Abstract
High levels of cytokines are associated with a poor prognosis in acute myeloid leukemia (AML). However, cytokines may induce, on one hand, survival factor expression and cell proliferation and, on the other hand, expression of inhibitory signals such as up-regulation of suppressors of cytokine signaling (SOCS) and induce apoptotic cell death. Because blasts from patients with AML express high procaspase protein levels, we asked whether granulocyte-macrophage colony-stimulating factor (GM-CSF) enhances procaspase protein production in AML cells. In the GM-CSF–responsive OCIM2 AML cell line, GM-CSF induced signal transducer and activator of transcription 5 (Stat 5) phosphorylation, up-regulated cyclin D2, and stimulated cell cycle progression. Concurrently, GM-CSF stimulated expression of SOCS-2 and -3 and of procaspases 2 and 3 and induced caspase 3 activation, poly(ADP[adenosine 5′-diphosphate]-ribose) polymerase (PARP) cleavage, and apoptotic cell death. The Janus kinase (Jak)–Stat inhibitor AG490 abrogated GM-CSF–induced expression of procaspase 3 and activation of caspase 3. Under the same conditions GM-CSF up-regulated production of BAX as well as Bcl-2, Bcl-XL, survivin, and XIAP. GM-CSF also increased procaspase 3 protein levels in OCI/AML3 and Mo7e cells, suggesting that this phenomenon is not restricted to a single leukemia cell line. Our data suggest that GM-CSF exerts a dual effect: it stimulates cell division but contemporaneously up-regulates Jak-Stat–dependent proapoptotic proteins. Up-regulation of procaspase levels in AML is thus a beacon for an ongoing growth-stimulatory signal.
Introduction
Apoptosis is an evolutionary conserved death program executed through the activation of a family of cysteine proteases termed “caspases.” Caspases are synthesized as latent intracellular proenzymes. Cleavage of the procaspase forms by either extra- or intracellular stimuli converts them into biologically active caspases. These caspases abrogate the effect of substrates that protect cellular integrity, such as the DNA-repair enzyme poly(ADP[adenosine 5′-diphosphate]-ribose) polymerase (PARP), thereby inducing apoptotic cell death.1-4 Recently we found that leukemia cells express high procaspase protein levels5-7 and that high levels of procaspase 2 and 3 are reliable prognostic factors in both acute myeloid leukemia (AML)7 and acute lymphoblastic leukemia (ALL).5 Whereas caspase activation and the role of various caspases in apoptotic cell death have been studied extensively,1-3 little is known about what enhances procaspase protein production.
It has been well established that myeloid leukemia cells produce cytokines that stimulate their proliferation.8 Furthermore, high cytokine levels are associated with a poor prognosis.9-11 However, several cytokines classified as “growth factors” also possess growth-inhibiting activities.12-14 For example, γ-interferon and epidermal growth factor (EGF) incite a dual effect: They promote cell growth and induce apoptosis by triggering caspase activity.15,16 Cytokines act through cellular receptors that are associated with members of the Janus kinase (Jak) family of protein tyrosine kinases (PTKs). Upon phosphorylation and activation of Jak, proteins bound to Jak can initiate signaling pathways such as those regulated by Ras, phosphatidylinositol 3–kinase (PI3K), and signal transducers and activators of transcription (Stats).17 However, growth stimulatory signals often trigger a negative feedback loop. Suppressor of cytokine signaling (SOCS) proteins belong to a recently identified family of cytokine-inducible proteins that are expressed following activation of the Jak-Stat pathway to abrogate various cytokine signaling.18 We hypothesized that hematopoietic growth factors both induce AML cell growth and at the same time trigger proapoptotic signals such as expression of SOCS proteins and up-regulation of the caspase pathway. Because granulocyte-macrophage colony-stimulating factor (GM-CSF) is a prominent growth factor in AML, we chose it to test our hypothesis. We found that, concurrent with its stimulation of AML cell growth, GM-CSF up-regulated SOCS-2 and -3, procaspases 2 and 3, and caspase 3, and induced PARP cleavage. Inhibition of the Jak-Stat axis by AG490 inhibited GM-CSF–induced expression of the apoptotic cascade. These results indicate that expression of a proapoptotic signal may accompany the stimulatory effect of GM-CSF in AML cells.
Materials and methods
Cell lines
The AML cell lines OCIM2 and OCI/AML3 were kindly provided by M. D. Minden (Ontario Cancer Institute, Toronto, ON, Canada).19,20 OCI/AML3 was established from a patient with AML and OCIM2 was established from a patient with erythroleukemia. Both cell lines were maintained in RPMI 1640 (Sigma Chemical, St Louis, MO) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT). Both cell lines express the GM-CSF receptor (CD116) by flow cytometry (data not shown).
The growth factor–dependent AML cell line Mo7e was kindly provided by A. Ciarletta (Genetics Institute, Cambridge, MA), and the erythroleukemia line TF-1 was obtained from the American Type Culture Collection (ATCC; Rockville, MD).21,22 Both lines were maintained in RPMI 1640 supplemented with 20% FCS, 2 mM glutamine, and 10 ng/mL and 5 ng/mL recombinant human GM-CSF (rhGM-CSF; Immunex, Seattle, WA).
Prior to processing, cells were removed from their preferred culture conditions at the log phase of growth and placed in tissue culture flasks at a density of 105 cells/mL in RPMI 1640 for 2 hours, after which time GM-CSF was added to the cultures.
Cell line clonogenic assay
The clonogenic assay was performed as previously described.23 Briefly, OCIM2 and OCI/AML3 cells were cultured in 0.8% methylcellulose (Fluka Chemical, Ronkonkoma, NY), 10% FCS, and RPMI 1640 in 1% (vol/vol) methylcellulose. The culture mixture was placed in 35-mm Petri dishes (Nunc, Naperville, IL) in duplicate and maintained at 37°C with 5% CO2 in air in a humidified atmosphere. Colonies were counted after 7 days using an inverted microscope. A colony was defined as a cluster of more than 40 cells.
Cell cycle analysis
Cell cycle analysis was performed according to standard protocols. Briefly, 5 million cells were pelleted following incubation with growth factors. The cell pellets were washed and resuspended in 2 mL 1% paraformaldehyde in phosphate-buffered saline (PBS; Gibco BRL, Grand Island, NY). Cells were incubated for 15 minutes at 4°C and then washed again in PBS, resuspended in 2 mL absolute ethanol, and stored at -20°C until staining. The stored cells were washed twice in PBS, resuspended in 0.5 mL propidium iodide (PI) staining buffer (50 μg/mL PI, 10 mg/mL RNase in PBS), and then incubated for 1 hour at room temperature in the dark. Flow cytometric analysis was performed using a FACSCalibur and CellQuest software (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA). Data analysis was performed with CellQuest software (BDIS) and Modfit LT V2.0 software (Verity Software House, Topsham, ME).
Western immunoblotting
Western immunoblotting was performed on cell lysates as described previously.7 The following antibodies were used for the respective proteins: monoclonal mouse antihuman ICH-1L (procaspase 2), mouse antihuman CPP32 (procaspase 3), mouse antihuman Bcl-2, mouse antihuman BAX, rabbit antihuman Bcl-XL, mouse antihuman Stat 1, mouse antihuman Stat 5, mouse antihuman XIAP (Transduction Laboratories, Lexington, KY), mouse antihuman FLICE (procaspase 8), rabbit antihuman caspase 10, mouse antihuman cyclin D2, mouse antihuman PARP (Pharmingen, San Diego, CA), rabbit antihuman survivin (Novus Biologicals, Littleton, CO), rabbit antihuman cleaved caspase 3 (New England Bio Labs, Beverly, MA), mouse antihuman phospho–Stat 5 (Upstate Biotechnology, Lake Placid, NY), and rabbit antihuman SOCS-1, -2, and -3 (Zymed, San Francisco, CA). Normal mouse immunoglobulin G (IgG) and rabbit IgG (Sigma) were used as controls. To confirm the detection of these proteins, we used lysates of the following cell lines: Jurkat cells (ATCC; for the detection of procaspase 3, PARP, Bcl-2, survivin, and phospho–Stat 5), Hela cells (ATCC; for the detection of cleaved caspase 3, XIAP, and survivin), HL60 cells (ATCC; for the detection of procaspase 8), U937 cells (ATCC; for the detection of procaspase 9), HepG2 cells (ATCC; for the detection of BAX), Raji cells (ATCC; for the detection of cyclin D2), and A431 cells (ATCC; for the detection of Stat 1). We also used lysates of human endothelial cells (for the detection of Bcl-XL and Stat 5).
Cell lysates were assayed for protein concentration using the BCA Protein Assay Reagent (Pierce Chemical, Rockford, IL). Each set of paired samples was then adjusted to have the same protein concentration. Electrophoresis was conducted at constant wattage (10 W) in running buffer cooled to 4°C. Stacking gels contained 4% (wt/vol) acrylamide, and separating gels contained 12% (wt/vol) acrylamide. Approximately 50 μg of sample protein was loaded into each of the appropriate lanes. Proteins separated with the sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) technique were transferred to nitrocellulose membranes. Transfers were performed overnight at 30 V in a cooled (4°C) reservoir containing 25 mM Tris (tris(hydroxymethyl)aminomethane), 192 mM glycine, and 20% methanol (pH 8.3) transfer buffer. Nitrocellulose membranes were removed from the blot apparatus and placed in a solution of Ponceau S stain (0.5% Ponceau S and 1% glacial acetic acid in H2O) to verify equal loading of protein in control and treated samples. After membranes were stained for 5 minutes, they were rinsed for 2 minutes and examined. Equal loading of protein was verified, and the membranes were then rinsed for an additional 10 minutes and immunoscreened. The membranes were blocked in BLOTTO (5% dried milk dissolved in 50 mM PBS) for at least 1 hour at room temperature. They were then washed 3 times in PBS plus 0.5% Tween 20. Next, the membranes were incubated for 1 hour with the appropriate antibodies. After incubation, the membranes were subjected to three 15-minute rinses in PBS containing 0.5% Tween 20. Bound antibody was detected with the ECL Western Blotting Detection System (Amersham, Arlington Heights, IL). The membranes were incubated with antirabbit horseradish peroxidase–labeled antibody at a concentration of 1:200 in PBS plus 0.5% Tween 20 at room temperature for 1 hour. After this incubation, the membranes were washed in PBS containing 0.5% Tween 20, and bound antibody was detected according to the ECL protocol. Chemiluminescence of the membranes was detected with X-OMAT AR5 x-ray film (Kodak, Rochester, NY) in stainless steel exposure cassettes (Sigma). Protein levels were scored by densitometry using the UltraScan XL (Pharmacia, Uppsala, Sweden). Results were normalized by dividing the numerical value of a sample's signal by the numerical value of the signal from the corresponding value of the β-actin that served as a control.
RNase protection assay
Procaspase mRNA expression was analyzed with the RNase protection assay as previously described.24 RNA probes from RiboQuant multiprobe RNase protection system (hAPO-1c; Pharmingen) were used, and the assay was performed according to the manufacturer's instructions. Briefly, RNA was isolated from 1 × 106 cells using Tri Reagent (Sigma), and samples of 5 μg total RNA were used for hybridization with radiolabeled probes. RNA probes were radiolabeled by in vitro transcription in the presence of α-32P UTP. Hybridization and digestion of unprotected RNA were performed according to the manufacturer's instructions, and samples were resolved by electrophoresis on a polyacrylamide sequencing gel. The gel was dried and exposed to an x-ray film for 2 to 72 hours. The migration distance versus log nucleotide length of the RNA standards was plotted and used to identify the RNase-protected bands.
Apoptosis assay
To quantify the percentage of cells undergoing apoptosis, we used annexin V–fluorescein isothiocyanate (FITC; Pharmingen) as previously described.25 Briefly, GM-CSF–treated cells were washed twice with cold PBS and then resuspended in binding buffer (10 nM HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid), 140 nM NaCl, 5 nM CaCl2, pH 7.4) at a concentration of 1 × 106 cells/μL. After incubation, 100 μL of the solution was transferred to a 5-mL culture tube and 5 μL annexin V–FITC and 10 μL propidium iodide were added. The tube was gently vortexed and incubated for 15 minutes. at room temperature in the dark. At the end of incubation, 400 μL binding buffer was added and the cells were analyzed immediately by flow cytometry. Flow cytometric analysis was performed with a FACSCalibur using the CellQuest software (BDIS). Data analysis was performed with CellQuest and Modfit LT V2.0 (Verity Software House) software.
Results
GM-CSF stimulates OCIM2 colony proliferation
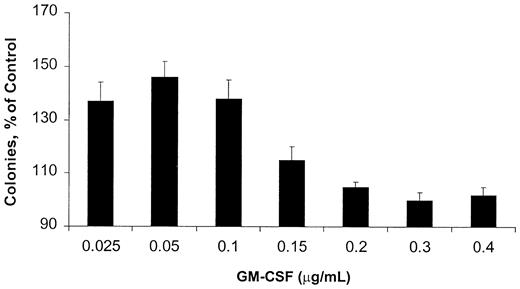
Because AML blasts respond to GM-CSF, but their growth is not dependent on this cytokine, we chose a GM-CSF–responsive, rather than a GM-CSF–dependent, cell line as a model. OCIM2 cells do not produce significant amounts of GM-CSF (< 1.5 pg/5 × 106 cells as measured by an enzyme-linked immunosorbent assay; data not shown), and they proliferate in the presence of culture media supplemented with FCS without the addition of an exogenous cytokine. We found that in a clonogenic assay, GM-CSF stimulated the proliferation of OCIM2 cells in a dose-dependent manner. However, the stimulatory effect of this cytokine reached its peak at a concentration of 0.050 μg/mL, and at higher GM-CSF concentrations the number of colonies decreased again (Figure 1). The bell-shaped growth curve suggests that at high concentrations, GM-CSF either did not stimulate OCIM2 colony growth or induced growth inhibition.
Effect of GM-CSF on OCIM2 colony-forming cell proliferation. Means ± SD of colony numbers from quadruplicate cultures are presented as percentages of control. Data from 3 different experiments are depicted. The mean numbers of colonies grown in the absence of GM-CSF (control cultures) were 320, 349, and 350.
Effect of GM-CSF on OCIM2 colony-forming cell proliferation. Means ± SD of colony numbers from quadruplicate cultures are presented as percentages of control. Data from 3 different experiments are depicted. The mean numbers of colonies grown in the absence of GM-CSF (control cultures) were 320, 349, and 350.
GM-CSF phosphorylates Stat 5, up-regulates cyclin D2, and stimulates cellular proliferation
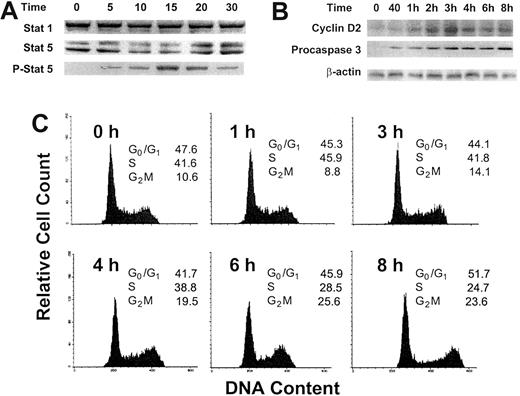
Because OCIM2 cells were deprived of any growth factor for 2 hours before GM-CSF was added to the culture, we sought to determine whether the addition of this cytokine activated the GM-CSF signaling pathway and stimulated the proliferation of these leukemia cells. We found that incubation with GM-CSF at a concentration of 0.025 μg/mL induced Stat 5 phosphorylation (Figure 2A). Within 5 minutes, Stat 5 protein levels dropped and the levels of its phosphorylated form were up-regulated. After 20 minutes Stat 5 levels were up-regulated, and after 30 minutes phosphorylated Stat 5 levels were down-regulated, whereas the level of Stat 1, which is usually affected by cytokines such as interferon, remained unchanged. Similarly, the incubation with GM-CSF resulted in an up-regulation of cyclin D2 and, as in our previous experiments, of procaspase 3 (Figure 2B). At the same time, 0.025 μg/mL GM-CSF stimulated OCIM2 cells to progress through the cell cycle (Figure 2C), as described previously in other GM-CSF–responsive cells.26-28 After 1 hour of exposure to GM-CSF, 45.9% of the cells were in S phase; at 6 hours, 25.6% were in G2M; and at 8 hours, 51.7% were in G0/G1. We could not demonstrate progression through the cell cycle after exposure of the cells to 0.1 and 0.25 μg/mL GM-CSF (data not shown).
Effect of GM-CSF on Stat 1, Stat 5, phosphorylated Stat 5, and cyclin D2 protein levels and on the progression of OCIM2 cells through the cell cycle. Stat 1, Stat 5, and phosphorylated Stat 5 (A) and cyclin D2 (B) proteins were detected by Western immunoblotting before and after OCIM2 cells were incubated with 0.025 μg/mL GM-CSF for 5, 10, 15, 20, and 30 minutes. Levels of procaspase 3 were measured to verify that procaspase 3 levels were up-regulated under the same conditions. A band of β-actin, demonstrating equal protein loading, is presented as a loading control. (C) Growth factor–deprived OCIM2 cells were incubated with 0.025 μg/mL GM-CSF for 8 hours and the cell cycle status of cell samples was analyzed at different time points. As shown, the fractions of cells entering S and G2M phases increase over time; then they decrease and the fraction of cells in G0/G1 phase of the cell cycle increases again. Representative data from 3 different experiments are shown.
Effect of GM-CSF on Stat 1, Stat 5, phosphorylated Stat 5, and cyclin D2 protein levels and on the progression of OCIM2 cells through the cell cycle. Stat 1, Stat 5, and phosphorylated Stat 5 (A) and cyclin D2 (B) proteins were detected by Western immunoblotting before and after OCIM2 cells were incubated with 0.025 μg/mL GM-CSF for 5, 10, 15, 20, and 30 minutes. Levels of procaspase 3 were measured to verify that procaspase 3 levels were up-regulated under the same conditions. A band of β-actin, demonstrating equal protein loading, is presented as a loading control. (C) Growth factor–deprived OCIM2 cells were incubated with 0.025 μg/mL GM-CSF for 8 hours and the cell cycle status of cell samples was analyzed at different time points. As shown, the fractions of cells entering S and G2M phases increase over time; then they decrease and the fraction of cells in G0/G1 phase of the cell cycle increases again. Representative data from 3 different experiments are shown.
GM-CSF up-regulates procaspase protein levels
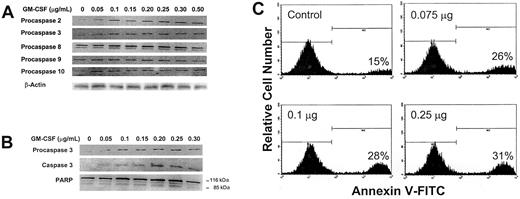
We first sought to determine whether increasing concentrations of GM-CSF would affect procaspase protein levels. To do this, we incubated OCIM2 cells at a low density for 2 hours with only RPMI 1640 in order to eliminate the stimulatory effect of FCS and cell-to-cell interaction. We then incubated the cells for 1 hour in the presence of increasing concentrations of GM-CSF. We analyzed procaspase protein levels of the “executioner” procaspase 3 and of procaspases belonging to both the intrinsic (procaspases 2 and 9) and extrinsic (procaspases 8 and 10) caspase pathways. Using Western immunoblotting, we found that at 0.050 μg/mL (a dose that stimulates cellular proliferation in the clonogenic assay) and at higher concentrations, GM-CSF up-regulated the protein levels of procaspases 2 and 3, but not of procaspases 8, 9, and 10 (Figure 3A). In other experiments (Figures 2 and 4), procaspase 3 levels were increased at GM-CSF concentrations of 0.025 μg/mL.
Effect of GM-CSF on procaspase protein levels, caspase 3 activation, and induction of apoptosis. (A) Growth factor–deprived OCIM2 cells were incubated for 1 hour in the absence of GM-CSF and in the presence of increasing concentrations of GM-CSF. Changes in the protein levels of procaspase 2, 3, 8, 9, and 10 were determined by Western immunoblotting. A band of β-actin, demonstrating equal protein loading, is presented as a loading control. (B) Growth factor–deprived OCIM2 cells were incubated in the absence of GM-CSF and in the presence of increasing concentrations of GM-CSF. The levels of procaspase 3, activated caspase 3, PARP, and cleaved PARP were analyzed by Western immunoblotting. (C) Growth factor–deprived cells were incubated for 6 hours in the absence of GM-CSF and in the presence of increasing concentrations of GM-CSF. The percentage of cells undergoing apoptotic cell death was quantitatively determined by flow cytometry, using annexin V–FITC. The percentage of cells undergoing apoptotic cell death is shown at the lower right of each box. Representative data from 3 different experiments are shown.
Effect of GM-CSF on procaspase protein levels, caspase 3 activation, and induction of apoptosis. (A) Growth factor–deprived OCIM2 cells were incubated for 1 hour in the absence of GM-CSF and in the presence of increasing concentrations of GM-CSF. Changes in the protein levels of procaspase 2, 3, 8, 9, and 10 were determined by Western immunoblotting. A band of β-actin, demonstrating equal protein loading, is presented as a loading control. (B) Growth factor–deprived OCIM2 cells were incubated in the absence of GM-CSF and in the presence of increasing concentrations of GM-CSF. The levels of procaspase 3, activated caspase 3, PARP, and cleaved PARP were analyzed by Western immunoblotting. (C) Growth factor–deprived cells were incubated for 6 hours in the absence of GM-CSF and in the presence of increasing concentrations of GM-CSF. The percentage of cells undergoing apoptotic cell death was quantitatively determined by flow cytometry, using annexin V–FITC. The percentage of cells undergoing apoptotic cell death is shown at the lower right of each box. Representative data from 3 different experiments are shown.
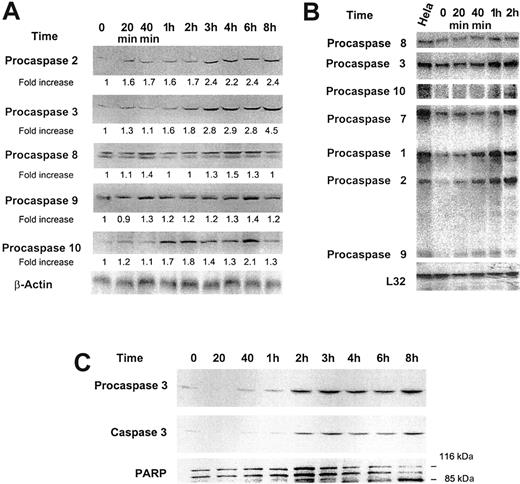
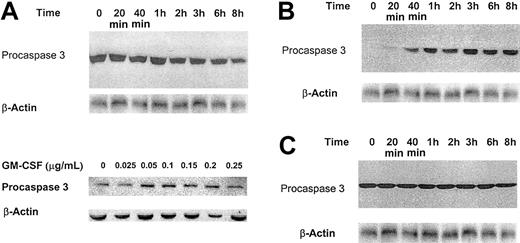
Effect of low-dose GM-CSF on procaspase protein levels, mRNA expression, and caspase 3 activation and PARP cleavage. (A) Growth factor–deprived OCIM2 cells were incubated in the absence of GM-CSF and in the presence of 0.025 μg/mL GM-CSF for 20 minutes, 40 minutes, and 1, 2, 3, 4, 6, and 8 hours. Procaspase protein levels were scored by densitometry and their numerical values were divided by the densitometry values obtained from the corresponding loading controls. The signals obtained at different time points following incubation with GM-CSF are depicted. A band of β-actin (upper band) is presented as loading control. (B) The changes in mRNA levels were recorded prior to and after incubation with 0.025 μg/mL GM-CSF for 20 minutes, 40 minutes, and 1 and 2 hours. RNA obtained from Hela cells was used as a positive control, and L32 was the loading control. The figure depicts mRNA of procaspases 8, 3, 10, 7, 1, 2, and 9. (C) Growth factor–deprived OCIM2 cells were incubated with 0.025 μg/mL GM-CSF as in previous experiments. Procaspase 3, caspase 3, and PARP protein levels were detected at different time points by Western immunoblotting. Representative data from 3 different experiments are shown.
Effect of low-dose GM-CSF on procaspase protein levels, mRNA expression, and caspase 3 activation and PARP cleavage. (A) Growth factor–deprived OCIM2 cells were incubated in the absence of GM-CSF and in the presence of 0.025 μg/mL GM-CSF for 20 minutes, 40 minutes, and 1, 2, 3, 4, 6, and 8 hours. Procaspase protein levels were scored by densitometry and their numerical values were divided by the densitometry values obtained from the corresponding loading controls. The signals obtained at different time points following incubation with GM-CSF are depicted. A band of β-actin (upper band) is presented as loading control. (B) The changes in mRNA levels were recorded prior to and after incubation with 0.025 μg/mL GM-CSF for 20 minutes, 40 minutes, and 1 and 2 hours. RNA obtained from Hela cells was used as a positive control, and L32 was the loading control. The figure depicts mRNA of procaspases 8, 3, 10, 7, 1, 2, and 9. (C) Growth factor–deprived OCIM2 cells were incubated with 0.025 μg/mL GM-CSF as in previous experiments. Procaspase 3, caspase 3, and PARP protein levels were detected at different time points by Western immunoblotting. Representative data from 3 different experiments are shown.
GM-CSF activates caspase 3, cleaves PARP, and induces apoptotic cell death
Because GM-CSF up-regulated the production of procaspase 3, we investigated whether it also cleaves caspase 3 and triggers apoptosis. As shown in Figure 3B, we found that GM-CSF induces both caspase 3 activation and PARP cleavage. We then used the annexin V–FITC assay to detect cells that had undergone apoptotic cell death. We found that under similar culture conditions, GM-CSF induced apoptosis in cells that were incubated with this cytokine for 6 hours (Figure 3C). Apoptosis was detected in 28% and 31% of OCIM2 cells incubated with 0.1 μg/mL and 0.25 μg/mL GM-CSF, respectively, and in 15% of the growth factor–deprived (background) cells. No significant change was found in apoptosis levels at concentrations of 0.025 μg/mL and 0.050 μg/mL GM-CSF.
Low-dose GM-CSF up-regulates procaspase levels, activates caspase 3, and induces PARP cleavage in a time-dependent manner
To study whether GM-CSF affects procaspase protein levels, we added 0.025 μg/mL GM-CSF to the growth factor–deprived cells and measured procaspase protein levels at different time points. We found that GM-CSF did not affect levels of procaspases 8 and 9 but significantly stimulated the production of procaspases 2, 3, and 10 in a time-dependent manner (Figure 4A). Because GM-CSF also stimulated procaspase protein production in a dose-dependent fashion, it is unlikely that growth factor deprivation was responsible for this effect.27-29 Up-regulation of procaspase 3 was reported to be associated with its activation.30 Therefore, we questioned whether a growth-stimulating dose of GM-CSF would induce caspase 3 cleavage. We found that incubation of OCIM2 cells with 0.025 μg/mL GM-CSF resulted in activation of caspase 3 (Figure 4C). Furthermore, GM-CSF also induced PARP cleavage in OCIM2 cells that did not undergo apoptosis under identical culture conditions (data not shown) but rather progressed through the cell cycle and multiplied.
To validate our findings, we also examined the effect of GM-CSF on procaspase mRNA. Using the culture conditions described above, we found that incubating OCIM2 cells with GM-CSF up-regulated the expression of procaspases 1, 2, 3, 8, 9, and 10 (Figure 4B). The changes in procaspase expression levels were noticeable after short incubation times (20 and 40 minutes).
GM-CSF up-regulates levels of SOCS-2 and -3 in OCIM2 cells
SOCS proteins are negative regulators of cytokine signaling. To test whether GM-CSF up-regulates SOCS protein levels, we incubated OCIM2 cells with increasing concentrations of GM-CSF and at 0.050 μg/mL for 20 minutes to 6 hours. We found that the levels of SOCS-2 and -3 were up-regulated in a dose- and time-dependent manner. The maximum level of expression occurred at 40 minutes to 1 hour, after which levels either declined (SOCS-3) or remained stable (SOCS-2; Figure 5).
Effect of GM-CSF on expression levels of SOCS-1, -2, and -3 as detected by Western immunoblotting. OCIM2 cells were incubated with GM-CSF at a concentration of 0.05 μg/mL in a time-dependent fashion ranging from 20 minutes to 6 hours. Upon stimulation with GM-CSF, levels of proteins of SOCS-2 and -3 increased, with a maximum response at 40 minutes to 1 hour. Thereafter, protein levels either remained stable (SOCS-2) or declined (SOCS-3). Representative data from 3 different experiments are shown.
Effect of GM-CSF on expression levels of SOCS-1, -2, and -3 as detected by Western immunoblotting. OCIM2 cells were incubated with GM-CSF at a concentration of 0.05 μg/mL in a time-dependent fashion ranging from 20 minutes to 6 hours. Upon stimulation with GM-CSF, levels of proteins of SOCS-2 and -3 increased, with a maximum response at 40 minutes to 1 hour. Thereafter, protein levels either remained stable (SOCS-2) or declined (SOCS-3). Representative data from 3 different experiments are shown.
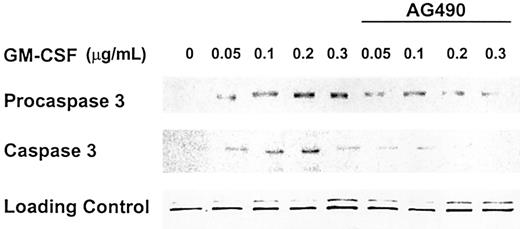
The Jak-Stat inhibitor AG490 down-regulates levels of procaspase 3 and inhibits the activation of caspase 3
Proteins of the Jak-Stat pathway constitute an instrumental component of GM-CSF signaling. To test whether the proapoptotic effect of GM-CSF is dependent on the activation of Jak-Stat proteins, we incubated OCIM2 cells with GM-CSF at concentrations of 0.050, 0.1, 0.2, and 0.3 μg/mL with and without 50 μM AG490 (the Jak-Stat inhibitor). As in our previous experiments, we found that GM-CSF up-regulated procaspase 3 and caspase 3 protein levels and that AG490 abrogates this effect (Figure 6).
Jak-Stat inhibitor AG490 abrogates expression of procaspase 3 and activation of caspase 3. OCIM2 cells were incubated with GM-CSF at concentrations of 0.05, 0.1, 0.2, and 0.3 μg/mL with and without addition of AG490 at a concentration of 50 μM. Representative data from 3 different experiments are shown.
Jak-Stat inhibitor AG490 abrogates expression of procaspase 3 and activation of caspase 3. OCIM2 cells were incubated with GM-CSF at concentrations of 0.05, 0.1, 0.2, and 0.3 μg/mL with and without addition of AG490 at a concentration of 50 μM. Representative data from 3 different experiments are shown.
GM-CSF up-regulates procaspase 3 protein levels in other cell lines
To determine whether the effect of GM-CSF on procaspase protein production is restricted to OCIM2 cells or can be found in other cells that respond to this cytokine, we studied other AML lines as well. We first examined the OCI/AML3 cell line. These cells do not produce significant amounts of GM-CSF (data not shown), and they grow in the presence of culture media supplemented with FCS without an exogenous growth factor. We found that OCI/AML3 cells proliferate in response to GM-CSF in a manner similar to that of the OCIM2 cell line (data not shown). After growth factor–deprived OCI/AML3 cells were incubated for 1 hour with increasing concentrations of GM-CSF as described above, procaspase 3 protein levels were detected by Western immunoblotting. As shown in Figure 7A, we found that GM-CSF increases procaspase 3 protein levels in OCI/AML3 cells. We then examined the effect of low-dose GM-CSF, as we did in our previous experiments. We found that 0.025 μg/mL GM-CSF did not increase the levels of procaspase 3 in OCI/AML3 cells. Instead, it slightly decreased them. Similarly, GM-CSF did not increase the levels of procaspase 3 in the GM-CSF–dependent TF-1 cells (Figure 7C), but it did induce a time-dependent increment in procaspase 3 protein levels in the GM-CSF–dependent Mo7e cell line (Figure 7B). Although growth factor dose and duration of incubation probably affected these results, our data indicate that the effect of GM-CSF on the caspase pathway is not restricted to a single leukemia cell line.
Effect of GM-CSF on procaspase 3 protein levels in OCI/AML3, Mo7e, and TF-1 cells. (A) Growth factor–deprived OCI/AML3 cells were incubated in the absence of GM-CSF and in the presence of increasing concentrations of GM-CSF. Procaspase 3 protein was detected by Western immunoblotting. Representative data from 3 different experiments are shown. (B-C) Effect of GM-CSF on procaspase 3 protein levels in Mo7e (B) and TF-1 (C) cells. Growth factor–deprived cells were incubated in the absence of GM-CSF and in the presence of 0.025 μg/mL GM-CSF. Procaspase 3 protein was detected at different time points by Western immunoblotting. A band of β-actin, demonstrating equal protein loading, is presented as loading control. Representative data from 3 different experiments are shown.
Effect of GM-CSF on procaspase 3 protein levels in OCI/AML3, Mo7e, and TF-1 cells. (A) Growth factor–deprived OCI/AML3 cells were incubated in the absence of GM-CSF and in the presence of increasing concentrations of GM-CSF. Procaspase 3 protein was detected by Western immunoblotting. Representative data from 3 different experiments are shown. (B-C) Effect of GM-CSF on procaspase 3 protein levels in Mo7e (B) and TF-1 (C) cells. Growth factor–deprived cells were incubated in the absence of GM-CSF and in the presence of 0.025 μg/mL GM-CSF. Procaspase 3 protein was detected at different time points by Western immunoblotting. A band of β-actin, demonstrating equal protein loading, is presented as loading control. Representative data from 3 different experiments are shown.
GM-CSF up-regulates protein production of Bcl-2 family members
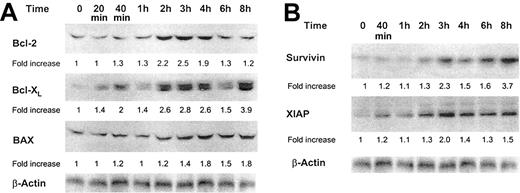
The Bcl-2 protein family is involved in the regulation of apoptotic cell death.31 Bcl-2, Bcl-XL, and BAX are localized at the outer mitochondrial membrane and either inhibit (Bcl-2 and Bcl-XL) or trigger (BAX) apoptosis.32 Because GM-CSF up-regulates procaspase 2, which is also a mitochondria-activated caspase, we sought to determine whether GM-CSF would affect the protein levels of these Bcl-2 family members. We found that GM-CSF increased the levels of Bcl-2, Bcl-XL, and, to a lesser extent, BAX (Figure 8A).
Effect of GM-CSF on Bcl-2, Bcl-XL, BAX, XIAP, and survivin protein levels. (A) Bcl-2, Bcl-XL, and BAX protein levels. (B) Survivin and XIAP protein levels. Growth factor–deprived OCIM2 cells were incubated in the absence of GM-CSF and in the presence of 0.025 μg/mL GM-CSF, and Bcl-2, Bcl-XL, BAX, XIAP, and survivin proteins were detected at different time points by Western immunoblotting. A band of β-actin, demonstrating equal protein loading, is presented as a loading control. Representative data from 3 different experiments are shown.
Effect of GM-CSF on Bcl-2, Bcl-XL, BAX, XIAP, and survivin protein levels. (A) Bcl-2, Bcl-XL, and BAX protein levels. (B) Survivin and XIAP protein levels. Growth factor–deprived OCIM2 cells were incubated in the absence of GM-CSF and in the presence of 0.025 μg/mL GM-CSF, and Bcl-2, Bcl-XL, BAX, XIAP, and survivin proteins were detected at different time points by Western immunoblotting. A band of β-actin, demonstrating equal protein loading, is presented as a loading control. Representative data from 3 different experiments are shown.
GM-CSF increases the levels of the antiapoptotic proteins survivin and XIAP
Because GM-CSF activated caspase 3 in OCIM2 cells without triggering apoptosis and also increased the levels of the antiapoptotic proteins Bcl-2 and Bcl-XL, we asked whether this growth factor also could increase the levels of downstream caspase inhibitors.33,34 As shown in Figure 8B, we found that GM-CSF increased the levels of both survivin and XIAP in a time-dependent fashion.
Discussion
Several cytokines, including GM-CSF, induce the proliferation of normal and leukemic hematopoietic cells and protect them from apoptotic cell death.35-39 In addition, high levels of circulating hematopoietic growth factors have been detected in patients with AML and are associated with an unfavorable prognosis.8-10 However, cytokines acting as growth factors also may elicit growth-inhibitory and proapoptotic signals.12-16 Furthermore, the identification of SOCS and other negative regulators such as SH2-containing phosphatase 1 (SHP1) and protein inhibitors of activated Stats (PIAS) lends weight to the observation that a growth-stimulatory signal is closely linked to the expression of inhibitory proteins. Therefore, we hypothesized that cytokines may up-regulate the caspase pathway.
It has been well established that fresh AML cells proliferate in response to GM-CSF. In addition, we demonstrated that high concentrations of GM-CSF elicit the opposite effect, that is, reduction of AML colony formation. However, AML cells are not GM-CSF–dependent. Accordingly, we chose the OCIM2 AML cell line to further analyze the mechanisms of this effect and test our hypothesis. OCIM2 cells do not produce GM-CSF, and they grow in the absence of this cytokine. In a clonogenic assay, GM-CSF stimulated OCIM2 colony proliferation. Interestingly, at concentrations higher than 0.050 μg/mL, GM-CSF did not increase colony growth further, and in fact, the number of colonies decreased. This suggests that either GM-CSF gradually lost its stimulatory effect or it inhibited at least a subpopulation of the cells. AML blasts express GM-CSF receptors with high or low affinity for the ligand and both OCIM2 and OCI/AML3 cell lines express GM-CSF receptors by flow cytometry. It is possible that differential expression of high- and low-affinity receptors may have accounted for the diverse effects of GM-CSF when tested at different concentrations.
GM-CSF activated the PTK-Stat pathway by phosphorylating Stat 5 (previously found to be activated in fresh AML cells),40 up-regulated the level of cyclin D2 (a cell cycle protein known to be activated by GM-CSF),41-45 and stimulated OCIM2 cells to progress through the cell cycle. Simultaneously, GM-CSF up-regulated the protein levels of procaspase 2 and 3 in both a dose-dependent and a time-dependent manner and induced caspase 3 activation and PARP cleavage without causing apoptotic cell death. GM-CSF induced apoptosis only at high concentrations. It is intriguing that 0.1 μg/mL GM-CSF induces apoptosis but at the same time still induces an increase in colony formation (Figure 1). This observation may point to a mixed population of cells in which the balance between proliferative and apoptotic signals is shifted to either side. Whether GM-CSF directly affected both the intrinsic and extrinsic caspase pathways is not clear; however, a cross-talk between these pathways is possible. For example, caspase 2 recently was reported to play a role in Fas-mediated apoptosis.46
Several investigators have detected activated caspases in viable and proliferating cells. For example, Jaattella et al47 found that cells could regain a normal growth rate in spite of cytochrome c efflux and the cleavage of caspase substrates such as PARP. Miossec et al48 and Posmantur et al49 noticed that caspase 3 could be activated in phytohemagglutinin (PHA)–stimulated T lymphocytes that did not undergo apoptotic cell death. Alam et al50 observed caspase activation and PARP cleavage in viable activated T and B cells. Furthermore, interleukin-16 production, along with caspase 3 activation, was detected in CD3+ and CD8+ T cells without apparent evidence of apoptosis.51,52 More important, Kennedy et al53 found that caspase activation is required for T-cell proliferation. Thus, although cleaved caspase 3 abrogates the activity of PARP and other substrates that protect cellular integrity, the “point of no return” in the apoptotic pathway is probably downstream of caspase 3. Furthermore, activated caspases may possess additional physiologic functions that are not related to the apoptotic process.54,55
Stat 5, which is activated by GM-CSF, was recently found to up-regulate the expression of the antiapoptotic protein Bcl-XL.56 Therefore, we asked whether GM-CSF affected the level of antiapoptotic proteins that either transduce antiapoptotic signals or block caspase signaling, thereby preventing cell death.56,57 Because GM-CSF increases the production of the mitochondria-bound procaspase 2, we first examined the effect of GM-CSF on members of the Bcl-2 family. Bcl-2 is thought to offset a decrease in the mitochondrial membrane potential, which is an early event associated with apoptosis.32 In addition, Bcl-2 can block the release of mitochondrial apoptogenic factors such as cytochrome c or sequester proforms of death-driving molecules, thereby inhibiting apoptotic cell death. In agreement with other studies,58,59 we found that GM-CSF up-regulated Bcl-2 protein levels. We also found that GM-CSF increased the levels of Bcl-XL, possibly by phosphorylating Stat 5, and that in contrast to its effect in mature neutrophils, GM-CSF up-regulates levels of the proapoptotic protein BAX.56,60 Interestingly, levels of Bcl-2 have been induced much more than those of BAX, indicating a stronger antiapoptotic effect on the level of mitochondrial membrane-based antiapoptotic proteins. As a caveat, however, this effect has been studied only at lower concentrations of GM-CSF and it is possible that the ratio of induction of Bcl-2 to BAX would be shifted in favor of an apoptotic response, commensurate with our results on expression of procaspase 3, at higher concentrations of the growth factor.
Similar to the action of Bcl-2 and Bcl-XL, other proteins that possess antiapoptotic activities could block the proapoptotic effects of activated caspase 3. We chose to study the effect of GM-CSF on 2 inhibitors of apoptosis (IAP) family members,34 XIAP61 and survivin.62 Cytokines, such as vascular endothelial growth factor, have been shown to induce the production of XIAP and survivin,63 and high levels of these 2 IAPs have been detected in AML.6,64 Our data show that GM-CSF up-regulates the protein levels of both survivin and XIAP. Thus it is possible that at a low concentration, the GM-CSF–induced activation of caspase 3 is not superseded by apoptosis, because of these antiapoptotic molecules, whereas at a high concentration, the proapoptotic signal prevails.
To determine whether GM-CSF affects procaspase protein production in other AML cell lines, first we used the GM-CSF–responsive OCI/AML3 cells, whose properties are similar to those of OCIM2. In these cells, a high dose of GM-CSF up-regulated procaspase 3 protein levels, but a low dose did not induce a time-dependent effect, as was found with the OCIM2 cells. Then we studied 2 GM-CSF–dependent cell lines and found a time-dependent up-regulation of procaspase 3 protein in one (Mo7e) but not the other (TF-1). Although the effects of GM-CSF depended on the concentration of GM-CSF, culture conditions, and incubation time, these data indicate that GM-CSF's up-regulation of procaspase protein production is not restricted to a single AML cell line.
The SOCS family of proteins has been implicated in the negative regulation of several cytokine pathways, in particular those that involve members of the Jak-Stat family of proteins.18 As GM-CSF not only activates cells to progress through the cell cycle but simultaneously induces proapoptotic proteins, we hypothesized that up-regulation of levels of SOCS proteins may constitute a growth-inhibitory feedback loop following stimulation of OCIM2 cells with GM-CSF.65-67 It has previously been shown that SOCS-2 functions as a component of a negative feedback mechanism in cells of patients with blast phase of chronic myeloid leukemia (CML).68 Here, we demonstrate that GM-CSF at low concentrations of 0.025 to 0.050 μg/mL up-regulates SOCS-2 and -3 in AML cells within 40 minutes following incubation with the growth factor. This observation is consistent with data from several investigators demonstrating that transcripts encoding SOCS-1, -2, or -3 can be induced within 15 to 30 minutes by stimulatory cytokines or hormones.69-76 Induction of expression of SOCS proteins following stimulation with GM-CSF may therefore constitute part of a negative feedback loop, albeit an inefficient one, in AML cells. Furthermore, the varying response of augmentation of SOCS protein levels also points to a differential role of SOCS proteins as components in a negative feedback loop.
As GM-CSF exerts its effects through activation of the PTK-Stat pathway, we asked what effect inhibition of this pathway would have on induction of procaspases and activation of caspase 3. We used the Jak-Stat inhibitor AG490 and observed inhibition of the expression of procaspase 3 and the activation of caspase 3. Jak-Stat thus represents one signaling route by which GM-CSF is linked to the apoptotic cascade in AML cells and establishes a connection between the growth-stimulatory and growth-inhibitory effects of GM-CSF.
Overall, our data suggest that GM-CSF induces a dual effect: it stimulates cell proliferation and, simultaneously, triggers proapoptotic signals in AML cells. Inhibition of the Jak-Stat pathway abrogates the GM-CSF–induced activation of the caspase cascade. Future studies will determine whether this phenomenon represents a growth factor–mediated intracellular feedback mechanism designed to negate excessive stimulatory signals. It remains to be seen whether antileukemia therapeutic strategies could take advantage of this phenomenon.
Prepublished online as Blood First Edition Paper, March 27, 2003; DOI 10.1182/blood-2002-06-1890.
Supported in part by grants from the National Institutes of Health (PO1 CA55164 and PO1 CA49639).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal