among recipients of CD34+-selected allogeneic transplants, the influence of the number of CD34+ cells on survival remains contradictory, since in CD34+-selected marrow transplantation higher cell doses lead to improved survival,3 while the opposite occurs with CD34+-selected PBSC transplants.11 Nevertheless, to the best of our knowledge, the impact of the CD34+ cell dose infused in patients undergoing reduced-intensity-conditioning (RIC) allogeneic transplantation has not been analyzed. Interestingly, in this subset of patients chemotherapy-induced toxicity is significantly reduced,12,13 thus allowing a better estimation of the impact of the CD34+ cell dose on GVHD and transplantation-related complications without the possible confounding effects of inflammatory cytokine production related to the use of high doses of chemotherapy during the conditioning regimen.14,15
Patients and methods
Patients
Patients eligible for entry into this study had myeloid or lymphoid malignancies that were potentially treatable with an allogeneic transplantation. Eligible patients were 45 years old or older and/or were at high risk of transplantation-related mortality (TRM) considering their pretransplantation evaluation. Between May 1998 and January 2002, 86 patients underwent RIC PBSC transplantation from a human leukocyte antigen (HLA)–identical sibling at 2 transplantation centers in Spain. Patients gave written informed consent for inclusion in the protocol, which was approved by all local ethical review boards and the Spanish Drug Agency (protocol 99-0151). Patient characteristics are shown in Table 1. Disease phase at transplantation was categorized as early (low risk) in 21 cases (acute leukemia or poor-risk myelodysplasia in first complete remission, untreated standard-risk myelodysplasia, first-chronic-phase chronic myelogenous leukemia [CML], lymphoid malignancy in first remission), intermediate in 26 patients (acute leukemia or myelodysplasia in second or higher complete remission, second-chronic-phase or accelerated-phase CML, lymphoid malignancy in second or higher remission) and advanced (high risk) in 39 cases (refractory or relapsed acute leukemia or myelodysplasia, blastic-phase CML, refractory or relapsed lymphoid malignancy, and all second transplantations).
Characteristics of patients (n = 86) at time of transplantation
Characteristics . | No. . | % . |
|---|---|---|
| Diagnosis and disease status | ||
| Acute myelogenous leukemia | 18 | 21 |
| Acute lymphocytic leukemia | 7 | 8 |
| Chronic lymphocytic leukemia | 7 | 8 |
| Chronic myelogenous leukemia | 6 | 7 |
| Non-Hodgkin lymphoma | 16 | 19 |
| Multiple myeloma | 17 | 20 |
| Myelodysplastic syndrome | 9 | 10 |
| Hodkgin disease | 6 | 7 |
| Disease status at transplantation | ||
| Low risk | 21 | 24 |
| Intermediate risk | 26 | 30 |
| High risk | 39 | 46 |
| Cytomegalovirus serology | ||
| Patient positive | 70 | 81 |
| Patient negative/donor positive | 7 | 8 |
| Patient and donor negative | 9 | 11 |
| Sex | ||
| Matched | 51 | 59 |
| Mismatched | 35 | 41 |
Characteristics . | No. . | % . |
|---|---|---|
| Diagnosis and disease status | ||
| Acute myelogenous leukemia | 18 | 21 |
| Acute lymphocytic leukemia | 7 | 8 |
| Chronic lymphocytic leukemia | 7 | 8 |
| Chronic myelogenous leukemia | 6 | 7 |
| Non-Hodgkin lymphoma | 16 | 19 |
| Multiple myeloma | 17 | 20 |
| Myelodysplastic syndrome | 9 | 10 |
| Hodkgin disease | 6 | 7 |
| Disease status at transplantation | ||
| Low risk | 21 | 24 |
| Intermediate risk | 26 | 30 |
| High risk | 39 | 46 |
| Cytomegalovirus serology | ||
| Patient positive | 70 | 81 |
| Patient negative/donor positive | 7 | 8 |
| Patient and donor negative | 9 | 11 |
| Sex | ||
| Matched | 51 | 59 |
| Mismatched | 35 | 41 |
Patients median age at time of transplantation was 52 years (range, 22-66 years)
Conditioning regimens and graft-versus-host disease prophylaxis
Two RIC regimens were used, one recommended for lymphoid malignancies and one for myeloid malignancies.12 The lymphoid RIC regimen consisted of fludarabine 30 mg/m2 administered intravenously on days -9 to -5, followed by melphalan 70 mg/m2 intravenously on days -3 and -2. The myeloid regimen consisted of the same doses of fludarabine together with busulphan 1 mg/kg for 10 doses (days -6 to -4, total 10 mg/kg), with phenytoin given as anticonvulsant prophylaxis. PBSCs were infused on day 0.
GVHD prophylaxis consisted of cyclosporine A (CsA) plus short-course methotrexate (MTX). CsA was given at a dose of 1 mg/kg per day intravenously from days -9 to -2, and then 3 mg/kg/d intravenously or orally from day -1. Levels were maintained in the therapeutic range until tapering. MTX was given at a dose of 15 mg/m2 intravenously on day 1 and a dose of 10 mg/m2 intravenously on days 3 and 6, followed by folinic acid rescue at the same dose per square meter intravenously every 6 hours for 4 doses starting 24 hours after each dose of MTX. Acute and chronic GVHD were graded by established criteria.16,17
Mobilization and harvesting of donor PBSCs
Donors received 10 μg/kg granulocyte colony-stimulating factor (G-CSF) daily for 5 to 6 days and mobilized PBSCs were collected from day 5 until an ideal target dose of at least 4 × 106 CD34+ cells per kilogram of recipient weight was collected. Stem cell apheresis was performed using a continuous flow cell separator, CS 3000 Plus, with a small-volume (50-mL) collection chamber (Baxter, Deerfield, IL).
Immunofluorescence analysis
The absolute number of CD34+ cells was measured in erythrocyte-lysed peripheral blood samples prior to initiating each apheresis as well as in the leukapheresis product. Cells were stained with an anti-CD34 monoclonal antibody (anti–HPCA-2) conjugated with phycoerythrin (PE) and analyzed by flow cytometry (FACScan; Becton Dickinson, San Jose, CA) as previously described.1,2 In order to increase the sensitivity and specificity of the measurement, a 2-step acquisition procedure was performed. In the first step, 20 000 events were acquired; in the forward scatter/side scatter (FSC/SSC) dot plot, granulocytes, monocytes, and lymphocytes were selected, eliminating debris and red blood cells (RBCs) in order to calculate the percentage of nucleated cells. In the second step, 300 000 events were acquired. A side-scatter/CD34-PE live gate was performed in which only cells with high intensity of PE fluorescence were selected; the cluster of CD34+ cells was then selected among these events around the low-intermediate side scatter. The proportion of CD34+ cells was then calculated as follows: number of CD34+ events selected in the second acquisition step × 100/300 000 events × percentage of nucleated cells obtained in the first acquisition step. Then the absolute count of peripheral blood (PB) CD34+ cells/μL was calculated by multiplying the proportion of CD34+ cells by the white blood cell (WBC) count.
In center B, this CD34+ cell assay was slightly modified according to the ISHAGE protocol.18
Chimerism studies
After transplantation, serial samples of whole PB, bone marrow (BM), and CD3+- and CD15+-selected cells, obtained using magnetic microbeads according to the manufacturer's protocols (MACS cell separation; Miltenyi Biotec, Bergisch Gladbach, Germany), were analyzed for degrees of donor-recipient chimerism using polymerase chain reaction (PCR) analysis of informative minisatellite regions. Samples were routinely analyzed soon after hematologic recovery (days 21 to 56), on days 90 to 100 and on days 180 to 200, and then every 3 months or in case of disease recurrence or suspicion of graft failure. Chimerism studies were performed with a commercially available automated kit (AmpFlSTR, SGM Plus; Applied Biosystems, Norwalk, CT) as previously reported.13,19
Statistical analysis
Events analyzed were calculated from the time of transplantation using Kaplan-Meier product-limit estimates. Patients who experienced TRM were censored at last follow-up. Event-free survival (EFS) was calculated from transplantation until disease progression or death, and those patients who did not reach disease response (complete or partial) any time after transplantation were considered to have experienced an event. Overall survival (OS) was calculated from transplantation until death from any cause, and surviving patients were censored at last follow-up. Univariate Cox regression was used to analyze the impact of time-dependent covariates on EFS and OS. Differences in hematologic recovery (days to reach an absolute neutrophil count [ANC] > 0.5 × 109/L, days to reach a platelet count > 20 × 109/L) between groups according to the different covariates were analyzed by Kaplan-Meier and log-rank tests. Significant factors were included in a multivariate analysis using a forward stepwise Cox proportional hazards regression model.
Since methods for CD34+ and CD3+ quantification by flow cytometry were not identical in the 2 centers, the data on the number of CD34+ and CD3+ cells infused were separately categorized according to each center's quartile values. Cases within the first quartile from each center were included under the same category (≤ percentile 25 [p25]), as were the cases within the second (p25-50), third (p50-75), and fourth (> p75) quartiles. Therefore 4 groups of CD34+ and CD3+ cells were established. Qualitative parameters and binary variables were analyzed using the analysis of variance (ANOVA) test and the multiple logistic regression model. Significance levels were set at .05.
Results
Characteristics of the product infused and impact on hematopoietic recovery
A median of 5.68 (range, 2.58-15.6) × 106 CD34+ cells/kg and 2.86 (range, 0.25-8.41) × 108 CD3+ cells/kg were infused. These data were 4.55 (range, 2.75-13.2) × 106 CD34+ cells/kg and 2.36 (range, 0.25-6.75) × 108 CD3+ cells/kg in center A and 7 (range, 2.58-15.6) × 106 CD34+ cells/kg and 3.41 (range, 1.9-8.4) × 108 CD3+ cells/kg in center B (P = .003 for CD34+ cells and P = .001 for CD3+ cells infused). Owing to these differences, both variables were grouped separately according to the quartile values for each center and then fused as previously specified.
No significant differences were observed in terms of CD3+ cells infused depending on the amount of CD34+ cells infused.
All evaluable patients reached levels of more than 500 granulocytes/mm3 at a median of 15 days (range, 6-23 days) and more than 20 000 platelets/mm3 at a median of 13 days (range, 0-23 days) after PBSC infusion. Neither the number of CD3+ cells infused nor the CD34+ cell dose infused significantly influenced the speed of hematopoietic recovery. The median (mean) time to neutrophil recovery (> 500/mm3) by quartile of CD34+ cells infused was 16 (15), 16 (16), 14 (15), and 15 (16) days, respectively, while the median (mean) time to platelet recovery (> 20 000/mm3) was 13 (12), 13 (13), 13 (14), and 11 (11) days, respectively. No differences in the speed of hematopoietic recovery were observed between the 2 centers. Regarding extrahematologic toxicity, overall the regimen was well tolerated: 7% of patients developed nausea of grade 3 or higher, 21% developed mucositis of grade 3 or higher, 7% developed liver toxicity of grade 3 or higher, and 11% developed diarrhea of grade 3 or higher.
Chimerism analysis in bone marrow, peripheral blood, T lymphocytes, and granulocytes was performed on days 21 to 28, 56, 100, 180, 270, and 365 after transplantation. Results on the kinetics of chimerism are shown in Table 2. The number of CD34+ cells infused did not influence the rate of complete chimerism in bone marrow and granulocytes. By contrast, 100% of patients receiving p75 or more of CD34+ cells reached complete chimerism in T lymphocytes by days 21 to 28, as compared with 44% among those receiving p75 or fewer cells (P = .046). A trend toward a faster rate of complete chimerism in unfractionated peripheral blood was also observed among those patients receiving higher doses of CD34+ cells (93% of complete chimerism in PB for those receiving p75 or more cells, vs 77% for those receiving p75 or fewer CD34+ cells; P = .4). Finally, we did not observe any impact from the CD3+ cell dose infused on the kinetics of chimerism.
Percentage of cases with complete chimerism after infusion of CD34+ and CD3+ cells
Source of sample analyzed . | Days after transplantation . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 21-28 . | 56 . | 100 . | 180 . | 270 . | 365 . | |||||
| Bone marrow | 73 | 81 | 83 | 88 | 86 | 88 | |||||
| Unseparated peripheral blood | 88 | 82 | 91 | 100 | 100 | 100 | |||||
| T lymphocytes | 57* | 58 | 73 | 88 | 100 | 100 | |||||
| Granulocytes | 92 | 88 | 92 | 94 | 100 | 100 | |||||
Source of sample analyzed . | Days after transplantation . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 21-28 . | 56 . | 100 . | 180 . | 270 . | 365 . | |||||
| Bone marrow | 73 | 81 | 83 | 88 | 86 | 88 | |||||
| Unseparated peripheral blood | 88 | 82 | 91 | 100 | 100 | 100 | |||||
| T lymphocytes | 57* | 58 | 73 | 88 | 100 | 100 | |||||
| Granulocytes | 92 | 88 | 92 | 94 | 100 | 100 | |||||
100% of patients receiving more than p75 vs 44% of patients receiving p75 or fewer CD34+ cells reached complete chimerism (P = .046); in all other instances, cell dose had no significant influence on the kinetics of complete chimerism
Impact of the product infused on clinical outcome
Overall, 37 patients (43%) developed acute graft-versus-host disease (aGVHD) at a median of 32 days (range, 10-125 days) and 26 (30%) of them developed grade 2 to 4 aGVHD. Among 83 evaluable patients, 48 (57.8%) developed chronic GVHD (cGVHD) at a median of 137 days after transplantation (range, 148 to 196 days). Twenty-two (26.5%) developed limited cGVHD and 26 (31.3%) extensive cGVHD.
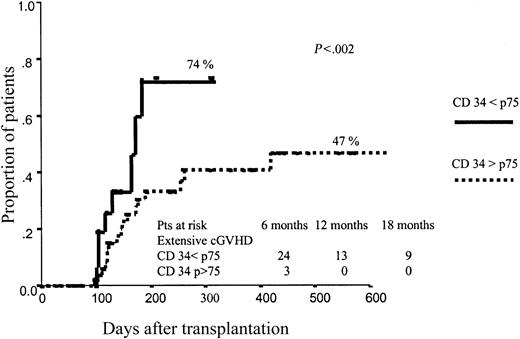
Neither CD34+ nor CD3+ cell dose significantly affected the incidence of aGVHD. By contrast, the dose of CD34+ cells infused had a significant influence on the development of cGVHD, with a cumulative incidence of extensive cGVHD of 74% vs 47% (P = .02) among patients receiving the higher dose (> p75) vs those receiving the lower dose (≤ p75) of CD34+ cells (Figure 1). Again, the dose of CD3+ cells infused did not influence the incidence of cGVHD. These results were confirmed when we analyzed the results from the 2 centers separately. Thus, in center A 83% of patients receiving more than p75 CD34+ cells developed extensive cGVHD, as compared with 56% among those receiving p75 or fewer CD34+ cells (P = .004), and the same trend was observed in center B: incidence of extensive cGVHD of 56% vs 39% for patients receiving high (> p75) vs low (≤ p75) doses of CD34+ cells, respectively.
No other variables were significantly associated with the occurrence of aGVHD. In terms of cGVHD, patients older than 51 years (percentile 50) had a significantly higher incidence of overall and extensive cGHVD than did younger patients (cumulative incidence for overall cGHVD, 100% vs 63%, log-rank P = .04; cumulative incidence for extensive cGVHD, 100% vs 43%, log rank P = .01). In addition, those patients receiving melphalan had a significantly lower incidence of overall and extensive cGVHD than did those receiving busulphan (cumulative incidence for overall cGVHD, 70% vs 87%, log-rank P = .008; cumulative incidence for extensive cGHVD, 42% vs 75%, log rank P = .01).
After a median follow-up of 385 days (range, 28-1312 days), 25 patients died, 17 (19.8% of the total) owing to TRM (8 patients owing to aGVHD, 2 owing to cGVHD, 4 owing to fungal or bacterial infection, and 3 owing to other toxicities) and 8 owing to disease progression. Neither the dose of CD34+ cells nor the dose of CD3+ cells infused significantly influenced TRM. We confirmed by both univariate and multivariate analysis that there were no significant differences between the 2 centers in terms of TRM, acute or chronic GVHD, or relapse.
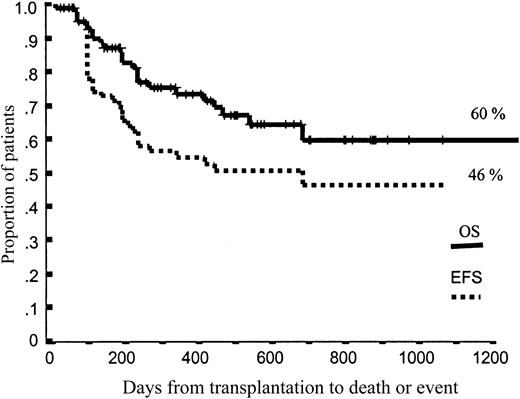
Projected OS and EFS at 43 months were 60% and 46%, respectively (Figure 2). Again, neither the dose of CD34+ cells nor the dose of CD3+ cells infused significantly influenced OS and EFS. Nevertheless, among patients categorized as high-risk according to disease status at transplantation, 36% of those receiving p75 or fewer CD34+ cells relapsed or progressed, compared with only 9% among those receiving more than p75 CD34+ cells (P = .07). Moreover, among patients receiving p75 or fewer CD34+ cells, 36% of high-risk patients relapsed, compared with 10% of low- and intermediate-risk patients (P = .004), while relapse rates were not significantly different between high-risk and low- or intermediate-risk patients when we infused more than p75 CD34+ cells (9% vs 30%, respectively; P = .22).
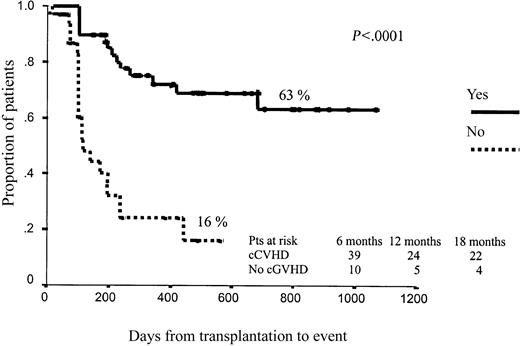
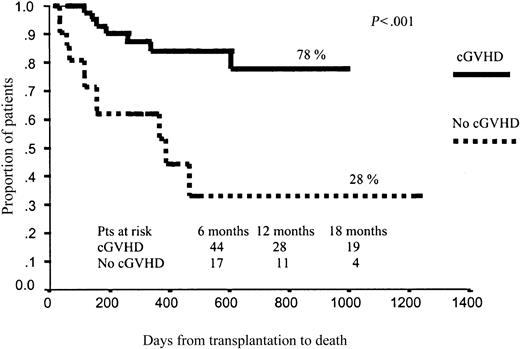
Regarding EFS, as expected, those patients who developed grade 3 to 4 aGVHD had significantly worse outcomes (15% vs 50% projected EFS at 43 months, P = .006). By contrast, cGVHD was associated with better EFS (63% vs 16% projected EFS at 43 months for those patients with and without cGHVD, respectively; P < .0001; Figure 3). No other variables affected EFS after RIC.
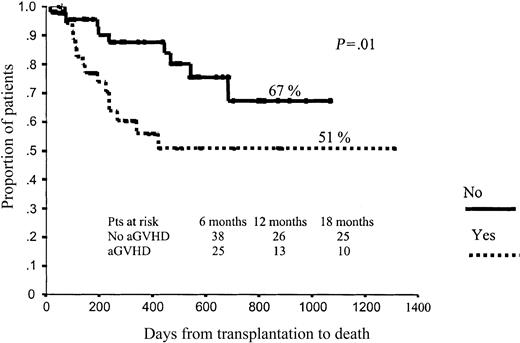
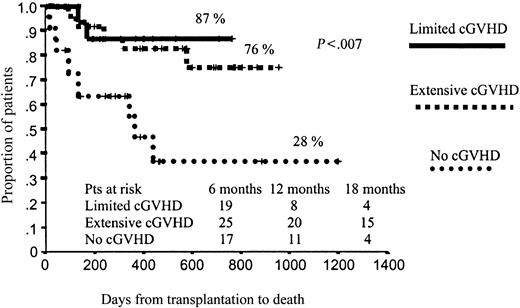
In terms of OS, those patients who developed aGVHD had a significantly worse prognosis than those without aGVHD (51% vs 67% projected OS at 43 months, respectively; P = .01; Figure 4). By contrast, those patients who developed cGVHD had significantly better outcomes than those who did not develop cGVHD (78% vs 28% projected OS at 43 months, respectively; P < .001), with a hazard rate (HR) of 6.41 (95% confidence interval [CI], 2.38-17.25; P = .0002; Figure 5). To further assess the effect of cGVHD on outcome, we grouped patients into 3 categories according to cGVHD (absent, limited, or extensive). Relapse rate was significantly higher among patients who did not develop cGVHD as compared with the other 2 categories: 50% vs 11% and 9%, respectively (P = .001). By contrast, no significant differences were observed in terms of TRM among these subgroups of patients, although a trend toward lower TRM was observed among those who developed limited cGVHD (5% TRM) as compared with those who developed extensive cGVHD (15% TRM) or those who did not develop cGVHD (20% TRM; P = .27). Finally, in terms of overall survival, patients who developed limited or extensive cGVHD had 87% and 76% projected OS at 43 months, while OS was only 28% for those patients who did not develop cGVHD (P = .004), with a hazard rate for patients who developed cGVHD of 5.78 (95% CI, 20.22-27.35; P = .02; Figure 6).
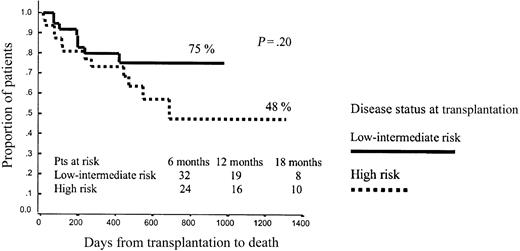
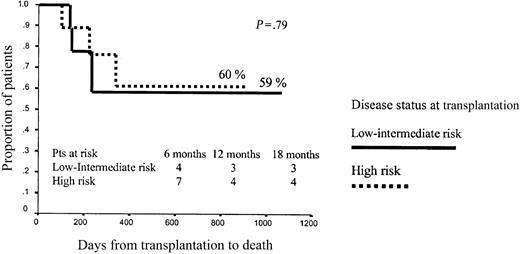
No other variables significantly influenced OS after RIC, although the dose of CD34+ cells infused showed a trend to influence outcome. Thus, among patients receiving p75 or fewer CD34+ cells, projected OS at 43 months was 75% versus 48% for low- or intermediate-risk and high-risk patients, respectively (P = .2), while among patients receiving more than p75 CD34+ cells, projected OS at 43 months was 60% versus 60%, respectively (P = .79; Figures 7 and 8).
Overall survival among patients receiving p75 or lower CD34+ cell dose.
Overall survival among patients receiving more than p75 CD34+ cell dose.
Discussion
Recent studies have been reported showing the impact of the CD34+ cell dose on the incidence of acute or chronic GVHD.9,10,11 Interestingly, this effect has been observed only after allogeneic PBSC transplantation (allo-PBSCT), while trials using bone marrow stem cells have found a positive influence on outcome without increasing the risk of GVHD after infusing high numbers of progenitor cells.6-8,20 In order to explain these differences, it should be noted that the range of CD34+ cells infused in allogeneic bone marrow transplantation (allo-BMT) is narrower than that used in allo-PBSCT, where the number of stem cells infused is usually higher.21-25 Moreover, the threshold level of CD34+ cells associated with an increased incidence of GVHD after allo-PBSCT has been high in all studies,9,10,11 and these levels have rarely been reached after allo-BMT.6-8,21-25 In addition, functional differences between CD34+ and CD3+ cells infused after treatment with G-CSF and bone marrow–harvested stem cells could also play a role,26-29 since mesenchymal stem cells, which may have an immunoregulatory effect,30 are infused after bone marrow but not after PBSC transplantation.31 Furthermore, Ryncarz and Anasetti32 have also reported on the capability of CD34+ hematopoietic progenitor cells to present certain antigens to autologous T lymphocytes, which could also explain the relationship between GVHD and CD34+ cells infused.
The present study has been performed in patients who received RIC, which decreases cell lysis and release of cytokines and inflammatory mediators induced by high doses of chemotherapy, thereby avoiding the confounding factor of these mediators of GVHD reactions.33-35 In this sense, in contrast to previous reports that analyzed the impact of CD34+ cell dose on GVHD after conventional conditioning regimens,9 we did not find an increase of aGVHD after infusing high doses of CD34+ cells, although we did find an increased risk of extensive cGVHD, in accordance with other studies.10 It is worth mentioning that while Urbano-Ispizua et al11 found that the infusion of high doses of CD34+ cells had a negative impact on survival, other reports did not find any impact of CD34+ cell dose on survival.7,9,10,36 This could be explained by the poor tolerance to GVHD in patients undergoing CD34+-selected allo-PBSCT.37 Interestingly, in our study the relapse rate among high-risk patients significantly decreased after infusion of high doses of CD34+ cells, thus favorably affecting outcome in this subgroup of patients. In addition, we found that, after a RIC regimen, cGVHD favorably affects event-free and overall survival, which has already been reported in previous studies performed on patients with acute myelogenous leukemia (AML) and myelodys-plastic syndrome (MDS) after RIC allogeneic transplantation.12,13 The positive impact of cGVHD on relapse rate has also been reported in some studies after conventional conditioning regimens,21,38,39 although it has not been found in other series.10,40,41 Since the therapeutic effect of RIC allogeneic transplantation relies mainly on graft-versus-leukemia (GVL) reactions, it is reasonable to find a link between cGVHD and event-free and overall survival in these type of transplantations.12,13 Moreover, the ability of allogeneic stem cell transplantation to eradicate leukemia is determined by both the intensity of the conditioning regimen and the GVL effect and, according to this and other studies based on RIC or truly nonmyeloablative conditioning regimens,42 the lower the conditioning regimen intensity, the more evident the GVL reaction. With our RIC regimen, chemotherapy could initially contribute to disease control while the immune system induces an adequate response, and at the same time a low extrahematologic toxicity and low TRM could be maintained. Nevertheless, it should be noted that since cGVHD is a time-dependent variable, studies showing any impact of cGVHD on survival must be interpreted cautiously, since this relationship could be explained by the fact that patients with longer survival have a higher risk of developing cGVHD and not the contrary. We performed a Cox regression analysis, which confirmed the favorable impact of cGVHD on event-free and overall survival in our study.
As previously reported,9,10,19-24,43,44 we did not find a relationship between the number of CD3+ cells infused and the incidence of acute or chronic GVHD. This finding could be attributed to the high doses of CD3+ cells infused in all patients, since, according to Kernan and colleagues,45,46 once the initial threshold of T cells has been exceeded, a further increase in T-cell numbers does not translate into more GVHD. Accordingly, Urbano-Ispizua et al47 and Wagner et al48 have reported that T-cell doses of more than 0.2 × 106 and more than 0.5 × 106 cells/kg are associated with an increased incidence of GVHD in both T cell–depleted allo-PBSCT and unmanipulated allo-BMT, respectively, without any differences reported for higher cutoff values. In the present study, all patients received doses much higher than those mentioned.
Finally, in terms of engraftment, we did not find a clear correlation between the number of CD34+ cells infused and the speed of hematopoietic recovery. As we have previously reported with regard to autologous transplantation,1,2 the higher the number of CD34+ cells infused the faster the engraftment, up to a dose ranging between 2.2 and 2.4 × 106 CD34+ cells/kg. In our experience, doses higher than that do not increase the speed of hematopoietic recovery, although the exact threshold varies among centers depending on the CD34+ quantification procedures. This observation concurs with the results reported by Zaucha et al10 and Gianni et al,49 who found that increasing the progenitor cells above a certain threshold had a limited potential to further accelerate neutrophil recovery. In addition, in a previous study including a small number of patients, we reported that after a RIC regimen the number of overall and myelomonocytic committed CD34+ cells does influence the speed of hematopoietic recovery, although only the myelomonocytic committed cell dose retained its influence in multivariate analysis,44 thereby suggesting that the number of the different CD34+ cell subpopulations is more relevant than the overall number of CD34+ cells infused for predicting the speed of hematopoietic recovery. In the present study, using a RIC regimen, we observed that stable engraftment can be ensured by infusing a CD34+ cell dose greater than or equal to 2.58 CD34+ cells/kg but that higher doses do not increase the speed of hematopoietic recovery. By contrast, the CD34+ cell dose may affect the kinetics of chimerism, since patients receiving higher doses reached complete chimerism in T lymphocytes more rapidly. This could also explain the higher incidence of extensive cGVHD among patients receiving the higher doses of CD34+ cells.
Since the CD34+ cell dose is the only pretransplantation variable that can be manipulated to affect cGVHD incidence, the number of CD34+ cells infused should be tailored cautiously to prevent extensive cGVHD among patients categorized as low-risk according to their disease status before transplantation, while high-risk patients, in which the benefit of GVL may determine disease outcome, should receive high doses of CD34+ cells. In conclusion, in the RIC allogeneic transplantation setting, the CD34+ cell dose, in addition to its impact on engraftment, may have a relevant influence on graft-versus-host disease and graft-versus-tumor effect.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-11-3503.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal