Abstract
Acute myeloid leukemias (AMLs) carrying inv(16)/t(16;16) chromosomal abnormalities are associated with a good prognosis. However, studies of this AML subtype have been hampered by the few number of patients reported, frequently collectively considered with those with AML carrying the t(8;21) translocation. We performed a retrospective study in 110 patients with inv(16)/t(16;16) AML (median age, 34 years) prospectively enrolled in 6 trials conducted in France between 1987 and 1998, with the aim to investigate prognostic factors for complete remission (CR) achievement and outcome of CR patients in this AML subtype. CR rate was 93%. Bad-prognosis factors for CR achievement were higher white blood cell count (WBC) and lower platelet count (optimal cutpoints at 120 and 30 × 109/L, respectively). At 3 years, estimated overall survival, disease-free survival (DFS), and cumulative incidence of relapse were 58%, 48%, and 42%, respectively. In multivariate analysis, (1) advanced age (optimal cutpoint, 35 years) was the only factor for shorter DFS and (2) advanced age and low platelet count were the 2 factors for shorter survival of CR patients. Outcome of CR patients (1) was not influenced by WBC and cytogenetic findings and (2) was similar among patients allocated to receive allogeneic transplantation, high-dose, or intermediate-dose cytarabine. Interestingly, advanced age was associated with a trend for more frequent additional chromosome abnormalities and predictive of higher cumulative incidence of relapse rather than death in first CR. These results markedly contrast with those reported in patients with t(8;21) AML in whom WBC, and not age, was the main high-risk factor for relapse, DFS, and survival.
Introduction
Core binding factors (CBFs) are a family of heterodimeric transcriptional regulators containing a common CBFβ (CBFB) subunit associated with 1 of the 3 CBFα (CBFA) members. The expression of CBFA2 (AML1) is restricted to myeloid and lymphoid tissues, and both CBFA2–/– and CBFB–/– embryos die without developing hematopoiesis.1-3 Recurrent chromosomal rearrangements involving the genes coding for CBFA2 and CBFB are observed in approximately 8% and 4% of patients up to 55 years of age with de novo acute myeloid leukemia (AML), respectively.4 These rearrangements may also be found in patients with therapy-related AML.5
The inv(16)(p13q22) fuses the CBFB gene located in 16q22 to the MYH11 gene located in 16p13, resulting in a chimeric protein product. Specific fluoresecent in situ hybridization (FISH) may be useful to confirm the presence of this rearrangement.6 The translocation t(16;16)(p13;q22) is an equivalent rearrangement of a much lower incidence. CBFB-MYH11 fusion transcripts may be evidenced by a specific reverse transcriptase–polymerase chain reaction (RT-PCR) assay, which provides a useful tool to estimate minimal residual disease.7 CBFB-MYH11 fusion protein blocks the differentiation process of myeloid leukemic cells through a sequestration of CBFA2 in the cytoplasm.3 The CBFA2/CBFBMYH11 complex also acts as a transcriptional repressor through the recruitment of corepressors and chromatin-modifying histone deacetylase activities.8 Expression of the CBFB-MYH11 chimeric protein alone is, however, not sufficient for leukemogenesis, and additional mutations may be needed to lead to the development of AML.3
Acute myeloid leukemias carrying CBFB alteration (CBFBAML) exhibit some specific characteristics. This AML subset is morphologically associated with the French-American-British AML-M4 subtype with an abnormal eosinophil component (M4eo).9 It has been demonstrated that abnormal eosinophils carry the specific inv(16)(p13q22) rearrangement and thus derive from the leukemic clone.10 Cytogenetically, the CBFB-MYH11 rearrangement may be associated with trisomy 8, 21, and 22 and less frequently with deletion of the long arm of the chromosome 7.11-13 Finally, c-kit mutations have been observed both in CBFA2- and CBFB-AML.14,15 Clinically, CBFB-AML, like CBFA2-AML, has been associated with a high rate of complete remission (CR) and favorable outcome as compared with other AML subsets.4,11,13,16-19 However, no large prognosis study involving patients with CBFBAML only has been reported to date. So far, even in large chemotherapy or transplantation studies, patients with CBFB-AML are often collectively referred with those with CBFA2-AML as patients with CBF-AML.18-20 In these patients, prolonged CR may be achieved with intensive postremission chemotherapy including high-dose cytarabine (HDAC),13,18,19,21 raising the question of the value of allogeneic hematopoietic stem cell transplantation (HSCT) in first CR in those with a donor.20
We recently reported the prognostic impact of the white blood cell count (WBC) and a derived WBC index in 161 patients with t(8;21) AML enrolled in 6 French multicenter AML trials between 1987 and 1998.22 We report here the results of a parallel study of 110 patients with inv(16)/t(16;16) AML, all prospectively enrolled in the same trials during the same period. Data from all patients were retrospectively reviewed. The aims of this survey were (1) to investigate the prognostic factors for CR achievement and outcome in a large population of patients with this disease; (2) to compare the results of allogeneic SCT in first CR with those of chemotherapy alone; and (3) to compare the prognostic significance of age and WBC in inv(16)/t(16;16) versus t(8;21) AML patients. Interestingly, we found that older patients were at higher risk for relapse leading to a worse outcome in older as compared with younger CR patients, despite comparable compliance to planned therapy. This result markedly contrasts with the lack of prognostic value of age in patients with t(8;21) AML and suggests differences in inv(16)/t(16;16) AML biology in the older patient population.
Patients, materials, and methods
Patient selection and review of data
All patients have been prospectively enrolled in 1 of the 6 following French multicenter trials: LAME-91,23 ALFA-9000,24 BGMT-87,25 BGMT-91,26 GOELAM-01,27 GOELAM-02.28 The LAME-91 study was a pediatric AML study, while the 5 other studies were adult AML studies. Patients with newly diagnosed de novo previously untreated AML with 30% or more marrow blasts and a performance status of 2 or below may enter these trials if also meeting the following specific eligibility criteria: age between 10 and 55 years and AML classified in the French-American-British (FAB) classification for both BGMT trials; age between 10 and 50 years and AML classified in the FAB classification for both GOELAM trials; age younger than 20 years and AML classified in the FAB classification with the exception of AML-M0 and AML-M7 for the LAME-91 trial; and age between 15 and 65 years for the ALFA-9000 trial. Between May 1987 and August 1998, 110 patients diagnosed with AML carrying the inv(16) or the t(16;16) rearrangement on standard karyotype at diagnosis entered 1 of these 6 trials. Reverse transcriptase–polymerase chain reaction (RT-PCR) was not considered for patient selection.
A predefined set of data including demography, hematologic presentation, cytogenetics, postremission therapy, and outcome was collected for each patient, sent to a central coordinating center, and reviewed for consistency and completeness before analysis. Because inclusion criteria for the study were only based on karyotype analysis, morphology was not centrally reviewed. Immunophenotyping of the leukemic cells was not retained due to the lack of consistent data in many cases. Chromosome analysis was performed using 24-hour unstimulated bone marrow cultures. Two banding techniques (RHG and GTG) were applied. Standard criteria to define a clone were used, and chromosomal abnormalities were classified according to the International System for Human Cytogenetic Nomenclature.29 Only patients with a minimum of 5 metaphases analyzed were also classified according to the abnormal/normal (AN) or abnormal/abnormal (AA) status, depending on the presence or the absence of normal metaphases. In each participating cooperative group (Groupe Ouest-Est des Leucémies Aiguës Myéloblastiques [GOELAM], Leucémies Aiguës Myéloblastiques de l'Enfant [LAME], Acute Leukemia French Association [ALFA], and Bordeaux-Grenoble-Marseille-Toulouse [BGMT]), all cytogenetic data were prospectively and centrally reviewed by a distinct working committee. We have recently reported that a so-called WBC index was the main prognostic factor in patients with t(8;21) AML.22 This WBC index was calculated as the product of WBC by the ratio of marrow blasts (WBC index = WBC × [% of marrow blasts/100]), with the aim to adjust the WBC on the spontaneous differentiating capacity of the leukemic clone observed in patients with t(8;21) AML. This WBC index was not considered in patients from the present study. The main reason for that was the lack of biologic rational to consider the percentage of marrow blasts as a relevant marker of any level of leukemic maturation blockage in the inv(16)/t(16;16) AML subtype.
Follow-up observations extended from 1996 to 2000, depending of the trial, with a median follow-up of 5.5, 6.5, 8.4, 6.4, 3.4, and 3.3 years for the LAME-91, ALFA-9000, BGMT-87, BGMT-91, GOELAM-01, and GOELAM-02 studies, respectively. Overall, the median follow-up was 5.7 years. For statistical analysis, outcome data were censored at 3.3 years, corresponding to the shorter median follow-up of the GEOLAM-02 study. Actually, only 2 relapses and 2 deaths were observed after this time. Comparisons of prognostic impacts of covariates in inv(16)/t(16;16) versus t(8;21) AML patients were performed using the previously reported population of patients with t(8;21) AML prospectively included in the same trials during the same period.22 The present study was approved by the institutional review board (IRB), Hôpital Saint-Louis, Paris, France.
Induction therapy
Induction therapy varied among the 6 prospective trials considered. In the pediatric LAME-91 trial, all included patients received the same induction regimen comprising 12 mg/m2/d mitoxantrone for 5 days and 200 mg/m2/d cytarabine as continuous infusion for 7 days. In the adult ALFA-9000 trial, patients were randomized at inclusion to receive 1 of the 3 following reinforced induction regimens: arm 1 consisted of 80 mg/m2/d daunorubicin for 3 days and 200 mg/m2/d cytarabine as continuous infusion for 7 days; arm 2 consisted of the same regimen followed at day 20 by a second induction course comprising 12 mg/m2/d mitoxantrone for 2 days and 500 mg/m2/12-h cytarabine as 3-hour intravenous bolus infusion for 3 days; arm 3 consisted of 80 mg/m2/d daunorubicin for 3 days and 500 mg/m2/d cytarabine as continuous infusion for 3 days, followed at day 8 by 12 mg/m2/d mitoxantrone for 2 days and 500 mg/m2/12-h cytarabine as 3-hour intravenous bolus infusion for 3 days. In the adult BGMT-87 and BGMT-91 trials, all included patients received the same induction regimen comprising 60 mg/m2/d daunorubicin for 3 days and 100 mg/m2/d cytarabine as continuous infusion for 10 days. In the adult GOELAM-01 and GOELAM-02 trials, patients were randomized at inclusion to receive either 8 mg/m2/d idarubicin for 5 days or 200 mg/m2/d rubidazone for 4 days in combination with standard 200 mg/m2/d cytarabine as continuous infusion for 7 days. Standard National Cancer Institute (NCI) complete remission criteria were similarly used in all these trials.30 Deaths occurring during the induction course and resistant AML after the induction course were classified as induction failures.
Postremission therapy
The impact of postremission therapy on outcome was analyzed using the intent-to-treat principle (treatment allocated at CR achievement). Postremission therapy varied among the 6 prospective studies considered (Table 1). Differences concerned mainly the place of allogeneic HSCT in first CR, the number of intensive postremission cycles of chemotherapy, the dosage of cytarabine within these intensive cycles, and the use of autologous HSCT. Definition criteria used for intensive postremission cycles of chemotherapy, high doses of cytarabine (HDAC), and intermediate doses of cytarabine (IDAC) have been previously reported for t(8;21) AML.22 Because of strong interactions related to study designs, treatment modalities mentioned above cannot be considered as independent variables. On one hand, no patient was allocated to receive one single intensive postremission cycle but containing HDAC. One the other hand, all patients allocated to receive an autologous HSCT had previously received one HDAC cycle.
Design of postremission therapy
LAME-9123 | |
| Intensive consolidation 1* | DNR, 40 mg/m2/d for 4 d |
| VP16, 100 mg/m2/d for 4 d | |
| AraC, 100 mg/m2/d for 4 d | |
| Allogeneic SCT after consolidation 1 | |
| Intensive consolidation 2* | Amsa, 150 mg/m2/d for 3 d |
| IDAC, 1000 mg/m2/12 h for 4 d | |
| L-ASPA, 6000 IU/m2/d for 2 d | |
| Maintenance or not† | |
| ALFA-900024 | |
| Mild consolidation 1 | Amsa, 90 mg/m2/d for 1 d |
| AraC, 120 mg/m2/d for 5 d | |
| No allogeneic SCT in first CR | |
| Intensive consolidation 2‡ | MTZ, 12 mg/m2/d for 3 d |
| VP16, 200 mg/m2/d for 3 d | |
| IDAC, 500 mg/m2/d for 6 d | |
| No maintenance | |
| BGMT-8725 and -9126 | |
| Mild consolidation 1 | DNR, 60 mg/m2/d for 2 d |
| AraC, 100 mg/m2/d for 7 d | |
| Allogeneic SCT after consolidation 1 | |
| Intensive consolidation 2 | DNR, 45 mg/m2/d for 3 d |
| HDAC, 3000 mg/m2/12 h for 4 d | |
| Autologous SCT or maintenance§ | |
| GEOLAM-0127 and -0228 | |
| Allogeneic SCT before consolidation 1 | |
| Intensive consolidation 1 | IDA, 10 mg/m2/d for 2 d or RBZ, 200 mg/m2/d for 2 d |
| HDAC, 3000 mg/m2/12 h for 4 d | |
| Autologous SCT∥ | Amsa, 150 mg/m2/d for 5 d |
| or intensive consolidation 2 | VP16, 100 mg/m2/d for 5 d |
| No maintenance |
LAME-9123 | |
| Intensive consolidation 1* | DNR, 40 mg/m2/d for 4 d |
| VP16, 100 mg/m2/d for 4 d | |
| AraC, 100 mg/m2/d for 4 d | |
| Allogeneic SCT after consolidation 1 | |
| Intensive consolidation 2* | Amsa, 150 mg/m2/d for 3 d |
| IDAC, 1000 mg/m2/12 h for 4 d | |
| L-ASPA, 6000 IU/m2/d for 2 d | |
| Maintenance or not† | |
| ALFA-900024 | |
| Mild consolidation 1 | Amsa, 90 mg/m2/d for 1 d |
| AraC, 120 mg/m2/d for 5 d | |
| No allogeneic SCT in first CR | |
| Intensive consolidation 2‡ | MTZ, 12 mg/m2/d for 3 d |
| VP16, 200 mg/m2/d for 3 d | |
| IDAC, 500 mg/m2/d for 6 d | |
| No maintenance | |
| BGMT-8725 and -9126 | |
| Mild consolidation 1 | DNR, 60 mg/m2/d for 2 d |
| AraC, 100 mg/m2/d for 7 d | |
| Allogeneic SCT after consolidation 1 | |
| Intensive consolidation 2 | DNR, 45 mg/m2/d for 3 d |
| HDAC, 3000 mg/m2/12 h for 4 d | |
| Autologous SCT or maintenance§ | |
| GEOLAM-0127 and -0228 | |
| Allogeneic SCT before consolidation 1 | |
| Intensive consolidation 1 | IDA, 10 mg/m2/d for 2 d or RBZ, 200 mg/m2/d for 2 d |
| HDAC, 3000 mg/m2/12 h for 4 d | |
| Autologous SCT∥ | Amsa, 150 mg/m2/d for 5 d |
| or intensive consolidation 2 | VP16, 100 mg/m2/d for 5 d |
| No maintenance |
SCT indicates stem cell transplantation; DNR, daunorubicin; VP16, etoposide; AraC, cytarabine; Amsa, amsacrine; IDAC, intermediate-dose cytarabine; L-ASPA, L-asparaginase; MTZ, mitoxantrone; HDAC, high-dose cytarabine; IDA, idarubicin; and RBZ, rubidazone.
In infants, postremission therapy was amended in December 1993 for one IDAC cycle followed by allogeneic or autologous HSCT.
Randomization between maintenance therapy or not.
Half-dose VP16 and IDAC in patients aged 50 years or older.
Randomization between autologous SCT or maintenance therapy in the BGMT-87 trial; autologous SCT for all patients in the BGMT-91 trial.
Randomization between autologous SCT and intensive consolidation 2 in the GOELAM-01 trial; intensive consolidation 2 for all patients in the GOELAM-02 trial.
Consequently, postremission therapy was firstly classified as follows: (1) allogeneic HSCT (patients with an HLA-identical sibling enrolled in a study including allogeneic HSCT in first CR) (ALLO; n = 16 patients); (2) 1 HDAC cycle followed by autologous HSCT (HDAC-auto; n = 19 patients); (3) 2 intensive postremission cycles including 1 HDAC cycle (HDAC-2; n = 28 patients); (4) 2 intensive postremission cycles including 1 IDAC cycle but no HDAC (IDAC-2; n = 17 patients); and (5) 1 intensive IDAC cycle only (IDAC-1; n = 22 patients). Because patients belonging to the subgroups HDAC-auto and HDAC-2 (all from the 2 GOELAM studies) have similar outcome, even after adjustment on covariates (data not shown), the final classification retained to analyze the impact of postremission therapy was the following 4-group classification: (1) ALLO (n = 16 patients; median age, 23.6 years); (2) HDAC-2 (n = 47 patients including the 19 HDAC-auto patients; median age, 42.6 years); (3) IDAC-2 (n = 17 patients; median age, 5.4 years); and (4) IDAC-1 (n = 22 patients; median age, 35.4 years). In addition, the 16 patients from the ALLO group were also compared with the 72 patients up to 50 years of age (median age, 31.8 years) from the 3 chemotherapy (CHEMO) groups HDAC-2, IDAC-2, and IDAC-1.
Statistical methods
The Fisher exact test was used for binary variable comparisons. The t test was used for mean comparisons, and the Mann-Whitney test was used for median comparisons. Impacts of continuous variables on CR rate and multivariate analysis for CR achievement were tested using the maximum-likelihood model. Overall survival was calculated from the time of inclusion until death, and patients alive were censored at the time of last contact. Disease-free survival (DFS) was calculated from the date of CR achievement until first relapse or death in CR, and patients alive in CR were censored at the time of last contact. Data on treatment failure were estimated by the Kaplan-Meier method31 and compared using the log-rank test.32 In univariate evaluations of the prognostic impact of continuous variables (age, blood counts), optimal cutpoints were determined using a corrected minimum P value method based on an approximation to the improved Bonferroni inequality.33 In multivariate analyses, outcome comparisons were adjusted with the Cox model34 and tested by the likelihood-ratio test. All multivariate analyses systematically included the trial as covariate. Cumulative incidences of relapse and death in CR were calculated from the date of first CR achievement until the date of relapse or death, respectively, when occurring as first event. Patients were not censored at the date of death in CR when estimating cumulative incidence of relapse and inversely.35 Risks of relapse and death in CR were compared using the standard Kaplan-Meier method and the log-rank test. A P value less than .05 was considered to indicate statistical significance. Hazard ratios were given with 95% confidence intervals (CIs). All calculations were performed using the Stata software, version 7.0 (Stata, College Station, TX).
Results
Patient population
Outcome and prognostic factors for CR achievement, DFS, incidence of DFS events, and overall survival of CR patients were evaluated in 110 patients with inv(16)/t(16;16) AML. Baseline characteristics of these patients are indicated in Table 2. Median age was 34 years (range, 0.7-64 years) with 21 patients (19%) aged 15 years or younger, 15 patients (14%) aged older than 50 years, and only 3 patients aged older than 60 years. Of note, 99 patients had AML-M4 in the French-American-British classification, including 77 patients with AML-M4eo. Median WBC was 44 × 109/L. Interestingly, a trend for higher WBC in children as compared with adults was observed. The median WBC was 84.5 × 109/L in patients aged 15 years or younger as compared with 30 × 109/L in those older than 15 years (P = .06). Median platelet count was 46 × 109/L. Median hemoglobin (Hb) count was 90 × 109/L.
Patient characteristics
Patients, no. | 110 |
| Sex, M/F | 59/51 |
| Median age, y (range) | 34 (0.7-64) |
| FAB classification, no. of patients | |
| AML-M4 | 99* |
| AML-M2 | 7 |
| AML-M1 | 3 |
| Unclassified | 1 |
| Study, no. of patients | |
| LAME-91 | 23 |
| ALFA-9000 | 26 |
| BGMT-87 | 12 |
| BGMT-91 | 12 |
| GOELAM-01 | 10 |
| GOELAM-02 | 27 |
| Median marrow blast percentage (range) | 64 (22-100) |
| Median WBC, × 109/L (range) | 44 (3-390) |
| 75% percentile upper limit | 89 |
| 90% percentile upper limit | 173 |
| 95% percentile upper limit | 222 |
| Median platelet count, × 109/L (range) | 46 (6-215) |
| 25% percentile lower limit | 30 |
| 10% percentile lower limit | 19 |
| 5% percentile lower limit | 17 |
| Median Hb count, g/L (range) | 90 (49-150) |
| 25% percentile lower limit | 77 |
| 10% percentile lower limit | 60 |
| 5% percentile lower limit | 58 |
Patients, no. | 110 |
| Sex, M/F | 59/51 |
| Median age, y (range) | 34 (0.7-64) |
| FAB classification, no. of patients | |
| AML-M4 | 99* |
| AML-M2 | 7 |
| AML-M1 | 3 |
| Unclassified | 1 |
| Study, no. of patients | |
| LAME-91 | 23 |
| ALFA-9000 | 26 |
| BGMT-87 | 12 |
| BGMT-91 | 12 |
| GOELAM-01 | 10 |
| GOELAM-02 | 27 |
| Median marrow blast percentage (range) | 64 (22-100) |
| Median WBC, × 109/L (range) | 44 (3-390) |
| 75% percentile upper limit | 89 |
| 90% percentile upper limit | 173 |
| 95% percentile upper limit | 222 |
| Median platelet count, × 109/L (range) | 46 (6-215) |
| 25% percentile lower limit | 30 |
| 10% percentile lower limit | 19 |
| 5% percentile lower limit | 17 |
| Median Hb count, g/L (range) | 90 (49-150) |
| 25% percentile lower limit | 77 |
| 10% percentile lower limit | 60 |
| 5% percentile lower limit | 58 |
WBC indicates white blood cell count; Hb, hemoglobin.
Including 77 AML-M4eo in the FAB classification.
As indicated in Table 3, cytogenetic analysis showed the inv(16) in 109 patients and the t(16;16) translocation in 1 patient (inclusion criteria). There was a trend for more frequent persistent normal metaphases (AN status) in patients younger than 35 years as compared with older patients (51% versus 32%; P = .08). Associated chromosome abnormalities were observed in 34 patients, usually as part of a relatively simple karyotype. Overall, trisomy 8, trisomy 22, trisomy 21, and del(7q) were observed in 10, 10, 4, and 4 patients, respectively. Five patients presented only trisomy 8, 5 patients only trisomy 22, 2 patients only trisomy 21, and 4 patients only del(7q) as additional anomaly. Other abnormalities were del(16q) in 3 patients and +9, del(9q), del(3p), t(11;16)(q23;q22), t(16;18)(q22;q12), t(3;5)(q11;p11), t(5;22)(p15;q11), or t(9;22)(q34; q11) in 1 patient each. Molecular analysis for MLL gene rearrangement was not performed in the patient with associated t(11;16)(q23; q22). An m-bcr BCR-ABL transcript was identified in the patient with associated t(9;22)(q34;q11).36 Finally, a complex karyotype (defined as 3 or more unrelated abnormalities) was observed in 7 patients. However, 5 of these 7 patients had only limited chromosome number anomalies in addition to the inv(16) (+8, +22 in 3 patients; +22, –18 in 1 patient; +8, +13, +14, +21 in 1 patient). Overall, there was a trend for more frequent additional chromosome abnormalities in patients aged 35 years or older as compared with younger patients (P = .06) (Table 3). Interestingly, this difference came from a higher incidence of associated chromosome structure rather than chromosome number abnormalities in older patients (P = .05 and .33, respectively).
Cytogenetic features
. | All . | Aged younger than 35 years . | Aged 35 years or older . | P . |
|---|---|---|---|---|
| Patients, no. | 110 | 51 | 59 | — |
| inv(16)/t(16;16), no. | 109/1 | 51/0 | 58/1 | — |
| AN/AA status,* no. | 45/64 | 26/25 | 19/39 | .08 |
| Associated abnormalities, no. | 34 | 11 | 23 | .06 |
| Number abnormalities only, no. | 18 | 7 | 11 | .33† |
| Including +8 | 9 | 2 | 7 | — |
| Including +22 | 9 | 3 | 6 | — |
| Including +21 | 3 | 3 | 0 | — |
| Including structure abnormalities, no. | 16 | 4 | 12 | .05† |
| del(7q) | 4 | 1 | 3 | — |
| del(16q) | 3 | 1 | 2 | — |
| Other | 9 | 2 | 7 | — |
. | All . | Aged younger than 35 years . | Aged 35 years or older . | P . |
|---|---|---|---|---|
| Patients, no. | 110 | 51 | 59 | — |
| inv(16)/t(16;16), no. | 109/1 | 51/0 | 58/1 | — |
| AN/AA status,* no. | 45/64 | 26/25 | 19/39 | .08 |
| Associated abnormalities, no. | 34 | 11 | 23 | .06 |
| Number abnormalities only, no. | 18 | 7 | 11 | .33† |
| Including +8 | 9 | 2 | 7 | — |
| Including +22 | 9 | 3 | 6 | — |
| Including +21 | 3 | 3 | 0 | — |
| Including structure abnormalities, no. | 16 | 4 | 12 | .05† |
| del(7q) | 4 | 1 | 3 | — |
| del(16q) | 3 | 1 | 2 | — |
| Other | 9 | 2 | 7 | — |
— indicates not applicable.
In 109 patients with at least 5 analyzed metaphases.
Using 1-sided Fisher exact test.
A total of 102 patients (93%) reached CR without difference among trials. Six patients (5%) died during the induction course of chemotherapy, at day 3, 5, 11, 19, 23, and 47. Of note, most of these patients died from multiorgan failure secondary to treatmentinduced acute tumor lysis syndrome and coagulopathy. The remaining 2 patients with primary resistant AML died 3 and 12 months after inclusion. Overall, 3-year overall survival estimate was 58% (95% confidence interval, 49%-67%), and 3-year DFS estimate was 48% (95% confidence interval, 38%-57%). At 3 years, the cumulative incidence of relapse was 42% and the cumulative incidence of death in CR was 10%. Estimated 3-year postrelapse survival was 34% (95% confidence interval, 20%-49%).
Prognostic factors for CR achievement
Univariate analysis. Advanced age was not a bad-prognosis factor for CR achievement, either considered as a continuous variable (P = .29) or with an age cutpoint at 15 years (P = .35) or 35 years (P = .28). Conversely, higher WBC was significantly predictive of induction failure when considered as a continuous variable (P < .001). The median WBC of the 8 patients who failed to reach a CR was 185 × 109/L (range, 18-390 × 109/L) as compared with 36 × 109/L (range, 3-240 × 109/L) in those who achieved a CR (P = .001). The optimal WBC cutpoint was 120 × 109/L (CR rate, 68% in the 19 patients with a WBC of 120 × 109/L or more as compared with 98% in the remaining patients; P < .001). Lower platelet count was also significantly predictive of induction failure when considered as a continuous variable (P = .015), and a similar trend was noted for lower Hb count (P = .052). The optimal platelet count cutpoint was 30 × 109/L (CR rate, 81% in the 26 patients with a platelet count less than 30 × 109/L as compared with 96% in the remaining patients; P = .02). Finally, the presence of an associated trisomy 22 was significantly associated with a lower CR rate (70% vs 95%, P = .03). The presence of other additional chromosome structure or number abnormalities or persistence of normal metaphases had no significant impact on CR rate.
Multivariate analysis. In a multivariate analysis also including adjustment on the 6 different trials and on the presence of a trisomy 22, higher WBC (P = .005, when considered as a continuous variable) and lower platelet count (P = .052, when considered as a continuous variable) remained the only 2 independent bad-prognosis factors for CR achievement.
Prognostic factors for DFS and overall survival in CR patients
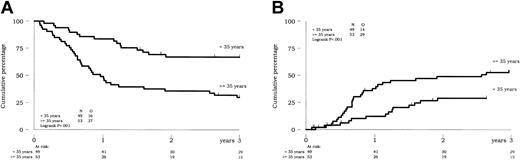
Univariate analysis. When considered as a continuous variable, advanced age significantly worsened DFS in the 102 CR patients (P = .001). The optimal age cutpoint was 35 years. As shown in Figure 1A, estimated 3-year DFS was 30% (95% CI, 18%-43%) in the 53 patients 35 years or older versus 67% (95% CI, 52%-78%) in the 49 younger patients (P < .001). Cumulative incidence of relapse according to these 2 age subgroups is indicated in Figure 1B. At 3 years, estimated cumulative incidence of relapse was 55% in older patients as compared with 29% in younger patients (P = .001). Conversely, cumulative incidences of death in CR did not significantly differ among both subgroups (15% versus 4% at 3 years, respectively; P = .31). Advanced age also influenced the survival of CR patients. At 3 years, estimated survival was 48% (95% CI, 34%-61%) in older patients as compared with 79% (95% CI, 64%-88%) in younger patients (P < .001). The only other baseline factor that influenced CR patients' outcome was low platelet count. Low platelet count significantly worsened the survival of CR patients (P = .01) but not their DFS (P = .20) (when considered with the cutpoint of 30 × 109/L). As a matter of fact, a platelet count less than 30 × 109/L did not influence the risk of relapse in these patients (P = .52) but was associated with a higher cumulative incidence of death in CR. At 3 years, estimated cumulative incidence of death in CR was 19% in the 21 CR patients with baseline platelet count less than 30 × 109/L versus 5% in the remaining CR patients (P = .005). Neither the presence of additional chromosome structure or number abnormalities nor the persistence of normal metaphases (AN status) had significant impact on CR patient outcome.
Prognostic impact of age on outcome of CR patients. (A) DFS. (B) Cumulative incidence of relapse.
Prognostic impact of age on outcome of CR patients. (A) DFS. (B) Cumulative incidence of relapse.
A comparison of the 4 IDAC-1, IDAC-2, HDAC-2, and ALLO postremission treatment groups is shown in Table 4 on an intent-to-treat basis. A strong interaction between median age and trial was obviously present, because of the fact that the pediatric study was the only to include IDAC-2 as postremission therapy (Table 4). Consequently, the 3 chemotherapy groups were not well balanced for median age (younger in the IDAC-2 group), median WBC (higher in the IDAC-2 group), presence of associated chromosome abnormalities (lower in the IDAC-2 group), and AN status incidence (higher in the IDAC-2 group). However, the 2 CHEMO and ALLO groups were comparable for all these covariates (P = .08, .89, .99, and .78, for age, WBC, presence of associated abnormalities, and AN status, respectively).
Comparison of intent-to-treat postremission treatment groups
. | IDAC-1 . | IDAC-2 . | HDAC-2 . | CHEMO* . | ALLO . |
|---|---|---|---|---|---|
| Patients, no. | 22 | 17 | 47 | 72 | 16 |
| Study, no. | |||||
| LAME-91 | 0 | 17 | 0 | 17 | 6 |
| ALFA-9000 | 22 | 0 | 0 | 17 | 0 |
| BGMT-87 | 0 | 0 | 6 | 6 | 5 |
| BGMT-91 | 0 | 0 | 10 | 8 | 1 |
| GOELAM-01 | 0 | 0 | 9 | 7 | 0 |
| GOELAM-02 | 0 | 0 | 22 | 17 | 4 |
| Sex, M/F | 10/12 | 8/9 | 27/20 | 36/36 | 9/7 |
| Median age, y (range) | 35 (15-64) | 5 (0.7-16) | 43 (18-59) | 32 (0.7-49) | 24 (1.9-39) |
| Median WBC, × 109/L (range) | 35 (3-228) | 84 (7-240) | 28 (3-186) | 43 (3-240) | 41 (8-238) |
| Associated chromosome abnormalities, no. | 8 | 3 | 15 | 20 | 4 |
| AN/AA status, † no. | 9/12 | 10/7 | 19/28 | 32/39 | 6/10 |
| Two cycles required for CR achievement, no. | 2 | 0 | 3 | 3 | 0 |
. | IDAC-1 . | IDAC-2 . | HDAC-2 . | CHEMO* . | ALLO . |
|---|---|---|---|---|---|
| Patients, no. | 22 | 17 | 47 | 72 | 16 |
| Study, no. | |||||
| LAME-91 | 0 | 17 | 0 | 17 | 6 |
| ALFA-9000 | 22 | 0 | 0 | 17 | 0 |
| BGMT-87 | 0 | 0 | 6 | 6 | 5 |
| BGMT-91 | 0 | 0 | 10 | 8 | 1 |
| GOELAM-01 | 0 | 0 | 9 | 7 | 0 |
| GOELAM-02 | 0 | 0 | 22 | 17 | 4 |
| Sex, M/F | 10/12 | 8/9 | 27/20 | 36/36 | 9/7 |
| Median age, y (range) | 35 (15-64) | 5 (0.7-16) | 43 (18-59) | 32 (0.7-49) | 24 (1.9-39) |
| Median WBC, × 109/L (range) | 35 (3-228) | 84 (7-240) | 28 (3-186) | 43 (3-240) | 41 (8-238) |
| Associated chromosome abnormalities, no. | 8 | 3 | 15 | 20 | 4 |
| AN/AA status, † no. | 9/12 | 10/7 | 19/28 | 32/39 | 6/10 |
| Two cycles required for CR achievement, no. | 2 | 0 | 3 | 3 | 0 |
WBC indicates white blood cell count.
This CHEMO group comprised all patients from the IDAC-1, IDAC-2, and HDAC-2 groups if aged 50 years or younger.
In 101 CR patients with at least 5 analyzed metaphases.
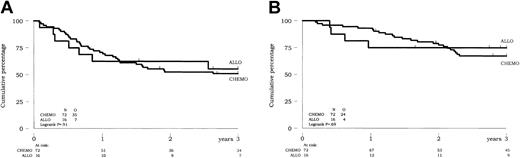
Using intent-to-treat analysis in the 88 CR patients aged 50 years or younger, no difference in DFS or survival was observed between patients allocated to receive allogeneic HSCT in first CR and those allocated to receive chemotherapy (P = .91 and .69, respectively) (Figure 2). Estimated 3-year DFS was 56% versus 51% in the ALLO and CHEMO groups, respectively. Estimated 3-year survival was 75% versus 66% in the ALLO and CHEMO groups, respectively. Of note, 14 of the 16 patients from the ALLO group and no patient from the CHEMO group were actually allografted in first CR. Similarly, no difference in outcome was observed between the ALLO and CHEMO groups in the more limited population of patients aged younger than 35 years (P = .73 for DFS and .87 for survival of CR patients).
Prognostic impact of allogeneic HSCT versus chemotherapy on outcome of CR patients up to 50 years of age (intent-to-treat analysis). (A) DFS. (B) Overall survival of CR patients.
Prognostic impact of allogeneic HSCT versus chemotherapy on outcome of CR patients up to 50 years of age (intent-to-treat analysis). (A) DFS. (B) Overall survival of CR patients.
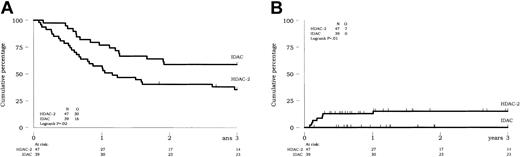
The outcome of the 86 CR patients allocated to receive 1 of the 3 classes of postremission chemotherapy was not easily comparable in univariate analysis, because of interactions mentioned above. Despite these interactions, patients from both IDAC-1 and IDAC-2 groups had comparable outcome, even after adjustment on covariate (not shown), and were considered in a single IDAC group of 39 patients. In univariate analysis, a better outcome was observed in these IDAC patients as compared with those patients from the HDAC-2 group (P = .008 and .02 for survival and DFS, respectively) (Figure 3A for DFS). This was related to a lower rate of death in CR (P = .01) (Figure 3B) rather than a lower cumulative incidence of relapse (P = .13).
Prognostic impact of the postremission chemotherapy (IDAC versus HDAC) on outcome of CR patients (intent-to-treat analysis). (A) DFS. (B) Cumulative incidence of death in CR.
Prognostic impact of the postremission chemotherapy (IDAC versus HDAC) on outcome of CR patients (intent-to-treat analysis). (A) DFS. (B) Cumulative incidence of death in CR.
Multivariate analysis. Finally, the Cox model was used to evaluate the following variables affecting survival of CR patients: age (as a continuous variable or with the cutpoint of 35 years), platelet count (with the cutpoint of 30 × 109/L), persistence of normal metaphases, postremission therapy according to the intent-to-treat ALLO/CHEMO classification, and trial. Platelet count was not included in a similar analysis for DFS. In the 86 CR patients not allocated to receive allogeneic HSCT in first CR, multivariate analysis was performed, including the following covariates for survival: age, platelet count, persistence of normal metaphases, trial, and postremission therapy according to the intent-to-treat IDAC/HDAC-2 classification. Again, platelet count was not included in a similar analysis for DFS.
Results are shown in Table 5. As indicated, advanced age was a bad-prognosis factor for DFS and survival in CR patients, either in the whole population or in the subgroup of patients from the CHEMO group. Low platelet count remained an independent prognostic factor for survival in these patients. In the population of patients not allocated to receive allogeneic SCT in first CR, patients from both IDAC and HDAC-2 chemotherapy groups had similar outcome in this multivariate setting.
Multivariate analyses for DFS and overall survival of CR patients: P values
. | All patients, n = 102 . | . | . | . | Patients from the CHEMO group, n = 86 . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | DFS . | . | Survival . | . | DFS . | . | Survival . | . | ||||||
| Age | ||||||||||||||
| Continuous variable | .009 | — | .01 | — | .02 | — | .03 | — | ||||||
| Cutpoint at 35 years | — | .002 | — | .02 | — | .003 | — | .05 | ||||||
| HR (95% CI) | — | 2.9 (1.5-5.6) | — | 2.8 (1.2-6.8) | — | 3.0 (1.5-6.2) | — | 2.5 (1.0-6.2) | ||||||
| Platelet count | ||||||||||||||
| Cutpoint at 30 g/L | — | — | .01 | .03 | — | — | .05 | .12 | ||||||
| HR (95% CI) | — | — | — | 2.3 (1.1-4.9) | — | — | — | — | ||||||
| AN status | .36 | .26 | .40 | .47 | .41 | .29 | .47 | .56 | ||||||
| Trial* | .23 | .22 | .57 | .42 | .68 | .57 | .79 | .66 | ||||||
| ALLO versus CHEMO | .53 | .62 | .71 | .62 | — | — | — | — | ||||||
| IDAC versus HDAC-2 | — | — | — | — | .31 | .36 | .54 | .46 | ||||||
. | All patients, n = 102 . | . | . | . | Patients from the CHEMO group, n = 86 . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | DFS . | . | Survival . | . | DFS . | . | Survival . | . | ||||||
| Age | ||||||||||||||
| Continuous variable | .009 | — | .01 | — | .02 | — | .03 | — | ||||||
| Cutpoint at 35 years | — | .002 | — | .02 | — | .003 | — | .05 | ||||||
| HR (95% CI) | — | 2.9 (1.5-5.6) | — | 2.8 (1.2-6.8) | — | 3.0 (1.5-6.2) | — | 2.5 (1.0-6.2) | ||||||
| Platelet count | ||||||||||||||
| Cutpoint at 30 g/L | — | — | .01 | .03 | — | — | .05 | .12 | ||||||
| HR (95% CI) | — | — | — | 2.3 (1.1-4.9) | — | — | — | — | ||||||
| AN status | .36 | .26 | .40 | .47 | .41 | .29 | .47 | .56 | ||||||
| Trial* | .23 | .22 | .57 | .42 | .68 | .57 | .79 | .66 | ||||||
| ALLO versus CHEMO | .53 | .62 | .71 | .62 | — | — | — | — | ||||||
| IDAC versus HDAC-2 | — | — | — | — | .31 | .36 | .54 | .46 | ||||||
Each column in the table represents a separate multivariate analysis.
HR indicates hazard ratio; CI, confidence interval; and —, not applicable.
LAME-91, ALFA-9000, BGMT-87, BGMT-91, GOELAM-01, or GOELAM-02.
Comparative significance of age and WBC in inv(16)/t(16;16) versus t(8;21) AML
In the 161 patients with t(8;21) AML included in the same trials during the same period of time, we have identified baseline WBC (and a derived WBC index taking into account the percentage of marrow blasts) as the sole prognostic factor for relapse and outcome after CR achievement.22 In these patients (median age, 28 years), age itself had no prognostic impact either on CR duration, DFS, or overall survival. Conversely, advanced age was here identified as the main prognostic factor for relapse and outcome after CR achievement in patients with inv(16)/t(16;16) AML, while WBC was of no prognostic value.
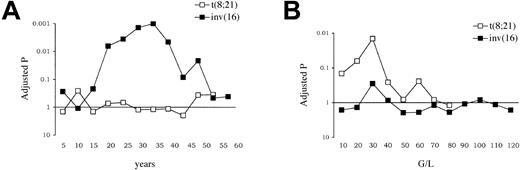
These contrary observations are summarized in Figure 4. Results of optimal age cutpoint analysis for DFS according to the cytogenetic subtype are shown in Figure 4A, which clearly indicates the prognostic impact of age in inv(16)/t(16;16) AML while not in t(8;21) AML patients. To study if this observation might be related to a lower compliance to the entire planned protocol in older patients, a comparative analysis of postremission therapy actually received was performed among 2 age subgroups using the optimal 35-year cutpoint. Among the 86 CR patients from the CHEMO group, 37 patients were aged younger than 35 years and 49 patients were aged 35 years or older. No significant differences in postremission therapy actually received at the planned dosage were found among these 2 subgroups of patients. Rates of first and second consolidation cycles (as defined in Table 1) delivered at the planned dosage were 95% and 80% in the younger group versus 94% and 75% in the older group (P = .99 and .60, respectively). Results of optimal WBC cutpoint analysis for DFS according to the CBF subtype are shown in Figure 4B, which conversely indicates the prognostic impact of WBC in t(8;21) AML while not in inv(16)/t(16;16) AML patients.
Comparative evaluation of age and WBC prognostic significance in t(8;21) and inv(16)/t(16;16) AML. (A) Adjusted P values for DFS according to selected age cutpoints. (B) Adjusted P values for DFS according to selected WBC cutpoints. P values for DFS comparison, after adjustment based on an approximation to the improved Bonferroni inequality,16 are given according to selected age (A) and WBC (B) cutpoints in each CBF-AML subtype. In inv(16)/t(16;16) AML, the optimal age cutpoint was 35 years (adjusted P value = .001). In t(8;21) AML, the optimal WBC cutpoint was 30 × 109/L (adjusted P value = .014).
Comparative evaluation of age and WBC prognostic significance in t(8;21) and inv(16)/t(16;16) AML. (A) Adjusted P values for DFS according to selected age cutpoints. (B) Adjusted P values for DFS according to selected WBC cutpoints. P values for DFS comparison, after adjustment based on an approximation to the improved Bonferroni inequality,16 are given according to selected age (A) and WBC (B) cutpoints in each CBF-AML subtype. In inv(16)/t(16;16) AML, the optimal age cutpoint was 35 years (adjusted P value = .001). In t(8;21) AML, the optimal WBC cutpoint was 30 × 109/L (adjusted P value = .014).
Discussion
Given relative rarity and comparable outcome, patients with inv(16)/t(16; 16) AML are frequently collectively studied with those with t(8;21) AML in a single group of good-risk CBF-AML.18-20 For instance, the 3-year DFS estimate of 48% observed in patients with inv(16)/t(16;16) AML from the present study is not significantly different than the 3-year DFS estimate of 55% we have recently reported in patients with t(8;21) AML treated in the same trials, even after adjustment on covariates including age, WBC, AN status, allogeneic SCT allocation, and trials.22,37 For this reason, it is often impossible to comparatively analyze prognostic factors in these patients. One may, however, suspect important differences in the prognostic significance of age and/or initial hematologic presentation between t(8;21) and inv(16)/t(16;16) AML patients. One may also discuss the value of HDAC as postremission therapy in inv(16)/t(16;16) AML as compared with t(8;21) AML patients. We recently reported the results of a large prognostic study in 161 patients with t(8;21) AML.22 To our knowledge, the present report is the largest prognostic study in those with inv(16)/t(16;16) AML.
The first and rather unexpected result of this study was the prognostic impact of age in patients with inv(16)/t(16;16) AML. Advanced age is commonly associated in patients with AML with lower CR rate and shorter survival. This well-known observation is related to the combination of 2 causal factors. First, different biologic features, such as unfavorable cytogenetics and multidrug resistance phenotype, may explain a poor response of elderly AML cells to current therapies and a higher risk of relapse. Secondly, older patients poorly tolerate intensive chemotherapy, with a higher rate of treatment-related deaths. From this point of view, it was actually surprising to find that advanced age was associated with a higher risk of relapse rather than a higher toxic mortality in such a well cytogenetically defined AML subgroup. This result, which was not observed in t(8;21) AML patients, suggests that inv(16)/t(16; 16) AML biology must differ in older as compared with younger patients with the same disease. First, persistent normal metaphases were less frequently observed in older patients. Second, we found more frequent additional chromosome abnormalities (especially chromosome structure abnormalities) in patients aged 35 years or older. This feature, which was not associated with a worse outcome, here or elsewhere,13 might nevertheless be a marker for increasing genetic instability in older patients, even within this inv(16)/t(16;16) AML subgroup. In this context, relevant gene alterations remain to be determined. The role of known tyrosine kinase receptor mutations (FLT3, c-kit) seems unlikely. Internal tandem duplications of the FLT3 gene did not appear to be frequent in the CBF-AML subset.38-40 The prognostic role of c-kit mutations, which have been reported in CBF-AML, warrants, however, further studies.14,15,41
The second main result of the present study was the observation that allogeneic SCT did not significantly improve the outcome of patients with inv(16)/t(16;16) AML in first CR. This result provides an additional support to data recently reported by the British Medical Research Council.20,42,43 Given these concordant observations and the higher morbidity known to be associated with allogeneic SCT, it may be suggested to use allogeneic SCT in second rather than in first CR in these patients. Definition of potential high-risk patients with inv(16)/t(16;16) AML that might benefit from transplantation in first CR remains very hazardous. With that respect, our results did not suggest that patients with high initial WBC were at higher risk for relapse and would thus have to be differently managed once the CR was achieved.
The Cancer and Leukemia Group B (CALGB) has emphasized the value of high doses of cytarabine (HDAC) as consolidation therapy in patients with CBF leukemia.21 The impact of HDAC and more generally of regimens used as postremission chemotherapy cannot be readily analyzed in our study, because of interactions between protocol designs and other important covariates, such as age. The higher toxicity associated with HDAC-containing cycles, reminiscent of results of the large randomized CALGB study,44 leading to a worse outcome in the present study was only observed in univariate analysis and needs to be confirmed by larger studies. On the other hand, it is quite impossible to compare the 78% 5-year DFS observed in the 18 patients with t(8;21) or inv(16) AML treated in the HDAC arm of the CALGB study18 with the 48% 3-year DFS reported here in 110 patients with inv(16)/t(16;16) AML. Numerous factors such as low number of patients and potential differential effect of HDAC in t(8;21) as compared with inv(16)/t(16;16) AML hamper such a comparison.
In conclusion, our study provides new data encouraging (1) more careful approaches to induce remission in patients with inv(16)/t(16;16) AML with high initial WBC and low initial platelet count; (2) remission consolidation based on repeated cycles of moderately toxic chemotherapy rather than allogeneic SCT in first CR; and (3) further investigations for additional genomic events in older patients with this specific subtype of AML.
Appendix
Participating investigators from the GOELAM were: JL Harousseau, N Milpied, P Moreau (Centre Hospitalier Universitaire [CHU], Nantes); F Witz, B Witz (CHU, Nancy); JY Cahn, F Garnache (CHU, Besançon); D Caillot, E Solary, F Mugneret (CHU, Dijon); B Lioure, A Falkenrodt (CHU, Strasbourg); T Lamy, B Drenou (CHU, Rennes); F Guilhot (CHU, Poitiers); M Delain (CHU, Tours); B Desablens, J Fernandes (CHU, Amiens); JF Abgrall, C Berthou (CHU, Brest); N Ifrah, S François, M Hunault-Berger (CHU, Angers); B Pignon, LF Vilque (CHU, Reims); D Guyotat, L Campos-Guyotat, C Mounier (CHU, Saint-Etienne); J Brière, C Gardin (Hôpital Beaujon, Clichy); P Casassus (Hôpital Avicenne, Bobigny); and B Audhuy (Centre Hospitalier, Colmar).
Participating investigators from the LAME group were: A Baruchel, T Leblanc, G Schaison (Hôpital Saint-Louis, Paris); AAuvrignon, J Landman-Parker, G Leverger (Hôpital Trousseau, Paris); G Michel, I Thuret (CHU, Marseille); JH Dalle, B Nelken (CHU, Lille); C Schmitt (CHU, Nancy); Y Perel (CHU, Bordeaux); V Gandemer (CHU, Rennes); JP Vannier (CHU, Rouen); JP Lamagnere (CHU, Tours); L de Lumley (CHU, Limoges); B Bader-Meunier (Hôpital du Kremlin-Bicetre, Paris); J Pico (Institut Gustave Roussy, Villejuif); G Couillaud (CHU, Dijon); F Mechinaud (CHU, Nantes); A Fischer (Hôpital Necker, Paris); S Lemerle (Centre Hospitalier Intercommunal, Créteil); C Berthou (CHU, Brest); and F Demeocq (CHU, Clermont-Ferrand).
Participating investigators from the ALFA were: S Castaigne, MT Daniel, L Degos, H Dombret, E Gluckman, JM Micléa (Hôpital Saint-Louis, Paris); E Archimbault, C Charrin, D Fière, X Thomas (Hôpital Edouard Herriot, Lyon); F Bauters, P Fenaux, JP Jouet, C Preudhomme (CHU, Lille); C Bastard, A Stamatoullas-Bastard, H Tilly (Centre Henri Becquerel, Rouen); G Auzanneau, T de Revel, G Nedellec, (Hôpital du Val de Grâce, Paris); D Bordessoule (CHU, Limoges); B Grosbois, R Leblay (Hôpital Sud, Rennes); G Tertian (Hôpital Antoine Béclère, Clamart); JM Zini, E Dupuy (Hôpital Lariboisière, Paris); M Janvier, F Turpin (Centre René Huguenin, Saint-Cloud); F Bauduer, M Renoux (Centre Hospitalier de la Côte Basque, Bayonne); J Jaubert (Hôpital Saint-Anne, Toulon); B Dupriez, P Morel (Centre Hospitalier, Lens); M Simon (Centre Hospitalier, Valenciennes); M Legros (Centre Jean Perin, Clermont-Ferrand); and M Schoenwald (Hôpital La Source, Orléans).
Participating investigators from the BGMT group were: JM Boiron, A Broustet, P Cony-Makhoul, G Marit, A Pigneux, J Reiffers (Hôpital Haut-Lévèque, Bordeaux); O Boulat, G Lepeu (Centre Hospitalier, Avignon); R Gressin, L Molina, JJ Sotto (CHU, Grenoble); D Blaise, R Bouabdallah, JA Gastaut, D Marininchi, AM Stoppa, N Vey (Institut Paoli Calmettes, Marseille); V Capdevilla, N Fegueux, E Jourdan, E Navarro, JF Rossi (CHU, Montpellier); and M Attal, N Dastugue, G Laurent, J Pris, F Rigal-Huguet (CHU, Toulouse).
All centers are located in France.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-11-3527.
A complete list of the members of the French Acute Myeloid Leukemia (AML) Intergroup appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ``advertisement'' in accordance with 18 U.S.C. section 1734.
We are indebted to Wim van Putten for providing Stata packages with facilities for Kaplan-Meier survival curves and cumulative incidence estimations and to Gérard Socié for critical review of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal