Abstract
Dendritic cells (DCs) are specialized antigen-presenting cells residing in tissues, from which they take up antigen. Activated DCs migrate through chemokine gradients from sites of inflammation to lymph nodes to stimulate T cells. At sites of inflammation, nucleotides, such as adenosine triphosphate (ATP), are released by activated or dying cells and can function as signaling molecules through P2 receptors (P2Rs). We investigated P2R expression in different DC populations and the effect of nucleotides on chemokine-directed migration. Exposure of monocyte-derived DCs (MoDCs) and CD1a+ dermal DCs to gradients of ATP inhibited their migratory capacity in a dose-dependent manner. Studies using P2R agonists and antagonists implicated signaling through the P2Y11R. On maturation, MoDCs down-regulated P2Y11R expression and were less sensitive to ATP-mediated inhibition of migration. In contrast, ATP did not inhibit the migration of CD1c+ peripheral blood (PB) DCs or interleukin-3 receptor-positive (IL-3R+) plasmacytoid DCs. Although all 4 DC populations expressed mRNA for P2Y11R, calcium-flux studies showed that blood DC types were unresponsive to P2Y11R agonists. In conclusion, DCs use distinct subtypes of P2R. The formation of ATP gradients at sites of inflammation may transiently inhibit the migration of local DCs, thus prolonging the time of antigen encounter. P2R inhibition may represent a new strategy to improve the migration of antigen-loaded DCs from the vaccination site to lymph nodes.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells whose primary function is to initiate and regulate T-cell responses. DCs are widely distributed in tissues, where they form a sentinel network to detect, capture, and process antigens.1 In recent years, it has become evident that DCs should be viewed as a system of antigen-presenting cells with diverse and distinct properties rather than as a single or unique cell type.2,3 In human blood, there are at least 2 different DC populations: CD1c+ myeloid peripheral blood DCs (PBDCs) and interleukin-3 receptor-positive (IL-3R+) plasmacytoid DCs (PDCs). Monocytes can differentiate into DCs in vitro when cultured in the presence of granulocyte macrophage–colony-stimulating factor (GM-CSF) and IL-4 or IL-13 for 5 to 7 days.4-6 Alternatively, a subset of CD16+ monocytes can transform into DCs after migration through an endothelial cell monolayer and ingestion of particulates in the collagen matrix below.7,8 Most of our knowledge about DCs has been gained from studying monocyte-derived DCs (MoDCs). However, identification of a physiologic counterpart in vivo remains unresolved. Therefore, a direct functional comparison of MoDC and physiologic DC types is essential to better understand DC biology.
DCs can be activated by an array of physiologic stimuli, such as those derived from microbes (lipopolysaccharide, bacterial DNA containing unmethylated CpG motifs), activated T cells (CD40 ligation), or mediators of inflammation (proinflammatory cytokines). Such stimuli induce DCs to undergo a process called maturation, which transforms them from cells specialized in antigen capture to cells specialized in activating T cells.1 Antigen uptake and T-cell activation are separate functions requiring the migration of DCs from the site of antigen encounter to regional lymphoid tissues. On activation, DCs up-regulate the chemokine receptors CXCR-4 and CCR-7, enabling them to migrate to gradients of the chemokines CXCL12 (SDF-1α) and CCL19/CCL21 (MIP-3β/6Ckine), respectively, which is important for lymph node homing. However, it has been demonstrated that chemokine receptor expression is not predictive of the migratory capacity of some DC types. For instance, prostaglandin E2 (PGE2) is an important regulator of migratory function of MoDCs.9,10 Recently, it has been reported that the activation of MoDCs by extracellular ATP induces the up-regulation of CXCR-4 and, to a lesser extent, CCR-7 and enables them to migrate to CXCL12 and CCL19, even in the absence of exogenous PGE2.11 Therefore, nucleotides may represent another class of important soluble factors regulating the migratory capacity of DCs.
Nucleotides such as ATP, adenosine diphosphate (ADP), and uridine triphosphate (UTP) can act as extracellular signaling molecules through the activation of membrane-bound P2 receptors (P2Rs), and they have been linked to the regulation of immune and inflammatory responses.12 P2Rs can be subdivided into P2XR, a family of ligand-gated ion channels, and G-protein–coupled 7-membrane spanning P2YR.13 MoDCs express mRNA for P2XR and P2YR, including P2X1, P2X2, P2X4, P2X5, P2X7, P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, and P2Y(13) (SP174).14-18 In MoDCs, nucleotide signaling through P2Rs has been associated with the up-regulation of activation markers and chemokine receptors, cytokine and chemokine secretion, and induction of apoptosis.11,14-17,19,20 Pharmacologic data point to the P2Y11R-mediating maturation of MoDCs by ATP.21 Studies on the cloned P2Y11R showed that the ATP-derivatives BzATP, ATPγS, and dATP are potent agonists, whereas suramin is a competitive antagonist.22 P2Y11R signals through phospholipase C and adenylyl cyclase pathways. The phospholipase C pathway mobilizes intracellular Ca2+ through IP3 and activates protein kinase C. The adenylyl cyclase pathway elevates cytosolic cyclic adenosine monophosphate (cAMP) levels, activating protein kinase A. Protein kinase A has been linked to the maturation-inducing effect of ATP on MoDCs.21 The role of phospholipase C in P2Y11R signaling on MoDC function has not yet been established. Nucleotides can also influence MoDC function by other P2Rs. For example, ATP concentrations that are 1000 times lower than those that induce maturation can act as chemoattractants for immature MoDCs.23 This effect is mediated by receptors, such as P2Y1R, P2Y12R, SP174 and P2Y2R, P2Y4R, P2Y6R, that are sensitive to ADP and UTP, respectively. Other investigators have established a role for the pore-forming P2X7R, a receptor that is activated by millimolar concentrations of ATP and that induces apoptosis.15 Together, these findings indicate that ATP effects on MoDC function are heterogeneous, depending on the P2R subtype used.
Our current knowledge regarding the role of nucleotides on human DC function has been predominantly gained from studying in vitro–generated MoDCs. Little information is available regarding P2R expression and signaling in physiologic DC types. In the present study, we examined the P2R expression profiles of different human DC types, such as in vitro–generated MoDCs, CD1a+ dermal DCs, CD1c+ PBDCs, and IL-3R+ PDCs, and we studied how these receptors are regulated during DC activation. Given that DCs, at sites of tissue damage or inflammation, are exposed to a complex array of proinflammatory mediators, cytokines, and nucleotides, we further investigated how exposure of these DC types to gradients of ATP impacts their capacity to migrate to chemokines in vitro.
Materials and methods
Media and reagents
DCs were cultured in RPMI 1640 supplemented with 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 60 mg/L penicillin G, 12.6 mg/L streptomycin, 2 mM l-glutamine, 1% nonessential amino acids, and 10% heat-inactivated fetal calf serum (FCS) (CSL Limited, Melbourne, Australia) in a 5% CO2 incubator. Adenosine 5′-triphosphate (ATP), uridine 5′-triphosphate (UTP), 2′- and 3′-O-(4-benzoylbenzoyl) ATP (BzATP), adenosine 5′-O-(3-thiotriphosphate) (ATPγS), 2′-deoxy ATP (dATP), 2-methylthio ATP (2-MeSATP), adenosine, oxidized ATP (2′,3′-dialdehyde ATP), suramin, pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), reactive blue 2 (RB-2), N-(2-[p-bromocin-namylamino]ethyl)-5-isoquinolinesulfonamide (H-89), Ro 31-8220 (bis-Indoylmaleimidine IX), U-73122 ((1-[6-[(17b)-3-Methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-1H-pyrrole-2,5-dione), phorbol 12-myristate 13-acetate (PMA), PGE2, and forskolin were all obtained from Sigma (St Louis, MO). The cAMP agonists dibutyryl cAMP and 8-bromo-cAMP were purchased from Wako (Tokyo, Japan).
Monoclonal antibodies and cytokines
Flow cytometric analysis was performed using fluorochrome-conjugated monoclonal antibody (mAb) against CD1a, CD1c, CD3, CD14, CD20, CD83, CD86, CD123w, HLA-DR, CXCR-4, and CCR-7 (PharMingen/Becton Dickinson, San Jose, CA) and against BDCA-1 and BDCA-4 (Miltenyi Biotech, Auburn, CA). The cytokines tumor necrosis factor-α (TNF-α), IL-4, and IL-3 were from PeproTech (Rocky Hill, NJ); GM-CSF (40 ng/mL) was from Schering-Plough (Sydney, Australia); and interferon-α2a (IFN-α2a) (Roferon-A) was from (Roche Products PTY, Sydney, Australia). Soluble CD40L-trimer (CD40L) was a gift from Immunex (Seattle, WA).
Generation of MoDCs and isolation of DC types from the peripheral blood
Human PBMCs were isolated from buffy coats from healthy volunteers provided by the Red Cross Blood Bank (Melbourne, Australia). PBMCs were prepared by Ficoll-Hypaque density gradient centrifugation (Amersham Biosciences, Uppsala, Sweden). MoDCs were generated as previously described.9 In short, CD14+ monocytes were positively selected using anti-CD14 mini-MACS (Miltenyi Biotech) and were cultured at a density of 5 × 105 cells/mL in RPMI-10% FCS in the presence of GM-CSF (40 ng/mL) and IL-4 (500 U/mL). On day 7, cells were pooled and readjusted to a cell density of 5 × 105 cells/mL. MoDCs were left untreated (immature MoDCs) or matured for 24 to 48 hours with CD40L (1 μg/mL) or ATP (250 μM) or with a combination of TNF-α (10 ng/mL), IFN-α (1000 IU/mL), and PGE2 (1 μg/mL). CD1c+ PBDCs were isolated using the BDCA-1 (CD1c) DC isolation kit, according to the manufacturer's protocol (Miltenyi Biotech). Purity of the isolated cells was enhanced by repeated positive selection (purity of CD1c+ HLA-DR+ cells was greater than 96%). CD1c+ PBDCs were cultured in RPMI-10% FCS in the presence of GM-CSF (40 ng/mL) and IL-4 (500 U/mL) at a density of 5 × 105 cells/mL. IL-3R+ (CD123w+) PDCs were isolated using the BDCA-4 DC isolation kit (Miltenyi Biotech). Purity was enhanced by repeated positive selection (purity of CD123w+ HLA-DR+ cells was greater than 95%). IL-3R+ PDCs were cultured in 96-well plates (2 × 105 cells/mL) in RPMI-10% FCS in the presence of IL-3 (10 ng/mL).
Enrichment of PBDCs from FL-treated patients
For some experiments, PBDCs were isolated from frozen PBMCs of patients with stage II, III, or IV melanoma who were enrolled in a phase 1 clinical study (LUD00-021) and received Flt-3 ligand (FL; Immunex) (20 μg/kg/d) for 10 consecutive days, alone or in combination with peptide vaccines. Blood for PBDC isolation was taken on day 15 of FL administration. The Protocol Review Committee of the Ludwig Institute for Cancer Research and the Human Research Ethics Committee of the Austin and Repatriation Medical Centre (Heidelberg, Australia) approved the protocol, and informed consent was obtained from all patients. CD1c+ PBDC and IL-3R+ PDCs were sorted on a MoFlo cell sorter (Cytomation; Fort Collins, TX), as described,9 or by positive selection using either the BDCA-1 or BDCA-4 isolation kit, as described.
Isolation of emigrant CD1a+ dermal DCs
Skin was obtained by methods approved by the Melbourne University and the Mercy Hospital Research ethics committee (Mercy Hospital, Melbourne, Victoria, Australia). Split-thickness skin was obtained using a Froud skin graft knife, and the explants were incubated in 2.5 mg/mL dispase 2 (Roche Biochemicals, Basel, Switzerland) in RPMI at 37°C for 1 hour before they were separated into epidermal and dermal sections. Dermal sheets were cultured in RPMI-10% FCS for 16 to 24 hours, and spontaneously migrating cells were harvested. This procedure yielded an enriched CD1a+ dermal DC population (50%-75% CD1a+CD14-HLA-DRbright). For mRNA isolation, migrated cells underwent fluorescence-activated cell sorting (FACS) analysis based on the expression of CD1a and HLA-DR.
Polymerase chain reaction
Total RNA was isolated from DCs using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Total RNA (0.15 μg) was used to synthesize cDNA using 1 μg random hexamers (Promega, Madison, WI), 1 mM dNTP (Amersham Pharmacia Biotech, Piscataway, NJ), 2 U RNase inhibitor (Promega), 5 mM MgCl2, PCR Buffer (Applied Biosystems, Foster City, CA) and 2 U M murine leukemia virus (MLV) reverse transcriptase (Life Technologies, Rockville, MD) in a 20-μL reaction for 60 minutes at 42°C. The enzyme was inactivated at 95°C for 5 minutes. Polymerase chain reaction (PCR) was carried out in 25 μL reactions using 0.2 mM dNTP, 0.625 U Amplitaq DNA polymerase, 1 × PCR Buffer II, 2 mM MgCl2 (all from Applied Biosystems), and 0.5 μM primers. Primers for P2XR and P2YR were previously described: P2X1, P2X7, P2Y1, P2Y5,
Quantitative real-time PCR
Gene expression levels were quantitated using ABI Prism 7700 Sequence Detection System (Applied Biosystems). Primers and probes for P2Y11R were designed by Primer Express software, version 1.5a (Applied Biosystems) (forward primer, 5′-TTGGTGGCCAGTGGTGTG-3′; reverse primer, 5′-TGAGCACCCGCATGATGT-3′; probe, 5′-FAM-CCCTCTACGCCAGCTCCTATGTGCC-3′). 18S rRNA PDAR was included for normalization. Multiplex reactions were set up in 96-well plates according to the manufacturer's instructions and were analyzed using the SDS program, version 1.7. Samples were tested in duplicate wells. Relative expression was calculated using the ΔCt method and is expressed relative to a calibrator—in this case MoDCs cultured with GM-CSF and IL-4.
Cell migration assay
Assays were performed as previously described.9 Briefly, lower chambers of transwell plates (8-μm pore size for MoDCs, 5-μm pore size for PBDCs and CD1a+ dermal DCs) (Costar, Corning, NY) were filled with 500 μL RPMI/10% FCS with or without the following chemokines: CCL19 (MIP-3β) (300 ng/mL), CCL21 (6Ckine) (100 ng/mL), CXCL12 (SDF-1α) (30 ng/mL), and CCL3 (MIP-1α) (50 ng/mL) (all from PeproTech). In some experiments, nucleotides were added to the chemokine-containing side of the transwells. Cells (1-2 × 104) in 100 μL RPMI/10% FCS were added to the upper chamber. After 2 hours of incubation at 37°C, cells in the lower chambers were harvested, concentrated to 50-μL volumes in Eppendorf tubes, and counted with a hemocytometer. Migration studies for all conditions were performed in triplicate wells.
Intracellular Ca2+ measurements
Changes of intracellular Ca2+ concentrations were measured by FACS.24 DCs (106 cells/mL or fewer) were incubated with fluo-3 acetoxymethyl ester (fluo-3/am)(4 μg/mL) and Fura Red (Fura Red/am) (10 μg/mL) in the presence of 0.02% Pluronic F-127 (all from Molecular Probes, Eugene, OR) for 30 minutes at 37°C. Cells were washed twice with assay buffer (145 mM NaCl, 5 mM KCl, 1 mM Na2HPO4, 0.5 mM MgSO4, 5 mM glucose, 1 mM CaCl2, and 10 mM HEPES) and were kept for 30 minutes at room temperature in the dark. For analysis, 20 μL cell suspension was transferred to tubes containing 200 μL buffer (37°C). Flow rate was adjusted to 100 to 150 events per second. After obtaining a baseline for 10 to 30 seconds, nucleotides were added and samples were measured for 102 to 204 seconds. Data were analyzed by plotting the LFL1/LFL3 ratios versus time using FloJo software (version 3.4; Tree Star, San Carlos, CA).
Statistical analysis
Data are expressed as mean values ± SEM. Statistical significance of differences was determined by the paired or unpaired 2-tailed Student t test. Differences were considered significant for P < .05 and are indicated by asterisks in the graphs.
Results
Regulation of migratory function of MoDCs differs from physiologic DC types
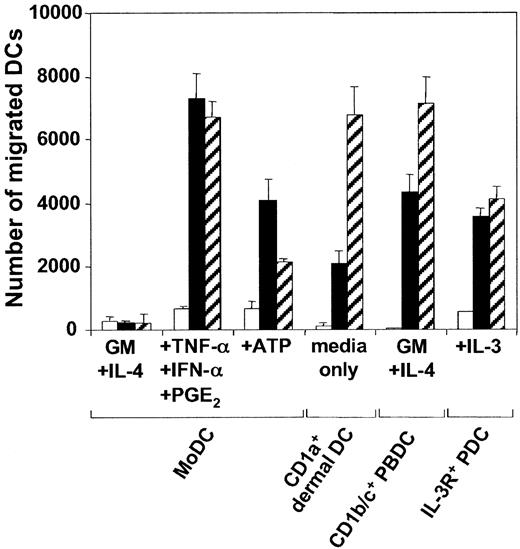
We and others9,10 have previously shown that though the expression of the chemokine receptors CXCR-4 and CCR-7 can be induced on MoDCs by proinflammatory cytokines, intact bacteria, or CD40L, receptor expression was not always predictive of efficient migratory function toward CXCL12 and CCL21. Maximal migratory function required MoDCs to be exposed to the above stimuli in the presence of PGE2. Interestingly, CD1c+ PBDCs and IL-3R+ PDCs differed from MoDCs in this respect. These DC types, which mature spontaneously during in vitro culture, migrated efficiently toward CXCL12 and CCL21 in the absence of exogenous PGE2 (Figure 1). An alternative inducer of MoDC migration toward CXCL12, and to a lesser extent CCL21, was found to be exposure to extracellular ATP.11 The data in Figure 1 confirm this finding. Moreover, as was seen with PGE2-containing stimuli, the present study found that ATP did not enhance the rapidly acquired migratory function of CD1c+ PBDCs or IL-3R+ PDCs (data not shown). To study the migratory properties of tissue-derived DCs, we isolated emigrant CD1a+ dermal DCs from split skin.25,26 Like the blood DC types, cultured CD1a+ dermal DCs expressed a mature phenotype (CD83+, CD86+, and HLA-DRbright) and migrated vigorously toward CXCL12 and CCL21, without the requirement for either exogenous PGE2 or ATP (Figure 1). Thus, of the 4 DC types studied, only the migratory function of MoDCs was regulated by exogenous ATP or PGE2.
Migration of MoDCs, CD1a+ dermal DCs, CD1c+ PBDCs, and IL-3R+ PDCs to the chemokines CXCL12 and CCL21. Day 7 MoDCs were cultured for 24 hours in the absence or presence of 250 μM ATP or a combination of TNF-α, IFN-α, and PGE2. CD1c+ PBDCs or IL-3R+ PDCs were cultured overnight in the presence of GM-CSF and IL-4 or IL-3, respectively. Emigrant CD1a+ dermal DCs were harvested from cultured dermal sheets. Migration of DCs (104 DCs/well) toward chemokines was assessed in a transwell chemotaxis assay. Representative experiments of at least 4 different donors are shown (mean ± SEM of triplicates). □ indicates medium; ▪, CXCL12; and ▧, CCL21.
Migration of MoDCs, CD1a+ dermal DCs, CD1c+ PBDCs, and IL-3R+ PDCs to the chemokines CXCL12 and CCL21. Day 7 MoDCs were cultured for 24 hours in the absence or presence of 250 μM ATP or a combination of TNF-α, IFN-α, and PGE2. CD1c+ PBDCs or IL-3R+ PDCs were cultured overnight in the presence of GM-CSF and IL-4 or IL-3, respectively. Emigrant CD1a+ dermal DCs were harvested from cultured dermal sheets. Migration of DCs (104 DCs/well) toward chemokines was assessed in a transwell chemotaxis assay. Representative experiments of at least 4 different donors are shown (mean ± SEM of triplicates). □ indicates medium; ▪, CXCL12; and ▧, CCL21.
Gradients of ATP inhibit chemokine-directed migration of MoDCs
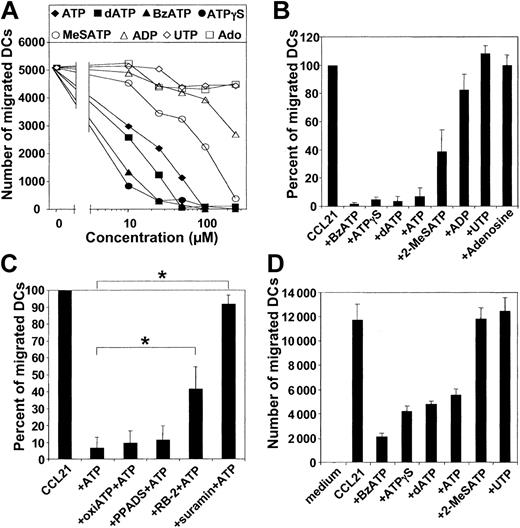
Because DCs are exposed to a complex array of proinflammatory mediators, cytokines, chemokines, and nucleotides at sites of inflammation, we addressed whether simultaneous exposure of MoDCs to a gradient of chemokines and ATP influences their capacity to migrate. For this purpose, ATP was added to the chemokine-containing chamber in transwell experiments. Under these conditions, ATP inhibited in a dose-dependent fashion the migration of MoDCs matured with TNF-α, IFN-α, and PGE2 to CCL21 (Figure 2B). An ATP concentration of 50 μM reduced migration by approximately 50%. This inhibitory effect of ATP on MoDC migration was mediated by a gradient of ATP because the addition of ATP to the DC-containing side of the migration chambers had no influence on migration (data not shown). To assess whether the ATP inhibition of migration was restricted to CCL21, we examined the influence of ATP on migratory-type MoDCs in response to CXCL12 and CCL19. Again, a potent inhibition of migration was observed (data not shown). This ATP effect was also not limited to a particular stimulation condition because ATP effectively inhibited the migratory capacity of MoDCs matured with CD40L, which by itself is a poor inducer of migratory function (Figure 2C). As little as 10 μM ATP was sufficient to inhibit 50% of the migration of CD40L-matured MoDCs compared with 25 to 50 μM for MoDCs matured with PGE2-containing stimuli (Figure 2B-C).
Exposure to a gradient of ATP inhibits chemokine-directed migration of specific DC types in a dose-dependent fashion. (A) Migration of MoDCs, CD1a+ dermal DCs, CD1c+ PBDCs, or IL-3R+ PDCs to CCL21 in the absence or presence of 100 μM ATP added to the chemokine side of the transwell chambers. Migration to CCL21 in the absence of ATP was normalized to 100% (mean ± SEM; n = 4 for CD1a+ dermal DCs; n = 6 for all other DC types). *P < .05. (B) Dose-dependent ATP-mediated arrest of migration to CCL21 of MoDCs matured with TNF-α, IFN-α, and PGE2 for 24 hours (mean ± SEM; n = 4). (C) MoDCs matured with CD40L for 24 hours. (D) Emigrant CD1a+ dermal DCs. Representative experiments, each performed in triplicate, are shown (n = 4).
Exposure to a gradient of ATP inhibits chemokine-directed migration of specific DC types in a dose-dependent fashion. (A) Migration of MoDCs, CD1a+ dermal DCs, CD1c+ PBDCs, or IL-3R+ PDCs to CCL21 in the absence or presence of 100 μM ATP added to the chemokine side of the transwell chambers. Migration to CCL21 in the absence of ATP was normalized to 100% (mean ± SEM; n = 4 for CD1a+ dermal DCs; n = 6 for all other DC types). *P < .05. (B) Dose-dependent ATP-mediated arrest of migration to CCL21 of MoDCs matured with TNF-α, IFN-α, and PGE2 for 24 hours (mean ± SEM; n = 4). (C) MoDCs matured with CD40L for 24 hours. (D) Emigrant CD1a+ dermal DCs. Representative experiments, each performed in triplicate, are shown (n = 4).
ATP inhibits chemokine-directed migration of CD1a+ dermal DCs but not CD1c+ PBDCs or IL-3R+ PDCs
Next, we examined the effect of ATP on the migratory capacity of 3 physiologic DC types—CD1a+ dermal DCs and the blood DC types, CD1c+ PBDCs and IL-3R+ PDCs. As observed for MoDCs, exposure to gradients of ATP inhibited the migration of CD1a+ dermal DCs to CCL21 (Figure 2D). A 50% inhibition of migration of CD1a+ dermal DCs was seen with 50 to 100 μM ATP. In contrast, migration of CD1c+ PBDCs and IL-3R+ PDCs was unaffected by the presence of ATP in the chemokine gradient (Figure 2A). ATP concentrations as high as 500 μM were ineffective, ruling out nonspecific effects such as buffer toxicity (data not shown).
Inhibition of migration by ATP is mediated by P2Rs
To determine whether the inhibitory effect of ATP on DC migration is mediated by P2Rs, we studied the effect of P2R agonists and antagonists on DC migration. For MoDCs and CD1a+ dermal DCs, the ranking order of potency of various P2R agonists was ATPγS ≈ BzATP > dATP > ATP > 2MeSATP (Figure 3A-B, D). As little as 10 μM ATPγS, BzATP, or dATP dramatically inhibited the migration of MoDCs (Figure 3A). UTP and the degradation products of ATP with activity on purinergic receptors, such as ADP and adenosine, induced no significant inhibition of migration (Figure 3A-B). Next, we studied the influence of several P2R antagonists on the ATP effect. The P2R antagonist suramin completely abrogated ATP-mediated inhibition of MoDC migration (n = 8; P < .01), whereas the antagonist reactive blue 2 (RB-2) was partially effective (n = 5; P < .01) (Figure 3C). In contrast, the P2R antagonist PPADS and oxidized ATP were ineffective. Interestingly, the profile obtained with P2R agonists and antagonists is characteristic for the cloned P2Y11R, as described by Communi et al.22
ATP inhibits DC migration through activation of P2R. (A) Nucleotides with specific binding affinities to P2R were tested at various concentrations for their ability to inhibit the migration of MoDCs, matured with TNF-α, IFN-α, and PGE2 for 24 hours, to CCL21. (B) Effect of nucleotides (at 100 μM) on the migration of migratory-type MoDCs to CCL21. Migration in the absence of nucleotides was normalized to 100% (mean ± SEM; n ≥ 5). (C) Effect of P2R antagonists on the inhibitory effect of ATP (100 μM) on MoDC migration. Migratory-type MoDCs were incubated with 30 μM suramin, 100 μM reactive blue-2 (RB-2), 100 μM PPADS, or 300 μM oxidized ATP (oxiATP) 45 minutes before the assessment of migration (mean ± SEM; n = 5-8). *P < .01. (D) Effect of P2R agonists (at 100 μM) on the migration of CD1a+ dermal DCs toward CCL21.
ATP inhibits DC migration through activation of P2R. (A) Nucleotides with specific binding affinities to P2R were tested at various concentrations for their ability to inhibit the migration of MoDCs, matured with TNF-α, IFN-α, and PGE2 for 24 hours, to CCL21. (B) Effect of nucleotides (at 100 μM) on the migration of migratory-type MoDCs to CCL21. Migration in the absence of nucleotides was normalized to 100% (mean ± SEM; n ≥ 5). (C) Effect of P2R antagonists on the inhibitory effect of ATP (100 μM) on MoDC migration. Migratory-type MoDCs were incubated with 30 μM suramin, 100 μM reactive blue-2 (RB-2), 100 μM PPADS, or 300 μM oxidized ATP (oxiATP) 45 minutes before the assessment of migration (mean ± SEM; n = 5-8). *P < .01. (D) Effect of P2R agonists (at 100 μM) on the migration of CD1a+ dermal DCs toward CCL21.
Inhibition of MoDC migration by ATP is mediated through phospholipase C
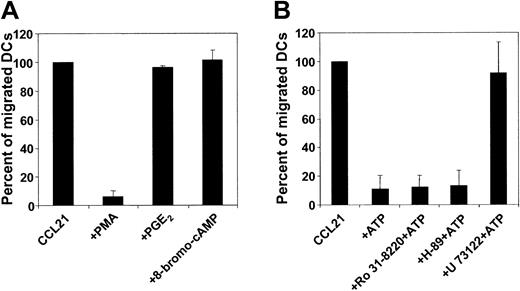
P2Y11R is dually coupled to phosphatidyl inositol/phospholipase C/protein kinase C and to adenylyl cyclase/cAMP/protein kinase A pathways.22 To examine whether protein kinase A activation could inhibit the migratory function of MoDCs, migratory-type MoDCs were exposed to activators of this pathway, such as PGE2, forskolin, db-cAMP, or 8-bromo-cAMP before or during the migration assay. As shown in Figure 4A, protein kinase A agonists did not affect DC migration. In contrast, PMA, a potent activator of protein kinase C, completely inhibited MoDC migration. Because these findings provide only indirect evidence of a possible mechanism of action for ATP, we examined the influence of these signaling pathways on the ATP effect using specific inhibitors. Inhibition of neither protein kinase C with Ro 31-8220 (0.5-5 μM) nor protein kinase A with H-89 (1-10 μM) antagonized the ATP-mediated arrest of MoDC migration (Figure 4B). Next, we investigated the role of phospholipase C. To this end, some methodological problems had to be resolved first because chemokines also signal through this pathway. As expected, preincubating MoDC with the phospholipase C inhibitor U-73122 (0.5 μM) resulted in a complete loss of migratory function (data not shown). However, exposing MoDCs to U-73122 (2-10 μM) by adding this inhibitor to the chemokine-containing side of the transwell did not interfere with migration. Under this condition, U-73122 antagonized the inhibitory effect of ATP on MoDC migration, suggesting that the inhibition of migration by ATP is mediated by phospholipase C (Figure 4B).
Influence of signal transduction pathways on MoDC migration. (A) Migration of migratory-type MoDCs to CCL21 in the absence or presence of protein kinase C or protein kinase A agonists. The protein kinase C agonist PMA (1 ng/mL) or the protein kinase A agonist PGE2 (10 μM) or 8-bromo-cAMP (10 μM) was added to the chemokine-containing side of transwells. Migration in the absence of activators was normalized to 100% (mean ± SEM; n = 3). (B) Migration of MoDC to CCL21 ± ATP in the absence or presence of protein kinase C or protein kinase A or of phospholipase C antagonists. MoDCs were incubated with the protein kinase C inhibitor Ro31-8220 (5 μM) or the protein kinase A inhibitor H-89 (5 μM) for 30 minutes before assessment was made of their migration to CCL21 in the absence or presence of 100 μM ATP. Incubation of MoDCs with the phospholipase C inhibitor U-73122 (0.5 μM) abolished chemokine-directed migration (not shown). Therefore, U-73122 (10 μM) was added to the chemokine-containing side of transwells (± ATP). Migration in the absence of ATP was normalized to 100% (mean ± SEM; n = 3).
Influence of signal transduction pathways on MoDC migration. (A) Migration of migratory-type MoDCs to CCL21 in the absence or presence of protein kinase C or protein kinase A agonists. The protein kinase C agonist PMA (1 ng/mL) or the protein kinase A agonist PGE2 (10 μM) or 8-bromo-cAMP (10 μM) was added to the chemokine-containing side of transwells. Migration in the absence of activators was normalized to 100% (mean ± SEM; n = 3). (B) Migration of MoDC to CCL21 ± ATP in the absence or presence of protein kinase C or protein kinase A or of phospholipase C antagonists. MoDCs were incubated with the protein kinase C inhibitor Ro31-8220 (5 μM) or the protein kinase A inhibitor H-89 (5 μM) for 30 minutes before assessment was made of their migration to CCL21 in the absence or presence of 100 μM ATP. Incubation of MoDCs with the phospholipase C inhibitor U-73122 (0.5 μM) abolished chemokine-directed migration (not shown). Therefore, U-73122 (10 μM) was added to the chemokine-containing side of transwells (± ATP). Migration in the absence of ATP was normalized to 100% (mean ± SEM; n = 3).
Human MoDCs and physiologic DC types express mRNA for the same P2XR and P2YR
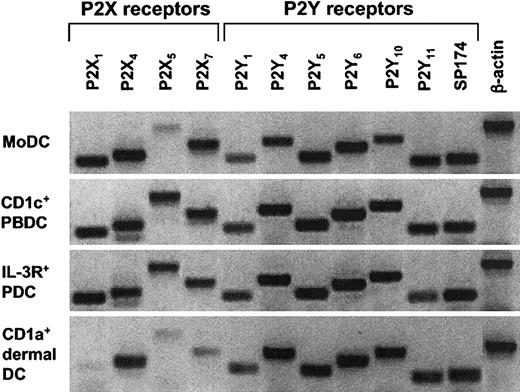
Given that ATP-mediated inhibition of migration was observed for MoDCs and CD1a+ dermal DCs, but not for CD1c+ PBDCs or IL-3R+ PDCs, we speculated that these DC types differ in the expression pattern of their P2R repertoire, especially with respect to P2Y11R. Because of the unavailability of mAb against most P2R, including P2Y11R, we analyzed the P2R expression of all 4 DC types by conventional RT-PCR. Surprisingly, we found similar mRNA expression patterns for P2R, including P2Y11R, in all 4 DC populations (Figure 5). However, there were donor-to-donor variations and semiquantitative differences in P2R expression between the 4 DC types.
Analysis of mRNA expression for P2XR and P2YR subtypes in different human DC populations. Profiles of P2R expression for MoDCs, CD1a+ dermal DCs, CD1c+ PBDCs, and IL-3R+ PDCs were generated by extracting mRNA from highly purified DC populations (purity, 95%-99%). Conventional RT-PCR was performed as described in “Materials and methods.” A representative experiment of at least 3 different donors is shown (for CD1a+ dermal DCs, n = 2).
Analysis of mRNA expression for P2XR and P2YR subtypes in different human DC populations. Profiles of P2R expression for MoDCs, CD1a+ dermal DCs, CD1c+ PBDCs, and IL-3R+ PDCs were generated by extracting mRNA from highly purified DC populations (purity, 95%-99%). Conventional RT-PCR was performed as described in “Materials and methods.” A representative experiment of at least 3 different donors is shown (for CD1a+ dermal DCs, n = 2).
CD1c+ PBDCs and IL-3R+ PDCs do not express functional P2Y11R
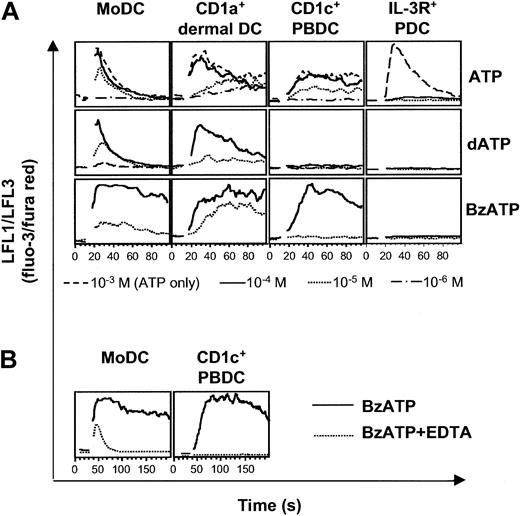
To assess whether the expression of P2Y11R mRNA reflects the expression of functional membrane receptor, we studied cytosolic Ca2+ transients in response to ATP and nucleotides with high affinity for P2Y11R, such as dATP and BzATP. Striking differences between the different DC types were seen (Figure 6A). First, MoDCs, CD1a+ dermal DCs, and CD1c+ PBDCs were sensitive to ATP at concentrations from 10 to 100 μM, whereas higher concentrations had no additional effect on Ca2+ mobilization. In contrast, IL-3R+ PDCs were 50 times less sensitive to ATP, requiring ATP concentrations of 500 to 1000 μM for the induction of Ca2+ signals. Interestingly, IL-3R+ PDCs were highly sensitive to UTP, inducing Ca2+ transients at concentrations as low as 0.1 μM (M.S. et al, unpublished data, 2002). Second, MoDCs and CD1a+ dermal DCs signaled in response to dATP, whereas CD1c+ PBDCs and IL-3R+ PDCs did not. Third, BzATP induced Ca2+ transients in MoDCs, CD1a+ dermal DCs, and CD1c+ PBDCs. However, in CD1c+ PBDCs, Ca2+ signaling to BzATP was dependent on extracellular Ca2+ because the presence of 0.5 mM EDTA (ethylenediaminetetraacetic acid) abolished signaling (Figure 6B). Therefore, CD1c+ PBDCs signaled only through P2XR in response to BzATP. In contrast, EDTA did not abolish signaling in MoDCs in response to BzATP (Figure 6B). Rather, Ca2+ transients with shorter duration and reduced amplitude were observed, indicating the additional activation of P2YR with mobilization of Ca2+ from intracellular stores. In conclusion, DC types differ in the use of their P2R repertoire, with CD1c+ PBDCs and IL-3R+ PDCs not responding to known inducers of P2Y11R signaling.
Differential sensitivity of DC types to nucleotides. Nucleotide-induced Ca2+ signaling of MoDCs, CD1a+ dermal DCs, CD1c+ PBDCs, and IL-3R+ PDCs was analyzed by flow cytometry. After establishing a baseline for 10 to 30 seconds, nucleotides were added at the indicated concentrations. (A) Each lane represents Ca2+ transients induced in DCs from the same donor and is representative of at least 4 separate experiments (for CD1a+ dermal DCs, n = 1). (B) Ca2+ signaling of MoDCs and CD1c+ PBDCs in response to 100 μM BzATP in the absence (solid line) or presence (dotted line) of 0.5 mM EDTA.
Differential sensitivity of DC types to nucleotides. Nucleotide-induced Ca2+ signaling of MoDCs, CD1a+ dermal DCs, CD1c+ PBDCs, and IL-3R+ PDCs was analyzed by flow cytometry. After establishing a baseline for 10 to 30 seconds, nucleotides were added at the indicated concentrations. (A) Each lane represents Ca2+ transients induced in DCs from the same donor and is representative of at least 4 separate experiments (for CD1a+ dermal DCs, n = 1). (B) Ca2+ signaling of MoDCs and CD1c+ PBDCs in response to 100 μM BzATP in the absence (solid line) or presence (dotted line) of 0.5 mM EDTA.
P2Y11R is down-regulated on maturation in MoDCs
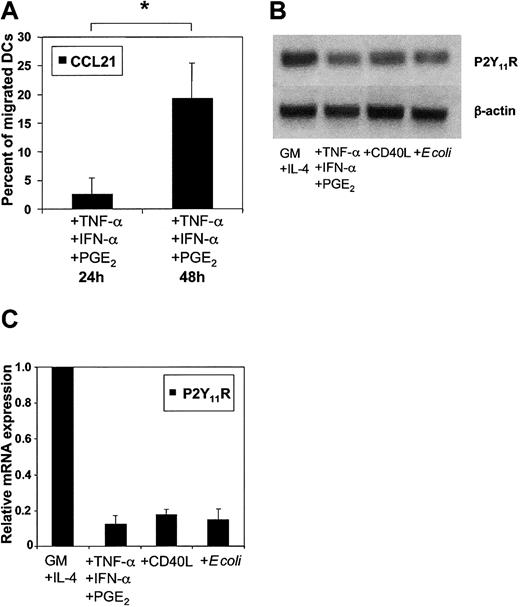
To further assess whether ATP-mediated inhibition of migration depends on the maturational stage of DCs, the migration of immature MoDCs toward CCL3 (MIP-1α) was examined. As little as 10 to 25 μM ATP inhibited CCL3-directed migration of immature MoDCs by 50% compared with 25 to 50 μM ATP for MoDCs matured with TNF-α, IFN-α, and PGE2 (data not shown). To assess whether sensitivity toward ATP is down-regulated during DC maturation, we examined the migration of MoDCs cultured in the presence of proinflammatory cytokines for up to 24 hours or 48 hours. As shown in Figure 7A, MoDCs matured for 48 hours were less efficiently inhibited in their migration by ATP (n = 7; P < .01). To address whether differences in P2R expression can account for this observation, we stimulated DCs with several classes of stimuli (proinflammatory cytokines, CD40L, or intact Escherichia coli). Nonquantitative conventional PCR showed similar mRNA profiles of P2XR and P2YR for unstimulated and stimulated MoDCs (data not shown). Because our data implicated signaling through P2Y11R as the major mechanism of the ATP-mediated arrest of DC migration, we analyzed P2Y11R mRNA expression in immature and mature MoDCs in more detail. Semiquantitative conventional RT-PCR using cDNA dilutions suggested down-regulation of P2Y11R mRNA expression during MoDC maturation (Figure 7B). This finding was confirmed by quantitative RT-PCR showing that P2Y11R mRNA expression after overnight stimulation was consistently reduced by more than 80%, regardless of the maturation stimuli used (Figure 7C).
Down-regulation of P2Y11R mRNA expression in matured MoDCs correlates with reduced ATP-mediated arrest of migration. (A) Effect of 100 μM ATP on the migration of MoDCs matured with TNF-α, IFN-α, and PGE2 for 24 or 48 hours. Migration to CCL21 in the absence of ATP was normalized to 100% (mean ± SEM; n = 7). *P < .01. (B) P2Y11R mRNA expression as measured by RT-PCR in immature MoDCs (GM-CSF + IL-4) or those matured overnight with TNF-α + IFN-α + PGE2, CD40L, or E coli. (C) Quantitative analysis of P2Y11R mRNA expression as measured by quantitative real-time PCR (RT-qPCR). Threshold was exceeded after 24 PCR cycles in immature MoDCs. Immature MoDCs were used as the calibrator to calculate relative P2Y11R mRNA levels in MoDCs matured in the presence of the indicated stimuli. Expression of 18S rRNA within each sample was used for normalization (mean ± SEM; n = 4).
Down-regulation of P2Y11R mRNA expression in matured MoDCs correlates with reduced ATP-mediated arrest of migration. (A) Effect of 100 μM ATP on the migration of MoDCs matured with TNF-α, IFN-α, and PGE2 for 24 or 48 hours. Migration to CCL21 in the absence of ATP was normalized to 100% (mean ± SEM; n = 7). *P < .01. (B) P2Y11R mRNA expression as measured by RT-PCR in immature MoDCs (GM-CSF + IL-4) or those matured overnight with TNF-α + IFN-α + PGE2, CD40L, or E coli. (C) Quantitative analysis of P2Y11R mRNA expression as measured by quantitative real-time PCR (RT-qPCR). Threshold was exceeded after 24 PCR cycles in immature MoDCs. Immature MoDCs were used as the calibrator to calculate relative P2Y11R mRNA levels in MoDCs matured in the presence of the indicated stimuli. Expression of 18S rRNA within each sample was used for normalization (mean ± SEM; n = 4).
Discussion
ATP is present in the cytosol at concentrations of 5 to 10 mM and in the intracellular compartments of platelets at concentrations of 100 to 1000 mM. It can be actively released by regulated exocytosis (activated platelets, endothelial cells, T cells), by traumatic cell lysis, or by passive leakage from damaged cells.27-29 Under physiologic conditions, the concentration of extracellular ATP is regulated by ecto-nucleotidases, such as ecto-ATP/ADPase (CD39), which hydrolyzes ATP and ADP to adenosine monophosphate (AMP), a nucleotide with no intrinsic activity on P2Rs.30 In inflamed tissues, the release of ATP and the down-regulation of CD39 can cause an accumulation of extracellular ATP.31 ATP signals through P2Rs and exhibits regulatory effects on immune and inflammatory responses.12 Given that under normal conditions high concentrations of ATP are restricted to intracellular compartments, the accumulation of extracellular ATP likely reflects tissue damage and may present a danger signal to the immune system.32 The influence of elevated extracellular ATP levels in vivo has been studied in CD39-/- mice, which lack the capacity to degrade extracellular ATP. Altered immune responses and defects in DC function indicate that nucleotides likely play an important role in immune responses in vivo.33 The first evidence that these effects could, at least in part, be a result of an impaired migratory function of leukocytes came from the same mice, which displayed a severe defect in cell migration with decreased cellular infiltrates and a complete failure of neovascularization at sites of tissue injury.34 The effects of ATP on human DCs have been studied using in vitro–generated MoDCs, and exposure to high micromolar concentrations of ATP has been linked to maturation, enhanced T-cell stimulatory capacity, cytokine production, and apoptosis.14-17,19-21 However, it is now clear that MoDCs are not functionally equivalent to their physiologic counterparts found in the blood.9 In the present study, we compared MoDCs with 3 physiologic DC populations—CD1a+ dermal DCs, CD1c+ myeloid PBDCs, and IL-3R+ PDCs—with respect to the regulation of their migratory function and P2R expression and to the influence of ATP on chemokine-directed migration.
Pronounced differences between MoDCs, CD1c+ PBDCs, and IL-3R+ PDCs were observed when studying the regulation of their migratory function. MoDCs gained full migratory capacity 24 hours after stimulation with PGE2-containing stimuli, as previously described.9,10 Interestingly, in those studies, PGE2 alone was a poor inducer of migratory function and necessitated additional activation with other stimuli, such as CD40L, proinflammatory cytokines, or microbial stimuli. It has been reported that MoDCs activated with ATP gain the ability to migrate in response to CXCL12 and CCL21, without exogenous PGE2 or additional stimuli.11 In the present study we confirmed these findings. We propose that different signaling pathways could account for the difference seen between the induction of migratory capacity mediated by ATP and PGE2. It has been shown that migratory function of MoDCs by PGE2 is mediated through elevated cytosolic cAMP levels and activated protein kinase A.9,10 In MoDCs, P2Rs and E-prostanoid 2/E-prostanoid 4 receptors signal through cAMP/protein kinase A. However, P2Rs also signal through phospholipase C. Simultaneous activation of protein kinase A and protein kinase C pathways by ATP may induce the migratory capacity of MoDCs, even in the absence of PGE2 and other stimuli. In contrast to MoDCs, the 2 blood DC types, CD1c+ PBDC and IL-3R+ PDC, rapidly acquired migratory capacity toward CXCL12 and CCL21 after overnight in vitro culture and without further stimulation. In contrast again to MoDCs, culturing these blood DC types in the presence of ATP did not enhance their migratory capacity. Similarly, mature emigrant CD1a+ dermal DCs migrated efficiently to CXCL12 and CCL21 without further stimulation. However, CD1a+ dermal DCs could have been exposed to nucleotides or PGE2 released within the traumatized skin during isolation. It is therefore possible that this DC type may still require exposure to such cAMP-elevating molecules in situ to attain its migratory function.
At sites of inflammation or tissue damage, it is likely that DCs are simultaneously exposed to gradients of ATP, chemokines, and maturation-inducing cytokines that permeate out of the epicenter of inflammation. We were interested in determining whether ATP within a chemokine gradient influences DC migration. We found that gradients derived from micromolar concentrations of ATP potently inhibited the migration of MoDCs and CD1a+ dermal DCs. By applying various P2R agonists and antagonists, we demonstrated that the inhibitory effect of ATP on DC migration was P2R mediated. Strikingly, the ranking order of potency of P2R agonists and antagonists was identical to the pharmacologic profile of the cloned P2Y11R.22 Because the human P2Y11R signals through phospholipase C/protein kinase C and adenylyl cyclase/protein kinase A, we studied the influence of both signaling pathways on DC migration. We found that ATP-mediated inhibition of MoDC migration was mediated by phospholipase C because the inhibition of phospholipase C with U-73122 abolished the ATP effect on migration. However, we cannot exclude the possibility that the results may be attributed to an unreported effect of U-73122 that potentially disrupts the ATP gradient, thereby abrogating the inhibitory effect of ATP. Interestingly, the inhibition of protein kinases A and C were ineffective in this respect. However, addition of the protein kinase C agonist PMA to the chemokine gradient completely inhibited the migration of MoDCs, indicating that this pathway could function as a regulator of DC migration. It is also possible that ATP-induced Ca2+ transients within the cell mediate the inhibition of migration. It is known that polarization of cytoskeletal mechanisms is a prerequisite for migrating cells.35 For example, the retraction of the rear part of a migrating cell and the protrusion of the lamellipodium are dependent on the polarization of intracellular Ca2+ concentrations, with higher concentrations in the rear end than in the lamellipodium. P2R signaling likely occurs at the DC surface most proximal to the ATP gradient (ie, the lamellipodium) and Ca2+ transients at this region of the cell body could disrupt the formation of intracellular Ca2+ gradients, thereby arresting cell motion. Together, our data demonstrate that the inhibition of DC migration by ATP is P2R mediated, most likely by P2Y11R, and that it involves signaling through phospholipase C.
When we examined the effect of ATP on the migration of blood DC types, we found that CD1c+ PBDCs and IL-3R+ PDCs were able to migrate efficiently in the presence of ATP, indicating DC type-specific use of the P2R repertoire. By RT-PCR we found that all DC populations expressed the same set of P2XR and P2YR, including P2Y11R. Despite these DC types expressing similar mRNA profiles for P2Rs, Ca2+-signaling studies revealed that their responsiveness to nucleotides differed substantially. Several observations have been made: first, MoDCs, CD1a+ dermal DCs, and CD1c+ PBDCs, 3 DC types expressing myeloid markers, were 50 to 100 times more sensitive to ATP than IL-3R+ PDCs, the latter signaling only in response to ATP concentrations close to the millimolar range; second, MoDCs and CD1a+ dermal DCs, but not CD1c+ PBDCs or IL-3R+ PDCs, signaled in response to dATP, an agonist of P2Y11R; and third, BzATP, an agonist of P2Y11R and P2X7R, induced strong and long-lasting Ca2+ transients in all 3 myeloid DC types. However, in CD1c+ PBDCs, BzATP signaling occurred only in the presence of extracellular Ca2+, indicating expression of the pore-forming P2X7R. In contrast, MoDCs also signaled to BzATP in the absence of extracellular Ca2+, revealing additional activation of a P2YR, such as P2Y11R. Taken together, these data provide the first evidence that functional P2Rs are differentially expressed in specific DC populations and that signaling through P2Y11R is absent in CD1c+ PBDCs and IL-3R+ PDCs. Because the inhibition of migration by ATP was most likely mediated by P2Y11R, a lack of functional P2Y11R in the blood DC types may explain why these DC populations migrated efficiently in the presence of ATP.
Inhibition of migration by ATP was also dependent on the maturational stage of the DCs, with immature MoDCs more sensitive to the ATP effect than mature MoDCs. This finding correlated with the down-regulation of P2Y11R mRNA expression on maturation, as assessed by quantitative RT-PCR. Thus, lower concentrations of ATP may be sufficient to inhibit the migration of immature DCs residing in tissues in vivo. The down-regulation of P2Y11R on maturation may be why relatively high concentrations of ATP were required to inhibit the migration of fully matured CD1a+ dermal DCs. As discussed above, we did not detect functional P2Y11R expression in cultured blood DC types. However, it is possible that CD1c+ PBDCs completely down-regulated P2Y11R expression at the cell surface during their rapid maturation in vitro. Interestingly, by quantitative RT-PCR, we found that P2Y11R mRNA expression was significantly down-regulated during overnight culture (data not shown). Therefore, P2Y11R signaling may play a role in CD1c+ PBDCs before their functional maturation in vitro. However, freshly isolated CD1c+ PBDCs were also unresponsive to P2Y11R agonists in Ca2+-flux studies (data not shown). Monoclonal antibodies directed against P2Y11R and P2Y11R-specific agonists to investigate this are unavailable.
Taken together, our data indicate that the physiologic consequences of exposure to ATP on DC migration depend on ATP concentration, DC type, and DC maturational stage (the latter 2 influence the P2R repertoire). The proposed working hypothesis is that low concentrations of ATP at the onset of inflammation may initially induce the recruitment of immature tissue-resident DCs (eg, in the dermis) into sites of inflammation, as previously reported.23 Here, chemotaxis is mediated by P2YR that are sensitive to nanomolar concentrations of ATP (or ADP and UTP), such as P2Y1R, P2Y12R and P2Y2R, P2Y4R, P2Y6R.23 However, once in the epicenter of inflammation, the accumulation of ATP transiently causes arrest in cell movement. This effect, most likely mediated by P2Y11R, can override the chemotactic effect of nucleotides and chemokines. Arrest of migration may prolong DC capacity to encounter and internalize antigens and may prolong exposure to maturation-inducing factors. Eventually, the degradation of extracellular ATP and the down-regulation of P2Y11R allows mature DCs to migrate from these sites of inflammation and to draining lymph nodes, where they can interact with T cells. In contrast, blood DC populations are insensitive to the regulatory effect of ATP on migration. Other, yet unidentified, factors are likely to regulate their migratory function.
In conclusion, we describe a novel mechanism by which ATP can regulate the trafficking of specific DC populations. Because the migration of DCs from the site of antigen capture to lymphoid tissue is a prerequisite for the induction and regulation of immune responses, ATP-mediated inhibition of migration could play an important role in inflammatory diseases and cancer. Pharmacologic targeting of P2Y11R may thus represent a new therapeutic strategy to improve the migration of in vitro–generated DCs or tissue-resident DCs to induce the trafficking of antigen from the vaccine site to the draining lymph nodes.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-12-3745.
Supported by the Sylvia and Charles Viertel Foundation, a Program Grant from the Australian National Health and Medical Research Council, the Ludwig Institute for Cancer Research, a grant from the Dr Mildred Scheel Stiftung (M.S.), the Austrian Science Fund (P-14949) (P.S.), and the Kyrle Fund of the Austrian Society of Dermatology and Venereology (P.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Mona Pfaender for helpful discussions.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal