Abstract
Studies comparing transfusion and nontransfusion patients suggest an increased risk of postoperative infections in transfusion groups. Supernatants of blood components have been shown to affect the function of T lymphocytes and natural killer cells. Here, we found that supernatants from stored red blood cells (RBCs) inhibit human neutrophil migration in response to formyl peptides and stimulate neutrophil locomotion. These effects can be observed with high dilutions of RBC supernatants, such as 1:5 × 106 (vol/vol), able to trigger locomotion as well as desensitization of the cells to alternative chemoattractants. The phenomenon might be mediated by chemoattractants present in the supernatants. As RBC supernatants failed to mobilize intracellular free calcium, the chemoattractants should belong to the group of pure chemoattractants, that is, soluble Fas ligand (sFasL) and transforming growth factor–β1 (TGF-β1), known to act without increasing calcium levels. Recombinant TGF-β1, but not sFasL, was found to reproduce the ability of RBC supernatants to both inhibit neutrophil response to formyl peptides and stimulate neutrophil locomotion. Moreover, TGF-β1–immunodepleted supernatants did not display neutrophil-directed activities. Finally, RBC supernatants from RBCs stored after depletion of leukocytes were incapable of affecting neutrophil function. With neutrophils acting as a first-line antimicrobial defense, the ability, shown here, of high dilutions of RBC supernatants to inhibit neutrophil chemotaxis through TGF-β1 may be a relevant determinant of infections in the postoperative period for transfusion patients. Consistently, the neutrophil chemotactic response to formyl peptide was inhibited by the plasma obtained from 5 transfusion patients.
Introduction
Although a causal relationship between blood transfusion complications, such as cancer recurrence and postoperative infections, has not been definitively proven by double-blind randomized controlled trials, various observational studies comparing patients receiving and not receiving blood transfusions support the hypothesis of an increased risk of postoperative complications in the transfusion group.1-9 Moreover, 4 out of 5 randomized controlled trials investigating the relationship between white blood cell–containing versus white blood cell–depleted allogeneic blood transfusions and the development of postoperative infections, show at least a trend toward an increased incidence of infections in association with white blood cell–containing transfusions.10-14 Consistent with the possible increased risk of postoperative infections in transfusion patients, various studies suggest that transfusions can induce immunomodulatory effects, including transient reduction in the CD4/CD8 T-cell ratio, reduced natural killer function, impaired lymphocyte mitogenic responses, and inhibition of delayed hypersensitivity.15 The detailed process whereby transfusions can induce immunosuppression is, however, still undefined and may include various mechanisms involving leukocyte-derived active mediators generated during blood storage, which may in turn impair the activity of immune cells.16-20 In this context, we have recently shown that nonleukodepleted stored red blood cells (RBCs) and hemoderivatives contain high levels of immunoregulatory molecules, such as soluble human leukocyte antigen (HLA) class I and Fas ligand.21,22 These molecules have been found to inhibit mixed lymphocyte responses and cytotoxic T-cell activity in allogeneic and in autologous combinations as well as to induce apoptosis in Fas+ cells.21,22 Nevertheless, the knowledge about the possibility that blood derivatives may influence the function of other cells, such as neutrophils, involved as first-line antimicrobial defenses, is lacking. The present study was therefore undertaken to uncover possible effects that supernatants from stored RBCs might have on neutrophil function.
Methods
Culture medium and reagents
Hanks balanced salt solution (HBSS) (EuroClone, Wetherby West, Yorkshire, United Kingdom), mixed with Dulbecco phosphate-buffered saline (PBS) (EuroClone) (HBBS/PBS = 3:1) containing 1 mg/mL bovine serum albumin (BSA) (Sigma Chemical, St Louis, MO), was used as incubation medium throughout the study. Ficoll-Hypaque (Lympholyte-I), Giemsa stain, and heparin were purchased from Cedarlane Laboratories (Hornby, ON, Canada), Merck (Darmstadt, Germany), and Roche (Milan, Italy), respectively. Fluorescein diacetate, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and N-formyl-methionyl-leucyl-phenylalanine (FMLP) were purchased from Sigma Chemical. Fluorescein isothiocyanate (FITC)–conjugated anti-CD11b monoclonal antibody (mAb) 44 (immunoglobulin G1 [IgG1]) was purchased from Biosource International (Camarillo, CA); antihuman Fas ligand (FasL) NOK-1 and NOK-2 mAbs were purchased from Pharmingen (San Diego, CA); and antihuman transforming growth factor–β1 (TGF-β1) mAb was purchased from R&D Systems (Minneapolis, MN). Soluble FasL (sFasL) was from Alexis (San Diego, CA). Human recombinant TGF-β1 was from R&D Systems. Fura-2 AM and 2′,7′-dichlorofluorescin-diacetate (DCFH-DA) were from Molecular Probes (Eugene, OR).
RBC supernatants
Blood units were obtained from 10 healthy volunteer donors scheduled to donate 2 units of blood on day zero and after 10 days. Blood units were submitted to the standard procedure for the preparation and storage of packed RBCs according to the Council of Europe's “Guide to the Preparation, Use and Quality Assurance of Blood Components” (Strasbourg, France, June 8, 1994). Briefly, whole blood (450 mL) was collected in plastic bags (Triplicate bag system; Baxter, Le Chartre, France) with 63 mL citrate phosphate dextrose (CPD) in the primary bag. After centrifugation (10 minutes, 3000 rpm, 20°C), packed RBCs were separated from plasma and transferred into the satellite bags, containing 80 mL saline-adenine-glucose-mannitol (SAG-M). The second blood donation was processed as described above, adding a prestorage leukodepletion (LD) step (LD-RBCs). Briefly, whole blood (450 mL) was collected in plastic bags (Triplicate bag system, with an integrated whole blood filter Leucoflex Sang Total 1 [LST1]; Macopharma, Tourcoing, France) with 63 mL CPD in the primary bag. Bags were stored at room temperature with filtration taking place 3 hours following donation. After centrifugation (10 minutes, 3000 rpm, 20°C), packed LD-RBCs were separated from plasma and transferred into the satellite bags containing 100 mL SAG-M. Storage time for RBCs and LD-RBCs before tests was 30 days. Supernatants were used at appropriate dilutions in incubation medium.
The supernatants of RBCs and LD-RBCs to be used in functional assays were extensively dialyzed to remove the additive solutions and, in some experiments, were immunodepleted with anti–TGF-β1 or anti-FasL monoclonal antibodies. The antibody concentration was chosen to achieve greater than 90% neutralization of cytokine activity, on the basis of neutralization assays performed by the manufacturer. After incubation (overnight, 4°C), supernatants were ultracentrifuged (10 000g, 45 minutes) and immediately tested in the chemotactic assays, which will be described.
Neutrophil and plasma preparation
Heparinized venous blood (10 U/mL heparin) was obtained after informed consent from healthy volunteers and from 5 patients before and after transfusion of 2 RBC units. The clinical characteristics of the patients are reported in Table 1. Plasma samples were separated by centrifugation (20 minutes, 4°C, 4000g) and then stored at -80°C. Final platelet counts were always below 3 × 106/mL. Neutrophils were isolated by dextran sedimentation and subsequent centrifugation on a Ficoll-Hypaque density gradient, as previously described.23 Contaminating erythrocytes were removed by hypotonic lysis.23 Then, neutrophils were washed 3 times with HBSS and resuspended in incubation medium at appropriate concentrations. Final cell suspension was more than 97% pure and more than 98% viable, as determined by usual assays.23
Characteristics of patients
. | Patient no. 1 . | Patient no. 2 . | Patient no. 3 . | Patient no. 4 . | Patient no. 5 . |
|---|---|---|---|---|---|
| Age, y | 61 | 86 | 71 | 65 | 70 |
| Sex | female | male | male | female | male |
| Hb, g/dL | 6.7 | 6.0 | 7.0 | 7.1 | 7.2 |
| Cause of anemia | melena | iron deficiency | hemorrhagic peptic ulcer | hip surgery, bleeding | hemorrhagic esophagitis |
| Other illnesses | diabetes | LVF, hiatal hernia | peripheral artery disease | no | no |
| Medications | H2A, insulin, fluconazole | diuretics, ACE inhibitor, PPI | ticlopidine, PPI | NSAID, PPI cephalosporin, | PPI, somatostatin |
| RBC units transfused | 4 | 3 | 3 | 2 | 2 |
. | Patient no. 1 . | Patient no. 2 . | Patient no. 3 . | Patient no. 4 . | Patient no. 5 . |
|---|---|---|---|---|---|
| Age, y | 61 | 86 | 71 | 65 | 70 |
| Sex | female | male | male | female | male |
| Hb, g/dL | 6.7 | 6.0 | 7.0 | 7.1 | 7.2 |
| Cause of anemia | melena | iron deficiency | hemorrhagic peptic ulcer | hip surgery, bleeding | hemorrhagic esophagitis |
| Other illnesses | diabetes | LVF, hiatal hernia | peripheral artery disease | no | no |
| Medications | H2A, insulin, fluconazole | diuretics, ACE inhibitor, PPI | ticlopidine, PPI | NSAID, PPI cephalosporin, | PPI, somatostatin |
| RBC units transfused | 4 | 3 | 3 | 2 | 2 |
Hb indicates hemoglobin; LVF, left ventricular failure; H2A, H2 receptor antagonist; ACE, angiotensin-converting enzyme; PPI, proton pump inhibitor; NSAID, nonsteroidal anti-inflammatory drug
Neutrophil locomotion
Neutrophil locomotion was studied by means of the leading-front method, as previously described.23 Tests were conducted in duplicate with the use of blind-well chambers (NeuroProbe, Gaithersburg, MD) with a 3 μm pore size cellulose ester filter (SSWPO 1300, lot no. H7PM21937; Millipore, Milan, Italy) separating the cells (4 × 105) from the chemoattractant. After incubation at 37°C for 45 minutes, the filters were removed, fixed in ethanol, stained with Harris hematoxylin, dehydrated, cleared with xylene, and mounted in Eukitt (Kindler, Freiburg, Germany). Duplicate chambers were run in each case, and the distance (in micrometers) traveled by the leading front of cells was measured at × 400 magnification; 5 randomly chosen fields were read for each filter.
Checkerboard analysis
Assays of cell migration with different doses of FMLP (0, 1, and 10 nM), in both the upper and bottom chamber and in the absence or presence of the appropriate dilution of RBC supernatant, were also performed. The results of these experiments were collected in checkerboard form, and chemokinesis (ie, change in the extent of random locomotion) and chemotaxis (ie, change in the directional response to the stimulus) were calculated according to Zigmond and Hirsch,24 as previously described.23
Intracellular Ca++ levels
The intracellular Ca++ levels were studied as previously described.23 Briefly, neutrophils (2.5 × 106), loaded with 2 μM Fura-2 AM (30 minutes, 37°C), were diluted 10-fold with HBSS-HEPES, incubated further for 30 minutes at 37°C, washed, and resuspended in HBSS-HEPES. Fluorescence changes before and after the addition of RBC supernatant or FMLP were monitored with Perkin-Elmer LS3 spectrofluorometer (Perkin Elmer Life Sciences, Shelton, CT) at an excitation wavelength of 338 nm and an emission wavelength of 550 nm.
Immunofluorescence neutrophil staining
Purified neutrophils (106 cells in HBSS) were incubated for 15 minutes at room temperature in the presence or absence of RBC supernatant or TGF-β–depleted RBC supernatant. Then, the cells were incubated for an additional 30 minutes at 37°C in the absence or presence of 100 nM FMLP. Finally, the cells were exposed to FITC-conjugated anti-CD11b mAb 44. The cells were the examined by means of a fluorescence Nikon Optiphot-2 microscope (Nikon, Melville, NY), and images were collected by a Hamamatsu Color–chilled 3 charge-coupled device (CCD) camera (Hamamatsu Italia, Varese, Italy), as previously described.25
Flow cytofluorimetric analysis of CD11b surface expression
The flow cytometric analysis of CD11b expression by neutrophils exposed or not to 100 nM FMLP were performed as previously reported.26 FITC-conjugated anti-CD11b mAb 44 and appropriate isotype-matched mAb of irrelevant specificity were used for the analysis. All of the flow cytometry experiments were carried out with the use of an EPICS XL flow cytometer (Beckman Coulter, Miami, FL).
Flow cytometric assessment of neutrophil oxidative metabolism
Flow cytometric analysis of neutrophil oxidative metabolism was carried out as previously described.23 Briefly, neutrophils were preincubated (15 minutes, 37°C) with DCFH-DA (5 μM). During the incubation time, DCFH-DA permeated the cells, where it was cleaved by intracellular esterases to produce nonfluorescent DCFH trapped within the cells. After washing in PBS, the cells were incubated for 15 minutes at room temperature in the presence or absence of RBC supernatant or TGF-β1–depleted RBC supernatant. Then, the cells were incubated for an additional 30 minutes at 37°C in the absence or presence of 100 nM FMLP. During this period, intracellular hydrogen peroxide oxidized DCFH to give a green fluorescent DCFH. At the end of the incubation, the reaction was stopped by keeping the samples on ice until flow cytometric analysis was carried out with an EPICS XL flow cytometer (Coulter).
Determination of sFasL and TGF-β1 concentrations
The concentrations of sFasL molecules were determined by double-determinant immunoassay (DDIA), as previously described,21 in samples of blood unit supernatants after centrifugation at 12 000g for 2 minutes.
The concentrations of active TGF-β1 were determined by DDIAs with a commercially available kit (Quantikine; R&D Systems) in samples of blood unit supernatants after centrifugation at 12 000g for 2 minutes.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) with Bonferroni posttest was performed by means of GraphPad InStat version 3.05 for Windows 95 (GraphPad Software, San Diego, CA). Differences were accepted as significant at P values less than .05.
Results
Inhibition of neutrophil chemotaxis by RBC supernatants
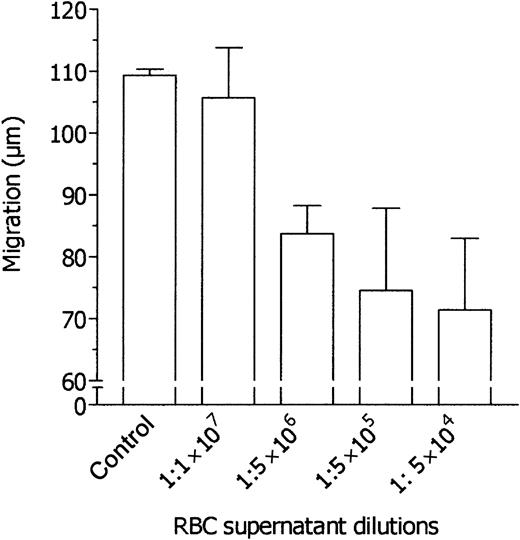
Neutrophil chemotaxis was studied by means of blind-well chambers, with a 3-μm pore filter, placed in the upper compartment of the chambers to separate the cells from the chemoattractant placed in the lower compartment. With 10 nM FMLP used as chemoattractant, the distance traveled by normal human neutrophils into the filters in 45 minutes was 125.6 ± 14.4 μm (mean ± 1 SD, n = 12). In preliminary experiments, the chemotactic response of neutrophils was found to be equally inhibited by RBC supernatants placed in the upper compartment of the migration chambers at dilutions ranging from 1:2 to 1:512 (vol/vol), and therefore higher dilutions of RBC supernatants were tested. As shown in Figure 1, neutrophil chemotaxis was inhibited by RBC supernatant up to 1:5 × 106 dilution. RBC supernatants did not affect neutrophil vitality as detected by the ethidium bromide–fluorescein diacetate assay (data not shown). These data suggest that RBC supernatants contain very potent inhibitory activities able to impair neutrophil chemotaxis at extraordinarily high dilutions.
Dose-dependent inhibition of FMLP-induced neutrophil migration by various dilutions of RBC supernatant. Neutrophils were incubated in the upper compartment of a chemotaxis chamber in the absence or presence of various dilutions of RBC supernatant, and their locomotory response to 10 nM FMLP in the lower compartment was tested after 45 minutes' incubation. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors.
Dose-dependent inhibition of FMLP-induced neutrophil migration by various dilutions of RBC supernatant. Neutrophils were incubated in the upper compartment of a chemotaxis chamber in the absence or presence of various dilutions of RBC supernatant, and their locomotory response to 10 nM FMLP in the lower compartment was tested after 45 minutes' incubation. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors.
Checkerboard analysis of neutrophil migration
Cell migration in response to a soluble compound can be related to 2 different locomotory mechanisms. The first one, chemokinesis, is the reaction by which the extent of random cell locomotion is enhanced by a compound in the environment. The other mechanism, the directional locomotion along the gradient of the solute in the environment is called true chemotaxis.24 To investigate whether RBC supernatant blocks chemokinesis or chemotaxis, experiments were carried out by means of the classic checkerboard analysis in the absence or presence of RBC supernatant. As shown in Table 2, RBC supernatant was found to inhibit the rate of cell locomotion in both chemokinetic and chemotactic conditions.
Checkerboard analysis of neutrophil locomotion in response to FMLP
. | FMLP below the filter, nM . | . | . | ||
|---|---|---|---|---|---|
| FMLP, above the filter, nM . | 0 . | 1 . | 10 . | ||
| Without RBC supernatant | |||||
| 0 | 51.5 ± 7.3 | 102.4 ± 9.8 (55.5 ± 5.5) | 112.6 ± 8.8 (61.2 ± 6.2) | ||
| 1 | — | 64.1 ± 2.9 | 91.3 ± 12.7 (70.2 ± 2.2) | ||
| 10 | — | — | 79.3 ± 3.9 | ||
| With RBC supernatant | |||||
| 0 | 40.5 ± 2.0 | 60.7 ± 3.2 (41.4 ± 2.0) | 66.5 ± 7.6 (43.5 ± 3.1) | ||
| 1 | — | 44.2 ± 2.2 | 55.8 ± 6.9 (46.4 ± 2.9) | ||
| 10 | — | — | 52.1 ± 5.5 | ||
. | FMLP below the filter, nM . | . | . | ||
|---|---|---|---|---|---|
| FMLP, above the filter, nM . | 0 . | 1 . | 10 . | ||
| Without RBC supernatant | |||||
| 0 | 51.5 ± 7.3 | 102.4 ± 9.8 (55.5 ± 5.5) | 112.6 ± 8.8 (61.2 ± 6.2) | ||
| 1 | — | 64.1 ± 2.9 | 91.3 ± 12.7 (70.2 ± 2.2) | ||
| 10 | — | — | 79.3 ± 3.9 | ||
| With RBC supernatant | |||||
| 0 | 40.5 ± 2.0 | 60.7 ± 3.2 (41.4 ± 2.0) | 66.5 ± 7.6 (43.5 ± 3.1) | ||
| 1 | — | 44.2 ± 2.2 | 55.8 ± 6.9 (46.4 ± 2.9) | ||
| 10 | — | — | 52.1 ± 5.5 | ||
Results are expressed as the distance migrated by the leading front of cells, μm/45 min. Numbers in parentheses show the calculated distance migrated if cells would have not responded to the gradient of FMLP, but would have moved randomly in the gradient. Calculations were performed according to Zigmond and Hirsch.24 Data are means ± 1 SD; n = 3. — indicates not done
Induction of neutrophil migration by RBC supernatants
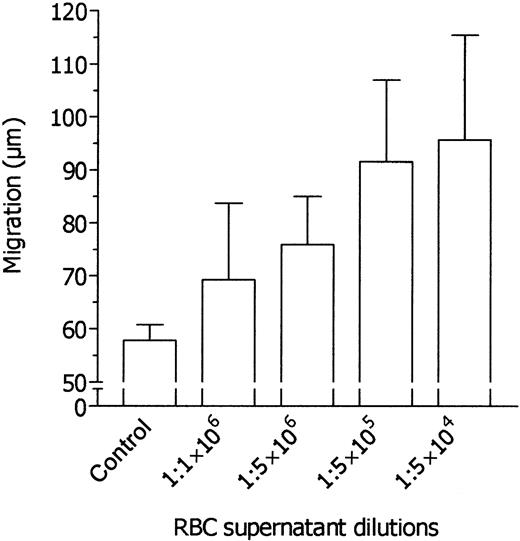
When normal neutrophils are incubated in the upper compartment of migration chambers without the addition of chemoattractant in the lower compartment, they migrate spontaneously. Under our experimental conditions, spontaneous neutrophil migration was 60.0 ± 12.6 μm (mean ± 1 SD, n = 12). In preliminary experiments, cell migration was stimulated by RBC supernatants added to the lower compartment of migration chambers at dilutions ranging from 1:2 to 1:512 (vol/vol), and higher dilutions of RBC supernatants were therefore tested. As shown in Figure 2, neutrophil migration was stimulated by RBC supernatant up to 1:1 × 106 dilution. It is noteworthy that the stimulatory activity of the supernatants was detected at dilutions comparable to those found to inhibit the chemotactic response to FMLP.
Dose-dependent induction of neutrophil migration by various dilutions of RBC supernatant. Neutrophils were incubated in the upper compartment of a chemotaxis chamber in medium, and their locomotory response in the absence or presence of various dilutions of RBC supernatant in the lower compartment was tested after 45 minutes' incubation. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors.
Dose-dependent induction of neutrophil migration by various dilutions of RBC supernatant. Neutrophils were incubated in the upper compartment of a chemotaxis chamber in medium, and their locomotory response in the absence or presence of various dilutions of RBC supernatant in the lower compartment was tested after 45 minutes' incubation. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors.
Effects of RBC supernatants on neutrophil activation
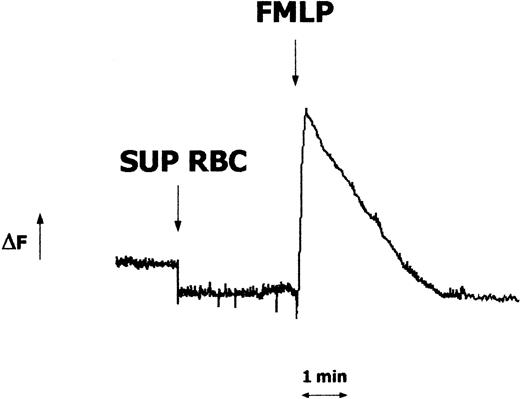
A series of experiments were then performed to test the capacity of RBC supernatants to trigger neutrophil activation and/or to interfere with FMLP-induced functional responses. First, we tested the effects of supernatants on the intracellular levels of calcium. As shown in Figure 3, no Ca++ mobilization was observed in Fura-2–loaded neutrophils exposed to RBC supernatant. Furthermore, RBC supernatant–treated neutrophils maintained their capacity to mount a rapid increase of Ca++ in response to FMLP. Then, oxidative metabolism, determined as flow cytometric analysis of intracellular oxidation of 2′-7′-dichlorofluorescin in DCFH-DA–loaded neutrophils, was studied. Consistent with data in the Ca++ assay, RBC supernatants did not stimulate the respiratory burst or impair the FMLP-triggered oxidative response by neutrophils (data not shown).
Effects of FMLP and RBC supernatants on the intracellular calcium levels in human neutrophils. Neutrophils (2.5 × 106) were loaded with Fura-2 AM, and fluorescence changes (ΔF) were monitored before and after exposure to RBC supernatant (1:1 × 105, vol/vol dilution) or FMLP (10 nM). Downward arrow indicates the stimulus addition. Results represent 1 of 3 experiments that yielded similar results.
Effects of FMLP and RBC supernatants on the intracellular calcium levels in human neutrophils. Neutrophils (2.5 × 106) were loaded with Fura-2 AM, and fluorescence changes (ΔF) were monitored before and after exposure to RBC supernatant (1:1 × 105, vol/vol dilution) or FMLP (10 nM). Downward arrow indicates the stimulus addition. Results represent 1 of 3 experiments that yielded similar results.
Effects of calcium-independent chemoattractants on neutrophil migration
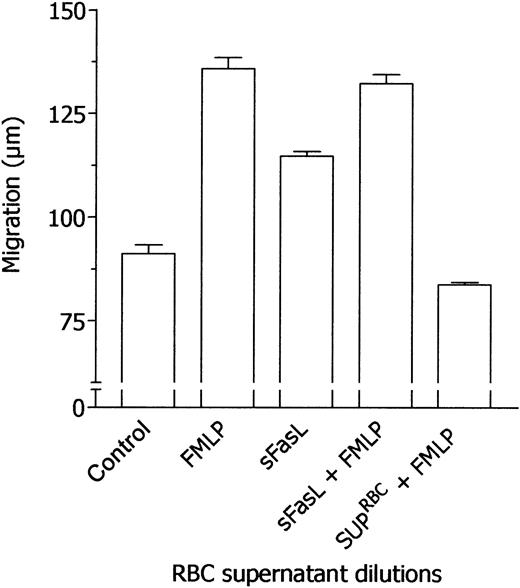
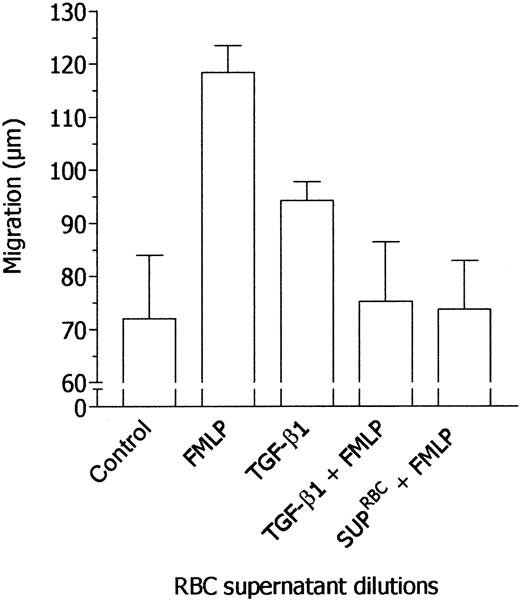
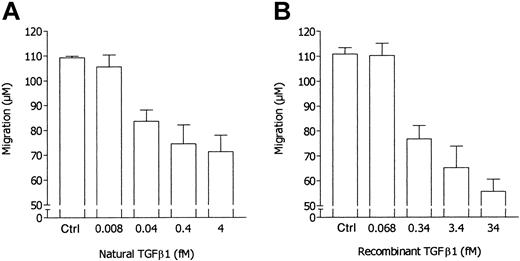
Taking into account the results reported so far, we hypothesized that the effects of RBC supernatants on neutrophil locomotion might be mediated by a calcium-independent chemokine able, on the one hand, to induce neutrophil migration and, on the other hand, to desensitize neutrophils to FMLP stimulation. At present, the major chemokines that exert their action without mobilizing intracellular calcium are sFasL23 and TGF-β1.27 Therefore, the concentrations of these chemokines in RBC supernatants have been determined. Mean concentrations from 10 different RBC supernatants were 21.15 ± 9.58 ng/mL sFasL and 4.85 ± 1.9 ng/mL active TGF-β1. In the present work, all experiments were performed with a sample of RBC units containing 18.7 ng/mL sFasL and 4.26 ng/mL TGF-β1. Moreover, these chemokines have been tested in parallel experiments with RBC supernatant to evaluate their ability to desensitize neutrophil chemotactic response to FMLP. As summarized in Figure 4, sFasL was incapable of inhibiting neutrophil responsiveness to FMLP whereas RBC supernatant was effective. On the contrary, TGF-β1 inhibited neutrophil locomotory response toward FMLP, simulating the effect of RBC supernatant (Figure 5). Indeed, when we take into account the concentration of natural TGF-β1 in RBC supernatant used in the assay, the inhibitory potency of recombinant TGF-β1 appeared to be 1 log lower than that of natural TGF-β1–containing RBC supernatants, as suggested by the comparison of dose-response experiments with recombinant TGF-β1 with data reported in Figure 1 expressed on a molar basis (Figure 6).
Failure of sFasL to desensitize neutrophils to the subsequent stimulation by FMLP. Locomotory activity of neutrophils incubated in the upper compartment of a chemotaxis chamber with medium and exposed in the lower compartment for 45 minutes to medium (Nil), 10 nM FMLP (FMLP), or 0.1 nM sFasL was compared with locomotory activity of cells incubated in the upper compartment with 10 nM sFasL (sFasL + FMLP) or RBC supernatant (1:1 × 105, vol/vol dilution, SUP-RBCs + FMLP) and exposed in the lower compartment to 10 nM FMLP. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors.
Failure of sFasL to desensitize neutrophils to the subsequent stimulation by FMLP. Locomotory activity of neutrophils incubated in the upper compartment of a chemotaxis chamber with medium and exposed in the lower compartment for 45 minutes to medium (Nil), 10 nM FMLP (FMLP), or 0.1 nM sFasL was compared with locomotory activity of cells incubated in the upper compartment with 10 nM sFasL (sFasL + FMLP) or RBC supernatant (1:1 × 105, vol/vol dilution, SUP-RBCs + FMLP) and exposed in the lower compartment to 10 nM FMLP. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors.
Desensitization of neutrophil response to FMLP by TGF-β1. Locomotory activity of neutrophils incubated in the upper compartment of a chemotaxis chamber with medium and exposed in the lower compartment for 45 minutes to medium (Nil), 10 nM FMLP (FMLP), or 40 fM TGF-β1 was compared with locomotory activity of cells incubated in the upper compartment with 40 fM TGF-β1 (TGF-β1 + FMLP) or RBC supernatant (1:1 × 105, vol/vol dilution, SUP-RBCs + FMLP) and exposed in the lower compartment to 10 nM FMLP. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors.
Desensitization of neutrophil response to FMLP by TGF-β1. Locomotory activity of neutrophils incubated in the upper compartment of a chemotaxis chamber with medium and exposed in the lower compartment for 45 minutes to medium (Nil), 10 nM FMLP (FMLP), or 40 fM TGF-β1 was compared with locomotory activity of cells incubated in the upper compartment with 40 fM TGF-β1 (TGF-β1 + FMLP) or RBC supernatant (1:1 × 105, vol/vol dilution, SUP-RBCs + FMLP) and exposed in the lower compartment to 10 nM FMLP. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors.
Comparison of dose-dependent inhibitory activity of natural TGF-β1 and recombinant TGF-β1 on FMLP-induced neutrophil locomotion. In response to 10 nM FMLP, the locomotory activity of neutrophils incubated in the upper compartment of the chemotaxis chamber in the absence or presence of natural TGF-β1 (nTGF-β1)–containing RBC supernatant (panel A) was compared with the locomotory activity of neutrophils incubated in the upper compartment of the chemotaxis chamber in the absence or presence of various doses of recombinant TGF-β1 (rTGF-β1) (panel B). Results in panel A are from data reported in Figure 1 and are expressed on a molar basis, with the concentration of natural TGF-β1 in RBC supernatant used in the assay (4.26 ng/mL) being taken into account. Ctrl indicates control.
Comparison of dose-dependent inhibitory activity of natural TGF-β1 and recombinant TGF-β1 on FMLP-induced neutrophil locomotion. In response to 10 nM FMLP, the locomotory activity of neutrophils incubated in the upper compartment of the chemotaxis chamber in the absence or presence of natural TGF-β1 (nTGF-β1)–containing RBC supernatant (panel A) was compared with the locomotory activity of neutrophils incubated in the upper compartment of the chemotaxis chamber in the absence or presence of various doses of recombinant TGF-β1 (rTGF-β1) (panel B). Results in panel A are from data reported in Figure 1 and are expressed on a molar basis, with the concentration of natural TGF-β1 in RBC supernatant used in the assay (4.26 ng/mL) being taken into account. Ctrl indicates control.
Effect of sFasL- and TGF-β1–depleted RBC supernatant on neutrophil chemotaxis
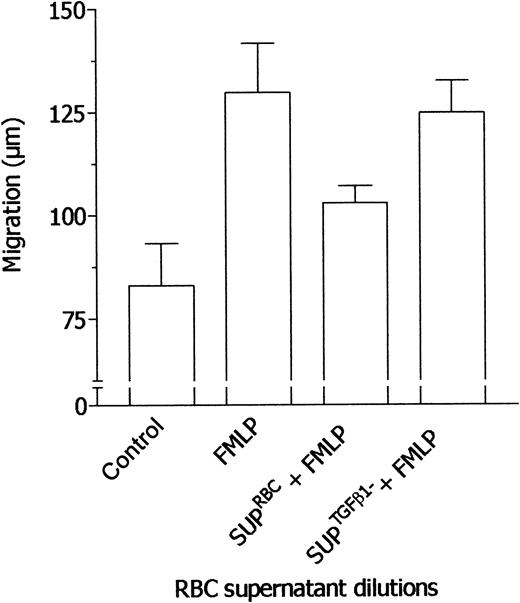
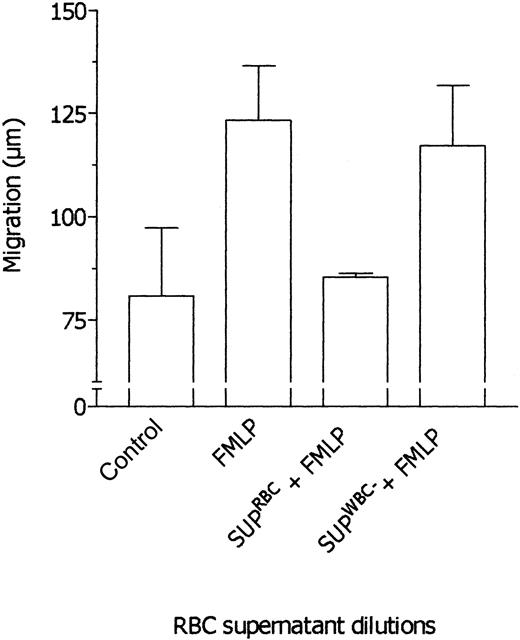
RBC supernatant was immunodepleted of TGF-β1 and sFasL, and after this procedure the level of these cytokines in the supernatant was undetectable. Then, the effects of undepleted and TGF-β1–depleted RBC supernatants were tested in parallel assays. As shown in Figure 7, TGF-β1–depleted RBC supernatant did not inhibit neutrophil chemotaxis in response to FMLP whereas the undepleted supernatant was effective. On the other hand, sFasL depletion did not affect the RBC supernatant properties (not shown). These findings strongly support the idea that the effects exerted by RBC supernatant on neutrophil locomotory responses are actually mediated by TGF-β1. Also, the results rule out the possibility that immune complexes generated by TGF-β1 immunodepletion may be responsible for reversal of inhibition. In ancillary experiments, supernatants from prestorage leukodepleted RBCs were tested in comparison with supernatants from nonleukodepleted RBCs. As shown in Figure 8, prestorage leukodepletion completely prevented the ability of RBC supernatant to inhibit neutrophil chemotaxis toward FMLP.
Effect of TGF-β1 immunodepletion on the ability of RBC supernatants to desensitize neutrophils to FMLP. Locomotory activity of neutrophils incubated in the upper compartment of chemotaxis chamber with medium and exposed in the lower compartment for 45 minutes to medium (Nil) and 10 nM FMLP (FMLP) was compared with locomotory activity of cells incubated in the upper compartment with RBC supernatant (1:1 × 105, vol/vol dilution; SUPRBCs + FMLP) or TGF-β1–depleted RBC supernatant (1:1 × 105, vol/vol dilution; SUPTGF-β1 + FMLP) and exposed in the lower compartment to 10 nM FMLP. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors.
Effect of TGF-β1 immunodepletion on the ability of RBC supernatants to desensitize neutrophils to FMLP. Locomotory activity of neutrophils incubated in the upper compartment of chemotaxis chamber with medium and exposed in the lower compartment for 45 minutes to medium (Nil) and 10 nM FMLP (FMLP) was compared with locomotory activity of cells incubated in the upper compartment with RBC supernatant (1:1 × 105, vol/vol dilution; SUPRBCs + FMLP) or TGF-β1–depleted RBC supernatant (1:1 × 105, vol/vol dilution; SUPTGF-β1 + FMLP) and exposed in the lower compartment to 10 nM FMLP. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors.
Effect of leukodepleted RBC supernatants on the neutrophil response to FMLP. Locomotory activity of neutrophils incubated in the upper compartment of the chemotaxis chamber with medium and exposed in the lower compartment for 45 minutes to medium (Nil) and 10 nM FMLP (FMLP) was compared with locomotory activity of cells incubated in the upper compartment with RBC supernatant (1: 1 × 105, vol/vol dilution; SUPRBCs + FMLP) or leukodepleted RBC supernatant (1:1 × 105, vol/vol dilution; SUPWBC + FMLP) and exposed in the lower compartment to 10 nM FMLP. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors.
Effect of leukodepleted RBC supernatants on the neutrophil response to FMLP. Locomotory activity of neutrophils incubated in the upper compartment of the chemotaxis chamber with medium and exposed in the lower compartment for 45 minutes to medium (Nil) and 10 nM FMLP (FMLP) was compared with locomotory activity of cells incubated in the upper compartment with RBC supernatant (1: 1 × 105, vol/vol dilution; SUPRBCs + FMLP) or leukodepleted RBC supernatant (1:1 × 105, vol/vol dilution; SUPWBC + FMLP) and exposed in the lower compartment to 10 nM FMLP. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors.
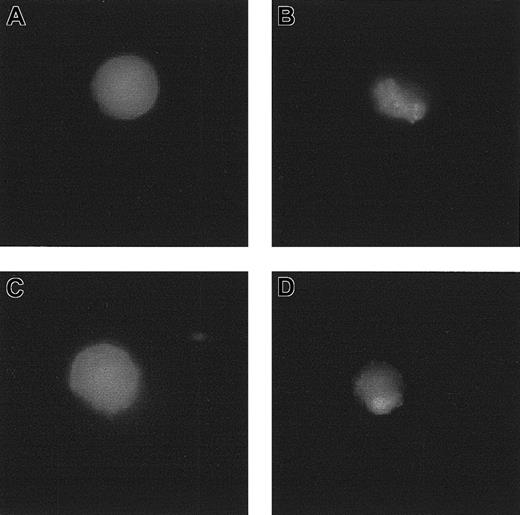
Effect of RBC supernatants on the FMLP-induced polarization and expression of CD11b
When incubated with a FITC-conjugated anti-CD11b mAb, purified neutrophils displayed the typical spherical morphology of resting cells, with a uniform fluorescence distribution (Figure 9). On the contrary, when the cells were first exposed to FMLP, before the incubation with the anti-CD11b mAb they displayed a polarized shape with CD11b redistribution (Figure 9). This is a typical response of locomotory cells engaged with chemotactic triggers. Nevertheless, as shown in Figure 9, this phenomenon was prevented by RBC supernatants but not by TGF-β1–depleted RBC supernatants. Finally, RBC supernatants did not influence the expression of CD11b on neutrophils and did not affect the CD11b up-regulation induced by FMLP (data not shown)
Effect of RBC supernatant on the FMLP-induced polarization and redistribution of CD11b. (A) Neutrophils exposed to FITC-conjugated anti-CD11b mAb. (B) Neutrophils exposed to 1 μM FMLP and labeled with FITC-conjugated anti-CD11b mAb. (C) Neutrophils preincubated with RBC supernatant (1:1 × 105, vol/vol dilution), exposed to 1 μM FMLP, and labeled with FITC-conjugated anti-CD11b mAb. (D) Neutrophils preincubated with TGF-β1–depleted RBC supernatant (1:1 × 105, vol/vol dilution), exposed to 1 μM FMLP, and labeled with FITC-conjugated anti-CD11b mAb.
Effect of RBC supernatant on the FMLP-induced polarization and redistribution of CD11b. (A) Neutrophils exposed to FITC-conjugated anti-CD11b mAb. (B) Neutrophils exposed to 1 μM FMLP and labeled with FITC-conjugated anti-CD11b mAb. (C) Neutrophils preincubated with RBC supernatant (1:1 × 105, vol/vol dilution), exposed to 1 μM FMLP, and labeled with FITC-conjugated anti-CD11b mAb. (D) Neutrophils preincubated with TGF-β1–depleted RBC supernatant (1:1 × 105, vol/vol dilution), exposed to 1 μM FMLP, and labeled with FITC-conjugated anti-CD11b mAb.
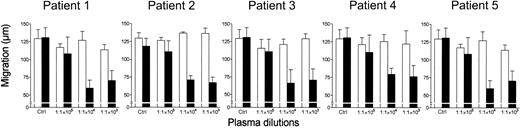
Effect of RBC transfusion on ex vivo neutrophil chemotaxis
To verify the clinical relevance of the present findings, the locomotory properties of neutrophils from healthy donors were tested in the presence of plasma obtained from 5 patients before and after RBC transfusion. As shown in Figure 10, the neutrophil chemotactic response to FMLP was inhibited only by the plasma obtained after RBC transfusion. Furthermore, neutrophils purified from patient no. 1 after RBC transfusion displayed an impaired locomotory response to FMLP (-85% versus control), whereas no significant differences with the control were observed with neutrophils obtained before the transfusion. Conversely, plasma samples from different healthy donors also did not inhibit FMLP-triggered neutrophil chemotaxis or stimulate neutrophil locomotion when tested to dilutions ranging from 1:4 to 1:1.5 × 106 (data not shown). Finally, at the same dilutions, plasma from healthy volunteers did not interfere with the capacity of RBC supernatants to modulate neutrophil locomotion (data not shown).
Effect of RBC transfusion on plasma modulation of FMLP-triggered chemotaxis. Neutrophils were incubated in the upper compartment of a chemotaxis chamber in the absence (Nil) or presence of various dilutions of plasma obtained from 5 patients before (open bars) and after (black bars) RBC transfusion. The locomotory response of neutrophils to 10 nM FMLP in the lower compartment was tested after 45 minutes' incubation. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors. Ctrl indicates control.
Effect of RBC transfusion on plasma modulation of FMLP-triggered chemotaxis. Neutrophils were incubated in the upper compartment of a chemotaxis chamber in the absence (Nil) or presence of various dilutions of plasma obtained from 5 patients before (open bars) and after (black bars) RBC transfusion. The locomotory response of neutrophils to 10 nM FMLP in the lower compartment was tested after 45 minutes' incubation. Results are expressed as mean ± 1 SD of 3 experiments with neutrophils from different donors. Ctrl indicates control.
Discussion
The results of the present study provide evidence that supernatants from stored RBCs contain factors capable of inhibiting both chemokinesis and chemotaxis of neutrophils. This inhibitory activity appears to be extremely potent in that a reduction of neutrophil responsiveness has been detected with the use of RBC supernatant up to 1:5 × 106 dilution.
The effects of supernatants from stored RBCs on several immune cell activities have been already studied. RBC supernatants have been shown to inhibit lymphocyte proliferative responses to mitogens and in allogeneic mixed cultures, to impair natural killer cell and cytotoxic T-cell activities, and to trigger apoptosis.15,21,22 Nevertheless, as compared with the present findings, these inhibitory activities were detected with the use of either undiluted or relatively low dilutions of RBC supernatant. Owing to the extraordinary potency of the inhibitory activity on neutrophil chemotactic responsiveness found in RBC supernatant, attempts were made to detect whether these supernatants would also affect neutrophil locomotion. We found that RBC supernatants are also endowed with chemotactic activity toward neutrophils. It is of note that the 2 activities, inhibition of the cell response to FMLP and stimulation of neutrophil migration, could be detected at similar supernatant dilutions. As a large body of literature shows that certain chemotaxins not only desensitize the cells toward a further stimulation with the same chemotaxin but also prevent the cell locomotory response toward other chemotaxins,28,29 we hypothesized that a similar factor might be responsible for these apparently opposite activities. Therefore, a chemotaxin might actually be the better candidate to explain the effects of RBC supernatant on neutrophils. In other words, a chemotaxin present in RBC supernatants might desensitize neutrophils toward stimulation by FMLP and, in parallel, be responsible for the chemotactic activity of supernatant.
Neutrophil chemotaxins can be classified into 2 functional groups.30 The first group, classical chemotaxins, includes FMLP, C5a, and interleukin 8 (IL-8) and is characterized by the ability to induce both chemotaxis and increase of intracellular free-calcium levels.28 The second group, pure chemotaxins such as TGF-β1, sFasL, substance P, and fibrinopeptide B,27,30,31 includes molecules able to induce chemotaxis without causing cell activation (ie, degranulation and oxidant generation) or increasing the intracellular calcium levels. As shown here, RBC supernatant did not modify the levels of free calcium in neutrophils or activate respiratory bursts. Therefore, one of the chemotaxins belonging to the second group appeared as an appropriate candidate to explain our observations. Various experimental findings are consistent with the conclusion that TGF-β1 is responsible for the effects displayed by RBC supernatant on neutrophil locomotion as follows: (a) Recombinant TGF-β1, but not sFasL, was capable as RBC supernatant to desensitize neutrophils toward FMLP chemotactic stimulation. (b) TGF-β1–depleted, but not undepleted, RBC supernatant failed to inhibit neutrophil chemotactic response toward FMLP. (c) TGF-β1 is undetectable in leukodepleted RGC supernatants that are, in turn, fully devoid of inhibitory activity. (d) The concentration of TGF-β1 detected in RBC supernatant was near to (approximately 1 log lower than) that of recombinant TGF-β1, which simulates the effects of supernatants on neutrophil behavior. This last quantitative discrepancy seems to be reasonably explained by a better neutrophil-directed activity of natural, as compared with recombinant TGF-β1. Other soluble mediators, that is, certain cytokines such as IL-1 or IL-4, may be involved in RBC supernatant activities. Unlike TGF-β1, which exerts its neutrophil-directed function at femtomolar concentrations, cytokines activate specific neutrophil responses at nanomolar concentrations very unlikely to be detected at the high dilutions of supernatants used in our settings. Nevertheless, we cannot rule out the existence of a putative mediator cooperating with TGF-β1 by priming neutrophil responses at subnanomolar concentrations.
Neutrophils are front-line agents in host defense against invading micro-organisms.32 They have several types of receptors, including chemoattractant receptors and integrins, by which neutrophils sense the pathogens and are recruited to sites of infections.33 When circulating into peripheral blood vessels, neutrophils display spherical morphology typical of resting blood cells, and integrins are uniformly distributed on the plasma membrane. After chemotactic factor triggering, neutrophils change their shape abruptly, acquiring a polarized morphology with the formation of a posterior tail, termed a uropod.33 At the same time, adhesion molecules, including β-integrins, undergo a topographic clustered redistribution in the plasma membrane.34 It is generally assumed that both of these phenomena are epiphenomena of the mechanical responses of cells engaged in locomotory responses. Thus, the present data showing that FMLP-induced neutrophil shape change and CD11b clustering are completely inhibited by native, but not by TGF-β1–depleted, RBC supernatant are supportive evidence for a major role of this cytokine in neutrophil-directed effects of RBC supernatant.
When we take into account the crucial role of neutrophils in host immune competence,32 the ability, shown here, of high dilutions of stored RBC supernatant to inhibit neutrophil chemotaxis appears as a potential mechanism contributing to the establishment of infections in transfusion patients in the postoperative period. Evidence for transfusions as a risk factor for bacterial infections after elective surgery is indeed increasing.2,5 In particular, various studies in both clean orthopedic and potentially contaminated abdominal surgery have all suggested significantly fewer infections in transfusion patients.6,10-12,14 Although posttransfusion lymphopenia is likely to contribute to the impaired host defense against infections, the inhibitory effect, shown here, of TGF-β1 on neutrophil chemotactic responsiveness appears to depict a new scenario. TGF-β1 is a 25-kDa homodimer that has been shown to be a natural and potent inhibitor of various immunologic responses,35 and it may contribute substantially to the reported immunosuppressive effects in recipients of RBC transfusions. In fact, the effects of this cytokine include the inhibition of the proliferation and function of T cells particularly by reducing IL-2 production and activity,36,37 the inhibition of B lymphocyte proliferation as well as the generation of cytotoxic T cells,38,39 and the inhibition of monocyte-macrophage activation.40 Many cells, including mononuclear cells, platelets, and granulocytes, are capable of synthesizing TGF-β1.41 These cells secrete a biologically inactive, latent form of the cytokine capable of binding to several proteins, mainly the so-called latency-associated peptide (LAP), but also α2-macroglobulin and extracellular matrix components.41 To exert its biologic activities, latent TGF-β1 must be activated by proteolytic cleavage (ie, plasmin) or by conformational changes induced by binding with thrombospondin and integrins.41 Currently, the reliability of available techniques to quantify TGF-β1 in plasma is questionable, mainly because of possible interference by plasmatic compounds.41 Nevertheless, our results show that there is no biologically detectable TGF-β1 activity in normal human plasma. This is suggested by 2 sets of evidence: (a) We could not observe any interference with neutrophil locomotory activity by plasma samples from different healthy donors. (b) Different dilutions of normal human plasma samples were found incapable of hindering TGF-β1–dependent modulation of neutrophil locomotion by RBC supernatants. On the contrary, our preliminary results hint that plasmatic TGF-β1 activity could be detected after RBC transfusion, when one takes into account that the effects of RBC supernatants on FMLP-triggered neutrophil chemotaxis have been mimicked by the plasma from 5 patients after RBC transfusion. Consistently, neutrophils purified from one patient after RBC transfusion displayed impaired locomotory capacities. Thus, these preliminary observations seem to suggest that our in vitro findings may be reproduced in vivo by RBC transfusion.
It is noteworthy that the inhibitory activity of RBC supernatants on neutrophil chemotaxis is completely prevented by prestorage leukodepletion of packed RBCs. This is conceivably explained by the ability of various leukocyte populations, particularly T lymphocytes and macrophages, to produce TGF-β1.42 The stimulus responsible for activation of TGF-β1 production by leukocytes during blood storage remains to be identified, although free hemoglobin, which has previously been shown to be capable of stimulating mononuclear leukocytes to release cytokines,43 is a putative stimulant. In any case, the findings presented here point toward the use of leukocyte-depleted blood for transfusion. This is consistent with the finding suggested by several prospective randomized trials that the avoidance of exposure to allogeneic leukocytes during preoperative transfusions reduces the incidence of bacterial infections.15
Supported by grants from Ministero dell' Università e della Ricerca Scientifica e Tecnologica (MURST) National Program 2002 “Apoptosis in human autoimmune and onco-haematological diseases. Possible diagnostic uses and therapeutic perspectives” (no. 2002065393) and from the University of Genoa (F.I., F.D., and F.P.).
M.G. and L.O. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal