Abstract
Activating mutations of FLT3 have been detected in patients with acute myeloid leukemia (AML). Two distinct types of FLT3 mutations are most common: internal tandem duplication (ITD) of sequences coding for the juxtamembrane domain and point mutations at codon 835 (Asp835) within the kinase domain. Both types of mutations constitutively activate the tyrosine kinase activity of FLT3 in experimental systems and result in factor-independent proliferation of Ba/F3 and 32D cells. Recently, novel mutations within the activation loop were identified in patients with AML: deletion of isoleucine 836 (Ile836del) and an exchange of isoleucine 836 to methionine plus an arginine insertion (Ile836Met+Arg). To examine whether the Ile836 mutations result in constitutive activation of the FLT3 receptor, we introduced both mutant FLT3 cDNAs transiently into HEK 293 cells. Both mutant FLT3 receptors were constitutively autophosphorylated in the absence of ligand and kinase activity led to constitutive activation of downstream signaling cascades as determined by activation of the STAT5 (signal transducer and activator of transcription 5) pathway. When stably expressed in the growth factor–dependent cell lines Ba/F3 and 32D, both deletion and insertion mutants led to factor-independent proliferation, indicating that both mutants have transforming capabilities. We then examined the sensitivity of the FLT3 ITD, FLT3 Asp835Tyr, and the novel FLT3 receptor mutants toward the kinase inhibitors AG1296, PKC412, and SU5614. We show that these FLT3 kinase inhibitors have distinct inhibitory potencies against different activating FLT3 receptor mutants. These results suggest that it may be useful to determine the exact kind of FLT3 mutation when applying receptor kinase inhibitors in clinical trials.
Introduction
The receptor tyrosine kinase (RTK) FLT3 belongs to the class III RTK subfamily that also includes KIT, FMS, and platelet-derived growth factor receptor (PDGF-R).1,2 These class III RTK members are characterized by an extracellular domain consisting of 5 immunoglobulin-like domains, a juxtamembrane domain, and 2 kinase domains (KDs) interrupted by a kinase insert.3 Ligand binding to the extracellular domain results in dimerization of the receptor followed by autophosphorylation on specific intracellular tyrosine residues. Subsequently, multiple downstream signaling pathways are activated.4-6 Activation of the FLT3 receptor with its ligand (FL) plays an important role in proliferation and differentiation of early hematopoietic progenitors.7-9
The FLT3 receptor tyrosine kinase is expressed on blast cells in most patients with acute myelogenous leukemia (AML), and activating mutations of FLT3 have been detected in approximately 30% of these patients.10 Two distinct groups of FLT3 mutations are most common: internal tandem duplications (ITDs) of the juxtamembrane coding sequence in 20% to 27% of patients with AML11-13 and point mutations at codon 835 (Asp835) within the second kinase domain in about 7% of patients with AML.12,14 Patients carrying the FLT3 ITD mutation seem to have a significantly worse prognosis, whereas the effect of point mutations on the prognosis of patients with AML has not yet been defined.11,12 Both types of mutations constitutively activate the FLT3 receptor, leading to activation of downstream signaling proteins, including STAT5 (signal transducer and activator of transcription 5) and MAP (mitogen-activated protein) kinase, and result in factor-independent proliferation of growth factor–dependent murine lymphoid and myeloid cells.14-16 Additional evidence in support of an oncogenic role of FLT3 mutations stems from studies demonstrating that mice receiving transplants of bone marrow retrovirally infected with FLT3 ITD develop a myeloproliferative disease.17
Recently, a series of novel mutations within the activation loop were identified in patients with AML. In that study, deletion of isoleucine 836 (Ile836del) occurred in 13 of 87 patient samples carrying point mutations in the kinase domain, and one sample was found to contain a novel point mutation, changing isoleucine 836 to methionine with an additional arginine inserted after codon 836 (Ile836Met+Arg).12 The effect of these mutations on FLT3 receptor signaling activity has not yet been investigated.
Because the FLT3 receptor is expressed in blast cells of most patients with AML and a significant fraction of these patients carry an activating mutation conferring a worse prognosis, targeting the molecule by specific inhibitors may establish new treatment options for this type of leukemia. Several FLT3 inhibitors have been shown to suppress growth factor–independent proliferation of FLT3 ITD-expressing cells and prolong the survival in a mouse model of FLT3 ITD–induced disease.18-20
In this study, we investigated the role of newly described FLT3 receptor mutations on receptor activation and cell growth. Our results indicate that both deletion and insertion mutants are sufficient to constitutively activate the FLT3 receptor and have transforming potential when expressed in the growth factor–dependent murine cell lines Ba/F3 and 32D. Because it has recently been demonstrated that different activating mutations of the closely related tyrosine kinase KIT respond differentially to KIT kinase inhibitors,21,22 we furthermore examined the sensitivity of the FLT3 ITD, FLT3 Asp835Tyr, and also the novel FLT3 receptor mutants toward the compounds AG1296, PKC412, and SU5614.
Materials and methods
Growth factors, antibodies, and inhibitors
Recombinant mouse FL and interleukin-3 (IL-3) were purchased from R&D Systems, Wiesbaden, Germany. Rabbit polyclonal antimouse-FLT3 antibody and rabbit–anti-STAT5 A/B antiserum were obtained from Upstate Biotechnology, Lake Placid, NY. Monoclonal phosphospecific STAT5 A/B antibody was a generous gift from Tom Wheeler, Hamilton, New Zealand.23 Antiphoshotyrosine antibodies were purchased from Upstate Biotechnology (4G10) and PharMingen, Heidelberg, Germany (pY20).
AG1296 and SU5614 were obtained from Calbiochem-Novabiochem, Schwalbach, Germany. PKC412 was a kind gift from Novartis Pharma AG, Basel, Switzerland. Each compound was dissolved in dimethyl sulfoxide (DMSO) to make an initial stock solution.
DNA constructs
The cDNA of murine wild-type FLT3 and FLT3 ITD was kindly provided by Hubert Serve (Münster, Germany).16 Site-directed mutagenesis of Asp835 and Ile836 (numbering is based on the human FLT3) was performed with QuikChange Site-Directed Mutagenesis Kit (Strategene, Heidelberg, Germany) according to the manufacturer's instructions. All constructs were confirmed by sequencing.
Cell culture and transfection methods
Ba/F3 and 32Dcl3 cells were cultured in RPMI 1640 (GIBCO-BRL, Karlsruhe, Germany) supplemented with 10% fetal calf serum (FCS; Biochrom KG, Berlin, Germany), glutamine, and IL-3. HEK 293 cells were maintained in Dulbecco modified Eagle medium (DMEM; GIBCO-BRL) supplemented with 10% FCS.
Ba/F3 and 32Dcl3 cells were transfected by electroporation. HEK 293 cells were transfected using Lipofectamine 2000 (Invitrogen, Karlsruhe, Germany) according to the manufacturer's recommendations.
Immunoprecipitation and immunoblotting
Cells were lysed in lysis buffer containing 10 mM Tris (tris(hydroxymethyl) aminomethane)–HCl (pH 7.4), 5 mM EDTA (ethylenediaminetetraacetic acid), 130 mM NaCl, 1% Triton X-100, 20 mM sodium phosphate (pH 7.5), 10 mM sodium pyrophosphate (pH 7.0), 50 mM NaF, 1 mM sodium orthovanadate, 1 mM glycerolphosphate, and protease inhibitors (Roche Diagnostics, Mannheim, Germany). After clarification by centrifugation and preclearing with protein A-Sepharose (Amersham/Pharmacia Biotech, Freiburg, Germany) 2 μg anti-FLT3 antibody was added. Antibody-protein complexes were precipitated with protein A-Sepharose. For immunoblotting and immunoprecipitation whole cell lysates and bound fractions, respectively, were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and blotting was performed on polyvinylidene fluoride (PVDF) membranes (Immobilon-P; Millipore, Eschborn, Germany). Detection of phosphotyrosine was performed using a mixture of the antiphosphotyrosine antibodies 4G10 and pY20. For detection of FLT3, p-STAT5, and STAT5, the indicated antibodies were used. After incubating the blots with horseradish peroxidase–conjugated secondary antibody, they were developed using SuperSignal chemoluminescent substrates from Pierce (Perbio Science, Bonn, Germany). Band analysis was performed using Quantity One 4.2 Software (BIO-RAD, Hercules, CA).
Proliferation and apoptosis assays
Cells (2 × 104/well) were plated into 96-well plates, and inhibitors were added as indicated. At the indicated time points cell viability was measured using the CellTiter96 Proliferation Assay (Promega, Mannheim, Germany) according to the manufacturer's instructions.
Apoptosis was examined using the Annexin V–FITC (fluorescein isothiocyanate) staining kit (PharMingen) according to the manufacturer's recommendations.
Results
Constitutive phosphorylation of FLT3 and activation of STAT5 in cells expressing mutant FLT3 receptor
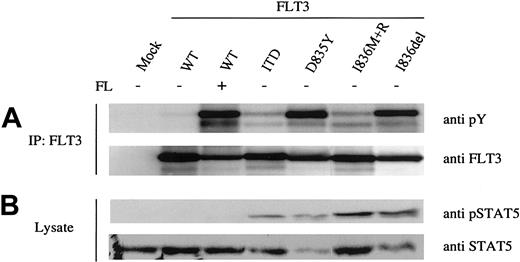
Amino acid changes within the activation loop of FLT3 have been shown to result in factor-independent phosphorylation of the FLT3 receptor.14,24 Recently, 2 novel mutations at codon 836 within the kinase domain were identified in patients with AML: deletion of isoleucine 836 (Ile836del) and the exchange of isoleucine 836 to methionine plus an arginine insertion (Ile836Met+Arg).12 To examine whether these Ile836 mutations result in constitutive activation of the receptor, we introduced both mutant FLT3 cDNAs transiently into HEK293 cells. As a control we established the same cell line expressing the wild-type (WT) FLT3 receptor and the ITD and Asp835Tyr mutants. Immunoblotting analysis using an antiphosphotyrosine antibody after immunoprecipitation of the FLT3 receptor revealed strong autophosphorylation of the deletion mutant Ile836del (Figure 1A), and constitutive phosphorylation was comparable to the Asp835Tyr mutant. In contrast, the Ile836Met+Arg mutant showed a weaker autophosphorylation signal, which was comparable to the FLT3 ITD mutant (Figure 1A).
Activation status of FLT3 receptor mutants. The indicated FLT3 constructs and vector alone (mock) were transiently overexpressed in HEK 293 cells. The FLT3 WT expressing cells were either untreated or stimulated with FLT3 ligand (FL). (A) FLT3 was immunoprecipitated from whole cell lysates and immunoblotted with antiphosphotyrosine antibody (upper panel). Subsequently, the blots were stripped and reblotted with anti-FLT3 antibody (lower panel). (B) Activation of STAT5 was demonstrated by immunoblotting the total cell lysates with anti-pSTAT5 antibody (upper panel). After stripping the membrane was reprobed with total anti-STAT5 antibody (lower panel).
Activation status of FLT3 receptor mutants. The indicated FLT3 constructs and vector alone (mock) were transiently overexpressed in HEK 293 cells. The FLT3 WT expressing cells were either untreated or stimulated with FLT3 ligand (FL). (A) FLT3 was immunoprecipitated from whole cell lysates and immunoblotted with antiphosphotyrosine antibody (upper panel). Subsequently, the blots were stripped and reblotted with anti-FLT3 antibody (lower panel). (B) Activation of STAT5 was demonstrated by immunoblotting the total cell lysates with anti-pSTAT5 antibody (upper panel). After stripping the membrane was reprobed with total anti-STAT5 antibody (lower panel).
Next, we investigated whether kinase activity of the mutants leads to constitutive activation of downstream signaling cascades. Because it has been reported that ITD as well as Asp835 mutations of the FLT3 receptor result in STAT5 activation,15,16,24 we examined the phosphorylation status of STAT5. Whole cell lysates were immunoblotted with phosphospecific STAT5 and STAT5 antibodies. As shown in Figure 1B, all mutant FLT3 receptors led to constitutive phosphorylation of STAT5. These results suggest that both Ile836del and Ile836Met+Arg are activating mutations of the FLT3 receptor. Interestingly, as published before,15 the WT FLT3 receptor despite strong activation in the presence of ligand failed to activate the STAT5 pathway (Figure 1B). Thus, specific mutations in the FLT3 receptor not only constitutively activate the receptor but also can lead to the activation of different signaling pathways.
Factor-independent growth of Ba/F3 cells expressing FLT3 Ile836del and Ile836Met+Arg
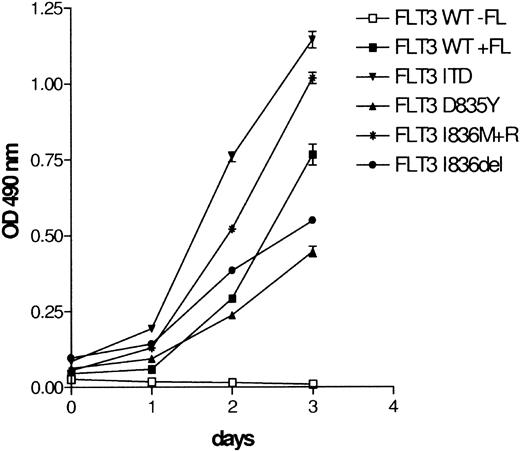
Next, we examined whether the deletion and insertion FLT3 mutants are capable of inducing growth factor–independent proliferation of Ba/F3 cells. Ba/F3 cells were stably transfected with WT or mutant FLT3 and cultured in the absence of IL-3. WT FLT3-transfected Ba/F3 cells were able to proliferate only in the presence of FL (Figure 2). In contrast, Ba/F3 cells expressing FLT3 Ile836del or Ile836Met+Arg grew factor independent, indicating that both mutants have transforming capabilities in these cells similar to the ITD and Asp835Tyr mutants (Figure 2). Similarly, 32D cells stably expressing both mutants also achieved growth factor–independent proliferation (data not shown).
FLT3 Ile836del and FLT3 Ile836Met+Arg induce IL-3–independent growth of Ba/F3 cells. Ba/F3 cells were stably transfected with WT or mutated FLT3 constructs. Mutant FLT3-transformed Ba/F3 cells were cultured in the absence of IL-3 and FL. FLT3 WT-transfected cells were grown with or without FL (100 ng/mL) as a control. Proliferation was determined daily by a colorimetric tetrazolium salt (MTS) assay. Data represent values ± SD of triplicates.
FLT3 Ile836del and FLT3 Ile836Met+Arg induce IL-3–independent growth of Ba/F3 cells. Ba/F3 cells were stably transfected with WT or mutated FLT3 constructs. Mutant FLT3-transformed Ba/F3 cells were cultured in the absence of IL-3 and FL. FLT3 WT-transfected cells were grown with or without FL (100 ng/mL) as a control. Proliferation was determined daily by a colorimetric tetrazolium salt (MTS) assay. Data represent values ± SD of triplicates.
Sensitivity toward tyrosine kinase inhibitors varies between different FLT3 mutations
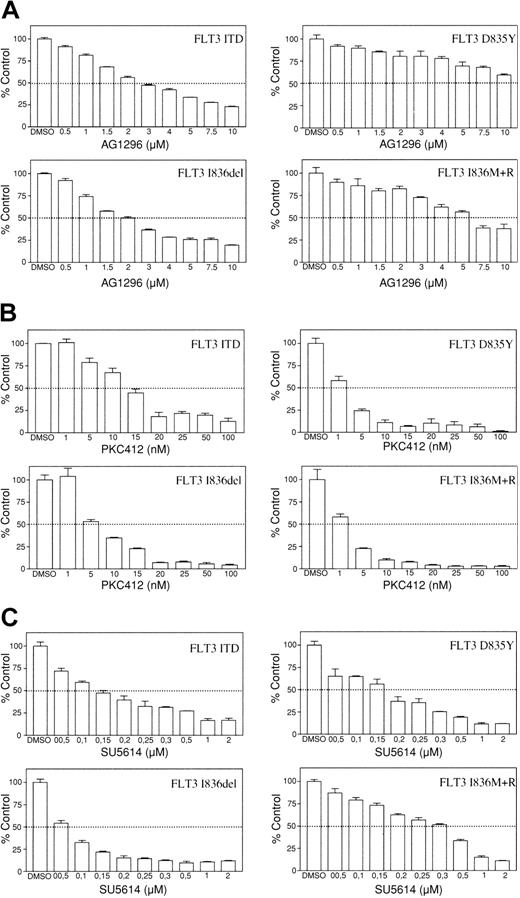
Several groups of tyrosine kinase inhibitors have been developed to inhibit kinase activity of RTK family members. AG1296, a compound from the class of tyrphostins, and the indolinone compound SU5614 both act as adenosine triphosphate (ATP)–competitive inhibitors.25,26 The staurosporin derivative PKC412 was originally identified to be an inhibitor of protein kinase C and subsequently was demonstrated to inhibit members of the class III RTKs.27 Tyrosine kinase inhibitors such as AG1296, SU5614, and PKC412 have been shown to suppress growth factor–independent proliferation of cells expressing constitutively activated FLT3.19,20 Therefore, we examined whether these inhibitors are also effective against novel FLT3 receptor mutants. To evaluate the inhibitory effect of these compounds, mutant FLT3-transformed Ba/F3 cells were grown in the presence of increasing concentrations of different inhibitors. After 24 hours proliferation of cells was determined by a colorimetric MTS assay (Figure 3).
Sensitivity toward tyrosine kinase inhibitors varies between different FLT3 receptor mutants. Ba/F3 FLT3 ITD, Asp835Tyr, Ile836del, and Ile836Met+Arg cells were incubated with increasing concentrations of (A) AG1296, (B) PKC412, and (C) SU5614. Cell viability was determined after 24 hours. Data are presented as percentage of control (DMSO treated) cells. Data represent values ± SD of triplicates. One representative of at least 3 independent experiments is shown.
Sensitivity toward tyrosine kinase inhibitors varies between different FLT3 receptor mutants. Ba/F3 FLT3 ITD, Asp835Tyr, Ile836del, and Ile836Met+Arg cells were incubated with increasing concentrations of (A) AG1296, (B) PKC412, and (C) SU5614. Cell viability was determined after 24 hours. Data are presented as percentage of control (DMSO treated) cells. Data represent values ± SD of triplicates. One representative of at least 3 independent experiments is shown.
The sensitivity toward the tyrphostin AG1296 differed significantly between the examined mutants (Figure 3A). FLT3 ITD– and FLT3 Ile836del–expressing Ba/F3 cells showed an IC50 (concentration that inhibits 50%) of approximately 2.5 μM, whereas Ba/F3 FLT3 Ile836Met+Arg required doses of approximately 6 μM for 50% inhibition. An IC50 could not be reached for the FLT3 Asp835Tyr-expressing cells in this assay (IC50 > 10 μM). FLT3 mutants also displayed different sensitivity to PKC412. Cells expressing FLT3 ITD required higher concentrations of PKC412 for inhibition of proliferation than cells expressing the catalytic domain mutants with IC50 values ranging from 5 nM to 12 nM (Figure 3B). As shown in Figure 3C, all FLT3 mutants were sensitive to growth inhibition by SU5614, but the sensitivity toward this compound also differed markedly between the mutants. Proliferation of Ba/F3 cells expressing the deletion mutant was most potently inhibited with an IC50 of approximately 0.06 μM, whereas 50% inhibition of cells transformed by the insertion mutant required more than 0.3 μM. Similar results were obtained using 32D cells stably expressing each FLT3 mutant (data not shown).
Addition of IL-3 abrogated the antiproliferative activity of each inhibitor as described previously,19,20,28 indicating that inhibitory effects were due to specific inhibition of FLT3 (data not shown).
Next, we performed flow cytometric apoptosis assays to further analyze the striking differences in growth inhibition by the compounds. Ba/F3 cells expressing each FLT3 mutant were exposed to several concentrations of AG1296, PKC412, and SU5614. Cells were harvested at daily intervals, and Annexin V/PI (propidium iodide) staining was carried out.
FLT3 ITD– and Ile836del–expressing cells showed a dose-dependent increase in apoptosis on exposure to AG1296. In contrast, treatment of Ba/F3 FLT3 Asp835Tyr and FLT3 Ile836Met+Arg with the tyrphostin did not result in any increase of apoptosis even at a concentration of 10 μM (Figure 4A). PKC412 at concentrations between 25 and 100 nM induced rapid apoptosis in cells expressing catalytic domain mutants. FLT3 ITD–expressing cells displayed far less apoptosis after PKC412 treatment for 24 hours (Figure 4B). SU5614 induced apoptosis most effectively in cells expressing the Ile836del mutant (Figure 4C).
Induction of apoptosis by AG1296, PKC412, and SU5614 varies between cells expressing different FLT3 receptor mutants. Ba/F3 cells expressing each FLT3 mutant were incubated in the presence of (A) AG1296 (2.5, 5, 10 μM) for 72 hours, (B) PKC412 (25, 50, 100 nM) for 24 hours, and (C) SU5614 (1, 5, 10 μM) for 24 hours. Flow cytometric analysis was performed after staining the cells with Annexin V/PI. Total percentage of Annexin-V/PI-negative cells (percentage of viable cells) is shown. One representative of at least 3 independent experiments is shown.
Induction of apoptosis by AG1296, PKC412, and SU5614 varies between cells expressing different FLT3 receptor mutants. Ba/F3 cells expressing each FLT3 mutant were incubated in the presence of (A) AG1296 (2.5, 5, 10 μM) for 72 hours, (B) PKC412 (25, 50, 100 nM) for 24 hours, and (C) SU5614 (1, 5, 10 μM) for 24 hours. Flow cytometric analysis was performed after staining the cells with Annexin V/PI. Total percentage of Annexin-V/PI-negative cells (percentage of viable cells) is shown. One representative of at least 3 independent experiments is shown.
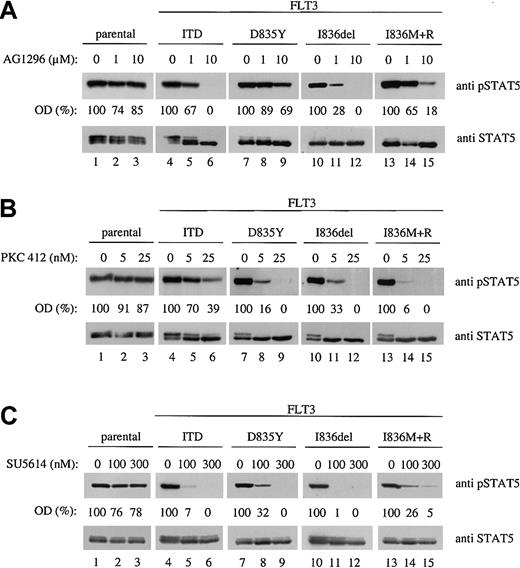
Finally, we wanted to determine whether the observed differences in growth inhibition and induction of apoptosis correlated with the phosphorylation status of STAT5. For that purpose mutant FLT3-expressing Ba/F3 cells were incubated with increasing concentrations of AG1296, PKC412, SU5614, or vehicle alone prior to cell lysis. As a control, parental Ba/F3 cells were treated likewise but in the presence of IL-3. The level of STAT5 activation was determined by Western blot analysis using a phosphospecific STAT5 antibody. AG1296 at 10 μM completely inhibited STAT5 phosphorylation in Ba/F3 FLT3 ITD as well as in Ba/F3 FLT3 Ile836del cells (Figure 5A, lanes 6 and 12). Cells expressing the Ile836Met+Arg mutant FLT3 receptor showed an incomplete reduction of 82% in STAT5 phosphorylation after treatment with AG1296 at 10 μM (Figure 5A, lane 15). In contrast, in Asp835Tyr-transformed Ba/F3 cells phosporylation level was only decreased by 31% in the presence of 10 μM AG1296 (lane 9). PKC412 at 25 nM completely inhibited STAT5 phosphorylation of cells expressing each catalytic domain mutant FLT3 receptor (Figure 5B, lanes 9, 12, and 15). In contrast, in Ba/F3 FLT3 ITD cells phosphorylation of STAT5 was only reduced by 61% (Figure 5B, lane 6). SU5614 at a concentration of 100 nM efficiently blocked FLT3-induced STAT5 phosphorylation in FLT3 ITD– and FLT3 Ile836del–expressing cells (Figure 5C, lanes 5 and 11). In cells expressing the FLT3 Asp835Tyr and the FLT3 Ile836Met+Arg mutants, the level of STAT5 phosphorylation was only decreased by 68% and 74%, respectively (Figure 5C, lanes 8 and 14). AG1296, PKC412, and SU5614 did not interfere with activation of STAT5 on IL-3 stimulation in parental Ba/F3 cells. Thus, there is a correlation between inhibition of proliferation and induction of apoptosis and inhibition of STAT5 activation by different FLT3 inhibitors.
AG1296, PKC412, and SU5614 inhibit FLT3-mediated STAT5 phosphorylation. Mutant FLT3-expressing Ba/F3 cells were incubated with the indicated concentrations of (A) AG1296, (B) PKC412, and (C) SU5614 or vehicle alone for 4 hours prior to cell lysis. Parental Ba/F3 cells were treated likewise but in the presence of IL-3. Activation of STAT5 was demonstrated by immunoblotting the total cell lysates with anti-pSTAT5 antibody (upper panel). After stripping the membrane was reprobed with total anti-STAT5 antibody (lower panel). Optical density (OD) ratios of STAT5 phosphorylation of treated versus untreated cells are shown below the upper panel.
AG1296, PKC412, and SU5614 inhibit FLT3-mediated STAT5 phosphorylation. Mutant FLT3-expressing Ba/F3 cells were incubated with the indicated concentrations of (A) AG1296, (B) PKC412, and (C) SU5614 or vehicle alone for 4 hours prior to cell lysis. Parental Ba/F3 cells were treated likewise but in the presence of IL-3. Activation of STAT5 was demonstrated by immunoblotting the total cell lysates with anti-pSTAT5 antibody (upper panel). After stripping the membrane was reprobed with total anti-STAT5 antibody (lower panel). Optical density (OD) ratios of STAT5 phosphorylation of treated versus untreated cells are shown below the upper panel.
Discussion
In healthy bone marrow the expression of the receptor tyrosine kinase FLT3 is restricted to stem cells and early progenitors.29 Activation of the receptor with its ligand (FL) contributes to proliferation and differentiation of these cells.7-9 FLT3 is also expressed at high levels on leukemic blasts in most cases of acute myeloid leukemia (AML).30,31 Stimulation of leukemia blasts with FL results in proliferation and prevents apoptosis, suggesting that FLT3 may play a pathophysiologic role in leukemogenesis.32,33 Indeed, activating mutations of the FLT3 receptor have been detected in approximately 30% of patients with AML and are believed to be of importance for the malignant phenotype.10
Recently, a series of novel mutations within the activation loop was identified in patients with AML. Deletion of isoleucine 836 (Ile836del) occurred in 13 of 87 patient samples carrying point mutations in the kinase domain, and one sample was found to contain a novel point mutation, changing isoleucine 836 to methionine with an additional arginine inserted after codon 836 (Ile836Met+Arg).12 In another study deletion of isoleucine 836 was detected in 2 of 32 patient samples carrying tyrosine kinase domain (TKD) mutations.34
In this study, we investigated the role of the recently described FLT3 receptor mutations Ile836del and Ile836Met+Arg on receptor activation and cell growth. Our results demonstrate that, when overexpressed in HEK 293, Ba/F3, and 32D cells, both mutants were able to constitutively activate the FLT3 receptor. Kinase activity of both mutants led to activation of downstream signaling pathways as implicated by activation of the STAT5 pathway. Most importantly, when stably expressed in the factor-dependent cell lines Ba/F3 and 32D, both deletion and insertion mutants grew factor independently, indicating that both mutants have transforming capabilities.
Recently, it has been demonstrated that a 2–amino acid elongation between codons 840 and 841 within the A-loop (840GlySer) also represents an activating mutation of the FLT3 receptor.35 Activating mutations in the juxtamembrane domain and within the activation loop of other class III RTK members have been reported.36,37 As is the case with Asp835 in FLT3, point mutations of the corresponding Asp816 of KIT and Asp802 of the colony-stimulating factor 1 (CSF-1) receptor also result in constitutive autophosphorylation in the absence of ligand.38,39 Furthermore, it has been demonstrated that a point mutation in the extracellular domain of the human CSF-1 receptor activates its transforming potential.40 Activating mutations within the extracellular domain of KIT are found in gastrointestinal stroma tumors (GISTs) and myeloproliferative disorders.41,42 Because novel mutations of FLT3 are continuously detected in patients with AML and other classes of mutations have been shown in closely related receptors, it is possible that leukemic cells from these patients could harbor additional activating mutations within the FLT3 receptor that are unknown presently. This hypothesis is supported by the fact that FLT3 is highly expressed in up to 100% of AML cases,29,31 and screening for mutations has been limited on restricted domains so far.
Because FLT3 is the most commonly mutated gene in AML, targeting the FLT3 receptor by specific inhibitors may establish new treatment options for this type of leukemia. Several FLT3 inhibitors have been shown to suppress growth factor–independent proliferation of FLT3 ITD–expressing cells and prolong the survival in a mouse model of FLT3 ITD–induced disease.18-20 In this study, we examined the sensitivity of Ba/F3 cells transformed by FLT3 ITD, FLT3 Asp835Tyr, and also the novel mutants FLT3 Ile836del and Ile836Met+Arg toward the compounds AG1296, PKC412, and SU5614. Our results indicate that these inhibitors have distinct activities against different mutants. It has been demonstrated that the tyrphostin AG1296 is able to inhibit factor-independent proliferation of TEL-FLT3–expressing Ba/F3 cells.19 In our experiments, AG1296 inhibited signaling from both the FLT3 ITD and FLT3 Ile836del at similar doses. FLT3 Ile836Met+Arg showed less sensitivity toward the tyrphostin. Remarkably, AG1296 had almost no influence on FLT3 Asp835Tyr-induced proliferation, inhibition of apoptosis, and phosphorylation of STAT5. Because this compound acts as an ATP competitor, it seems likely that the kind of mutation within the activation loop has a major influence on the grade of sensitivity. It has already been shown that different mutations within the kinase domain of BCR-ABL are responsible for tyrosine kinase inhibitor resistance in vitro,43,44 indicating that the binding of an inhibitor is strongly dependent on the exact molecular structure of the target.
Recently, it has been shown that PKC412 inhibited the proliferation of Ba/F3 FLT3 ITD cells with an IC50 of less than 10 nM.20 Although our IC50 values for inhibition of FLT3 ITD were slightly higher (IC50 = 12 nM), in agreement with this study we found a higher sensitivity of the Asp835Tyr mutant toward PKC412. Interestingly, treatment with PKC412 revealed less activity against the juxtamembrane domain than against the catalytic domain mutants.
To support the notion that the exact amino acid sequence can influence the sensitivity differentially against various tyrosine kinase inhibitors, we also used a third compound, the indolinone SU5614. This compound was recently tested for the inhibition of various c-KIT receptor mutants. The catalytic mutant KIT required higher doses of SU5614 for maximal inhibition than the juxtamembrane (JM) mutant.21,22 Our data demonstrate that differential response of catalytic domain and JM domain mutants toward the indolinone SU5614 observed in KIT does not occur in FLT3. Our findings suggest that shortening of the A-Loop (Ile836del) in the FLT3 receptor results in better response to SU5614, whereas elongating mutations (Ile836Met+Arg) cause less sensitivity, as indicated by IC50 values of 60 nM and 300 nM, respectively.
In conclusion we found that 2 recently described FLT3 receptor mutations detected in patients with AML indeed lead to constitutive activation and signaling of the FLT3 receptor and are able to transform murine lymphoid and myeloid cells in vitro. Furthermore, the data suggest that different mutations in the FLT3 receptor lead to a divergent sensitivity toward a series of inhibitors targeting the receptor molecule. Our data indicate that determining the precise nature of FLT3 mutation when applying receptor tyrosine kinase inhibitors in clinical trials may be of great importance, even more so because novel activating mutations are continuously being described.
Prepublished online as Blood First Edition Paper, March 27, 2003; DOI 10.1182/blood-2002-11-3441.
Supported in part by research funding from Novartis Germany (J.D.); and by grants from José-Carreras Stiftung and Mildred-Scheel-Stiftung (J.D.) and Bundesministerium für Bildung und Forschung (BMBF) (01-GS-0105 and 01-GS-0155) (J.D. and C.P). C.M. is supported by a fellowship from José-Carreras Stiftung.
PKC412 is a Novartis compound.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr T. Meyer (Novartis Pharma, Basel, Switzerland) and H. Gschaidmeier (Novartis Pharma, Nürnberg, Germany) for the generous gift of PKC412, Dr H. Serve (University of Münster, Department of Hematology/Oncology, Germany) for providing the murine wild-type FLT3 and FLT3 ITD cDNAs, and Dr T. Wheeler (Dairy Science, AgResearch, Ruakura Research Centre, Hamilton, New Zealand) for the phospho-STAT5 antibody.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal